?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The shortage of PFF2, N95, KN95, and FFP2 respirators in the COVID-19 pandemic required that alternative respiratory protection measures be adopted to reduce the number of cases of the disease. Given this scenario, with the purpose of proving that chemical cartridges offer some degree of protection against aerodispersoids, among these those of biological origin, such as SARS-CoV-2, the filtering efficiency of chemical cartridges was evaluated. The evaluation of the filtering efficiency of cartridges was performed using the air filter test method, which uses micrometric air particles for the penetration test. The research proved that chemical cartridges, in general, have an average efficiency above 85% for respiratory protection against aerodispersoids. The multigas cartridge and organic vapor/acid gas cartridge had an average filtration efficiency of 91%. Chemical cartridges associated with facial or semi-facial respirators can become powerful resources for respiratory protection for people exposed to the virus, when the use of PFF2 or equivalent respirators is not possible.
PUBLIC INTEREST STATEMENT
The shortage of PFF2 respirators and equivalents (N95, KN95, FFP2, P2, Korea 1st Class and DS) in several countries during the COVID-19 pandemic forced many public managers and scientists to look for alternatives for respiratory protection. This article provides valuable information on alternative respiratory protection that can provide levels of effectiveness close to PFF2 respirators in emergency situations.
1. Introduction
The COVID-19 pandemic caused by the SARS-CoV-2 virus and its variants has had catastrophic impacts on public health and the world economy (Duff et al., Citation2021; Ibn-Mohammeda et al., Citation2021). The rapid spread of the disease on several continents shows that respiratory protection measures, such as the use of N95 respirators or equivalent (PFF2, KN95, FFP2, P2, Korea 1st Class and DS), is essential to reduce and control the number of cases (Howard et al., Citation2021). The great demand for this product has generated an unprecedented worldwide shortage (Kirubarajan et al., Citation2020). To make up for the lack of personal protective equipment (PPE), alternative measures were adopted, such as the use of surgical masks, handmade masks, the reuse of respirators for long periods and even the development of research aimed at decontaminating for PPE, however, demand remains greater than supply (Cai & Floyd, Citation2020; Fischer et al., Citation2020; Morais et al., Citation2021).
The evaluation of the efficiency of N95 respirators and their equivalents is done through the exposure of the respirator, in a closed system, to aerodispersoids of liquid origin, on a micrometric scale, containing an aqueous solution of NaCl. These respirators are certified by the respective countries for respiratory protection against solid and liquid aerosols (dusts, fumes and mists) from this test (NBR ABNT 13698, Citation2011; Plana et al., Citation2021).
The nanometric nature of the SARS-CoV-2 virus gives it a great availability to form clusters with other particles in the air; therefore, despite the virus having a diameter of approximately 125 nm, under real conditions, it is possibly associated with other particles of different sizes, as well as other pathogens (Pan et al., Citation2019; Zhu et al., Citation2020). For this reason, N95 respirators and their equivalents are used as the main respiratory protection measure in the COVID-19 pandemic, even though they are certified to filter particles with a diameter greater than 0.3 µm.
Unlike these respirators, chemical cartridges coupled to a reusable facial or semi-facial respirator are effective in the respiratory protection of people exposed to certain types of gases and vapors, such as ammonia, methylamine, chlorine, mercury, formaldehyde, acid gases and organic vapors; however, they are not recommended by the manufacturers for respiratory protection against solid or liquid aerodispersoids, among these biological agents (Dharmarajan et al., Citation2001).
The pandemic scenario imposed on several nations the need to develop pharmacological and non-pharmacological alternatives for the prevention and control of the disease; in this sense, with the objective of proving that chemical cartridges offer some degree of protection for people exposed to aerodispersoids, the research evaluated the efficiency filtering chemical cartridges against solid aerodispersoids with a diameter close to the SARS-CoV-2 virus. The finding that chemical cartridges offer some degree of protection in the filtration of aerodispersoids, and specifically particles with a diameter close to that of SARS-CoV-2, significantly increases the number of respiratory protection measures that can be used, on an emergency basis, against respective virus causing COVID-19 and even against other pathogens (Wang et al., Citation2021).
To assess the efficiency of chemical cartridges against aerodispersoids, a method was developed that uses solid particles from atmospheric air itself as a test agent, given that biological agents are also solid aerodispersoids, but may also be available in the form of mist.
2. Method
The research of an experimental nature and analytical approach was developed at the Occupational Hygiene Laboratory of the Federal Institute of Pernambuco, located in the city of Abreu e Lima, State of Pernambuco, Brazil. The aim of the study was to evaluate the filtration efficiency of 5 types of chemical cartridges (Table ): ammonia/methylamine cartridge, formaldehyde cartridge, mercury vapor/chlorine gas cartridge, multigases cartridge, organic vapor/acid gas cartridge; against solid aerodispersoids with a diameter greater than 0.3 µm from the air filter test method. As biological agents are also aerodispersoids, it is possible that chemical cartridges are considered, on an emergency basis, to help protect individuals exposed to SARS-CoV-2.
Table 1. Characteristics of chemical cartridges used in efficiency tests
2.1. Experimental
The experiment used solid particles with a diameter: 0.3 µm—10 µm from the laboratory’s own air as a test agent. The research considers that, under real conditions, the virus is released into the air by aerosol and forms agglomerates of various sizes with other solid and liquid particles present in the mouth and nose of human beings and thousands of natural and anthropogenic particles suspended in the air (Asadi et al., Citation2020; Clapp et al., Citation2021; Gallo et al., Citation2021; Matuck et al., Citation2021; Niu et al., Citation2014).
To evaluate the filtering efficiency of chemical cartridges against aerodispersoids, an air filter test method was developed. The experimental apparatus of the method (figure ) was positioned at a height of 1.3 m from the ground, the mean height of the respiratory zone of a seated individual. Before starting the process of evaluating the filtration efficiency of each chemical cartridge, possible sources of air currents in the environment were eliminated.
Figure 1. Schematic diagram of the cartridges and PFF2 respirators efficiency evaluation system using micrometric particles of the air.
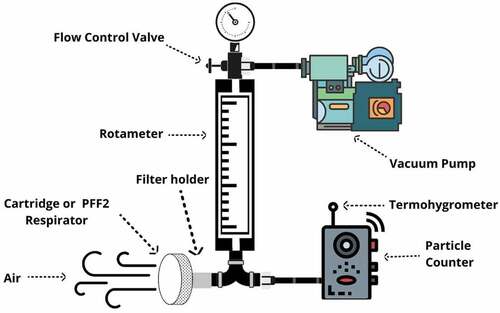
The particle counter was calibrated and then the chemical cartridge to be evaluated was placed in the chemical cartridge holder. The method starts from the air from the environment itself, which is sucked by the vacuum pump at a flow rate of 95 L/min. This flow rate was adopted because it is the flow rate used in the penetration test in evaluating the filtration efficiency of PFF2 respirators and because it simulates the breathing of an individual performing an extremely heavy activity (NBR ABNT 13698, Citation2011). Some particles dispersed in the air were filtered by the chemical cartridges; the particles that penetrated were quantified by the particle counter. The particle counter operated at a flow rate of 2.8 L/min for a fixed counting time of 10 minutes, that is, it sampled 28 liters of air. In addition to the requirements described above, this method was performed adopting the following operating conditions: Air temperature (T): 0°C < T < 50°C; Relative air humidity (U): 10% < U < 90%.
For each chemical cartridge evaluation performed, immediately, the same procedure was performed without the presence of the chemical cartridge. Having obtained the number of particles that pass through the cartridge and the number of particles counted without the cartridge, the filtration efficiency was calculated, according to equation:
PCWC = Number of particles counted with sample;
PCNCW = Number of particles counted without sample.
2.2. Control quality
To guarantee the quality control of the method, the filtration efficiency of 36 units of PFF2–9920 H respirators from 3 M manufacturer was evaluated, certified by the Ministry of Labor and Social Security of Brazil (Certificate of Approval: 17611) and approved by the Surveillance Agency Health in the country (Registration: 80284930200). The respirators used in the research have an average mass of 8 g, are foldable (2 panels), non-valved and have bacteriological filtration efficiency (BFE)>99%. This respirator is equivalent to the N95 (USA), FFP2 (European Union), KN95 (China), P2 (Australia/New Zealand), Korea 1st Class (South Korea) and DS (Japan) respirator.
The certification obtained in Brazil confers on this respirator official proof of a minimum efficiency of 94% for protection against solid and liquid aerodispersoids with a diameter greater than 0.3 µm. The evaluation of the efficiency to obtain certification is carried out in accordance with the guidelines of the Brazilian technical standard (NBR ABNT 13698, Citation2011).
Assuming that PFF2 respirators have a minimum filtration efficiency of 94% for aerodispersoids, the efficiency of these respirators was evaluated using the air filter test method. The test follows the same process as for chemical cartridge evaluations, with the exception of the filter holder that was adapted for the respirator. To evaluate the results, the statistical process control chart was used, in which efficiency values above 94% were established as satisfactory results and unsatisfactory if any value obtained was below 94%. A mobile range chart of respirator efficiency was also created to assess possible instabilities of the method during the evaluation process.
2.3. Scanning electron microscopy (SEM)
To perform the SEM micrograph by secondary electrons of the chemical cartridges, the Quanta 200 FEG, from the FEI manufacturer, and the Everhart Thornley detector (ETD) were used. The images were obtained in a high vacuum, at a temperature of 21° C and a relative humidity of 76%.
2.4. Statistical analysis
To assess the quality of the method, the raw data from the evaluation of the efficiency of the PFF2 respirators (N = 36), obtained by the air filter test method, was tested for normality (Shapiro-Wilk test) and subsequently elaborated a statistical process control chart and mobile range chart of efficiency, in which a lower and upper control limit was established. A student’s t test was performed for a level of 99% of hypothesis, significant as alternative hypothesis (H1) > 94.0 and null hypothesis (H0) .
The raw data of the filtering efficiency of chemical cartridges were treated using the concepts of descriptive statistics and tested for normality (Shapiro-Wilk test) and homogeneity of variances (Levene test). Afterwards, ANOVA was applied, followed by Games-Howell post-hoc test to verify which interactions differ or not. To perform the tests, the number of samples described in Table was used. All statistical tests performed in this research were performed considering α = 0.050, except a student’s t-test.
3. Results
3.1. Results control quality
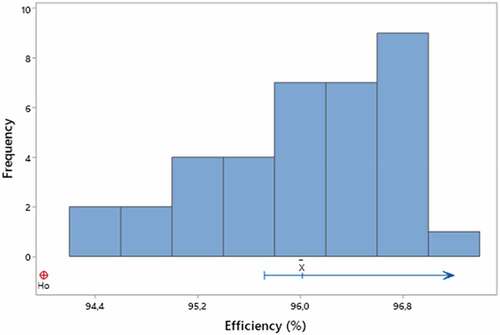
The data from the evaluation of the efficiency of PFF2 respirators presented normal distribution (p = 0.112) and values above 94% of efficiency ( = 96.0%; σ = 0.7). Based on the verification of data normality, a statistical process control chart was created to monitor the performance of the EPI in the efficiency evaluation process (figure ).
Figure 2. Statistical process control chart of the efficiency of PFF2 respirators taken by the air filter test method. LIC = Lower control limit. LSC = Upper control limit. N = Sample.
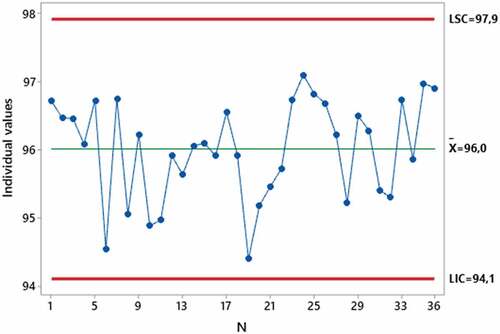
The statistical process control chart shows that the efficiency values obtained are between the lower control limit (LIC) and the upper control limit (LSC), which correspond to −3σ and +3σ respectively, so the results suggest a close probability 100% of the respirator’s efficiency values are within the range of −3σ to 3σ from the mean.
To monitor the variations in the process of evaluating the filtering efficiency of PFF2 respirators by the air filter test method, the quality control tool, mobile range chart, was used (Figure ).
Figure 3. Mobile range chart of the efficiency of PFF2 respirators obtained by the air filter test method. LIC = Lower control limit. LSC = Upper control limit. AM = moving range. N = Sample.
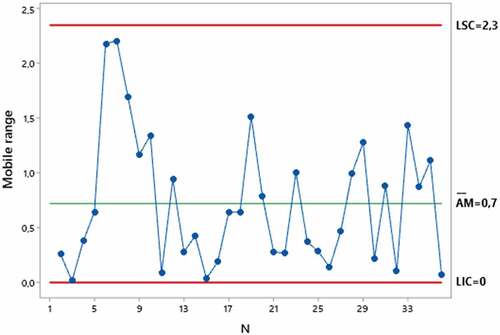
The results obtained demonstrate that there were no anomalous variations of the method during the process of evaluating the efficiency of respirators, as the data vary randomly around the center line and are within the upper and lower control limits; that is, no trends or patterns are present. Therefore, the variation of the efficiency evaluation process is stable.
To complement the result of the statistical process control chart, a student’s t-test was performed, considering as an alternative hypothesis (H1): > 94.0. The test result (p-value < 0.001) showed, for a significance level of 99%, that the sampled data set has a mean greater than 94.0; that is, the result obtained is the same as the certified value (
> 94.0; figure ).
3.2. Result of the efficiency of chemical cartridges
The results of the descriptive statistics of the filtering efficiency of chemical cartridges against aerodispersoids with a diameter greater than 0.3 µm (Ø > 0.3 µm; Table ) show that the average of all evaluated cartridges is above 85%.
Table 2. Descriptive statistics of chemical cartridge efficiency
Among the evaluated cartridges, the mercury vapor/chlorine gas cartridge stands out, which had the lowest average efficiency among all cartridges, even obtaining an average below the average of all cartridges. On the other hand, the multigas cartridges had the highest average efficiency, but it did not have the efficiency of a PFF2 or equivalent respirator.
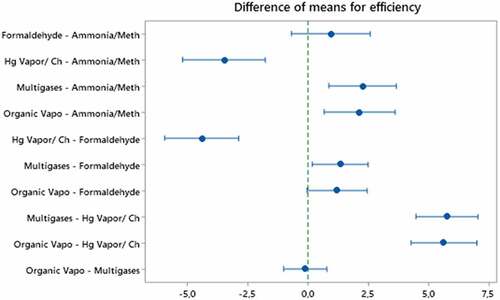
When analyzing the results of Table , it is inferred that there is a difference between the average efficiency of the evaluated chemical cartridges. To verify these inferences, data were tested for normality by the Shapiro-Wilk test (p =0.08) and for equality of variance by the Levene test (p <0.001), considering α =0.050. Once the normality condition was satisfied, the ANOVA was performed, which showed a significant difference between the cartridges in terms of efficiency (p <0.001). Not meeting the criterion of equality of variances, to verify which chemical cartridges present a significant difference between them, the Games-Howell post-hoc test was used, a statistical test similar to the Tukey test, which does not require equality of variances as a prerequisite (figure ).
Interpretation of the Games-Howell graph is simple. If an interval does not contain zero, it means that the corresponding means will be significantly different. Observing Figure , it can be seen that despite the ANOVA showing that the mean of chemical cartridges is not statistically equal, the Games-Howell test shows that the mean efficiency of formaldehyde cartridges does not differ statistically from the mean of organic vapor cartridges /acid gases and ammonia/methylamine, moreover, the average of multigas cartridges does not differ significantly from the average of organic vapor/acid gases cartridges. From the results presented, it appears that chemical cartridges have different levels of protection against aerodispersoids with a diameter greater than 0.3 µm and cannot have their generalized efficiency for respiratory protection purposes, especially against aerodisperoids of biological origin, such as the SARS-CoV-2 virus.
3.3. Micrograph results
Chemical cartridges consist of granular activated carbon, surrounded by a polymeric fibrous material and a plastic cartridge. Activated carbon is chemically treated to promote chemical adsorption of gas or steam. In the case of efficiency for protection against aerodispersoids, demonstrated in this research, by chemical cartridges, the chemical adsorption mechanism does not seem to be valid. show how chemical cartridges retain aerodispersoids.
Figure 6. Micrograph of the polymeric fibrous material surrounding the activated carbon within the organic vapor/acid gas cartridge analyzed by SEM. (A) Detail of fibers with adhesion of aerodispersoids. (B) Detail of the fiber exerting electrostatic capture of an aerodispersoid with a diameter smaller than 5 µm.
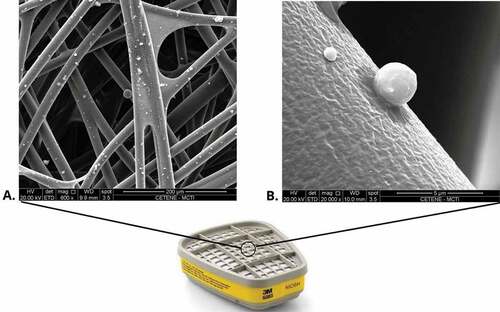
Figure 7. Detail of the capture of an aerodispersoid by one of the activated carbon pores of the cartridge.
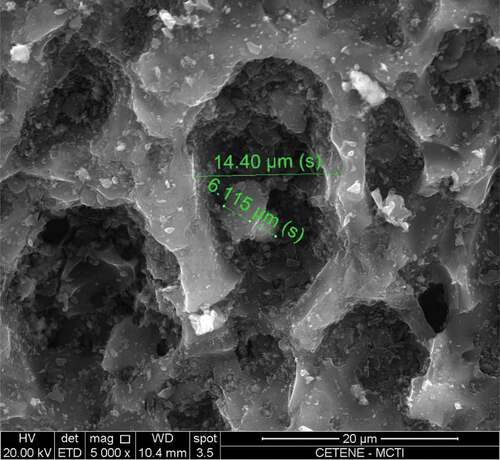
Analyzing the images, it can be seen that the polymeric fibrous material that surrounds the activated carbon inside the cartridge performs the function of a mechanical filter, electrostatically attracting aerodispersoids. It is also noticeable that some particles are trapped in the pores of the activated carbon, suggesting that, together with the fibrous material, they are responsible for the chemical cartridges offering some degree of efficiency in protecting against aerodispersoids.
4. Discussion
The statistical process control chart is a quality tool defined as a method of detecting problems in the evaluated processes. Its objective is to monitor a product during a process in order to identify non-conforming outputs (Ishikawa, Citation2012).
The results of the statistical process control chart were used to guarantee the quality control of the method used to assess the efficiency of the chemical cartridges and demonstrated that all evaluated PFF2 respirator units have efficiency above the LIC (94.1%) and below of the LSC (97.9%).
With all 36 units of PFF2 respirators evaluated above the LIC (94.1%), it appears that the result obtained is consistent with the product certification value, which establishes a minimum efficiency of 94%. Therefore, it appears that the air filter test method obtained the same efficiency result for the evaluated respirators as the method used by the manufacturer for the certification of respirators (NBR ABNT 13698, Citation2011).
The moving range chart is a quality management tool used to monitor process variation when working with individual observations (Ishikawa, Citation2012). For the purposes of this research, the moving amplitude chart was used to monitor possible anomalous variations in the efficiency evaluation process that could compromise the stability of the air filter test method over time. It is observed in the moving amplitude chart that none of the points is outside the control limits, and the points present a random pattern. Therefore, the process variation is under statistical control, that is, the air filter test method did not show anomalies during the process of evaluating the efficiency over time, indicating that this method is valid for evaluating the efficiency of filters.
The results of the efficiency of chemical cartridges showed that although all cartridges have the same shape and composition, with the exception of the activated carbon mass of the mercury vapor/chlorine gas cartridge, which has 5 g less than the other cartridges, the efficiency of filtration for aerodispersoids greater than 0.3 µm in diameter varies with cartridge type.
It is inferred that the difference between the means of chemical cartridges, evidenced by statistical tests, is explained by the type of chemical treatment that the activated carbon of each type of cartridge is subjected to. That is, some possibly favor the capture of aerodispersoids by means of its pores, such as the multigas cartridge and organic vapor/ acid gases cartridge, which had the highest average filtration efficiency.
In the case of the mercury vapor/chlorine gas cartridge, the lowest average for efficiency was observed among all evaluated cartridges. This fact is possibly associated with a smaller mass of activated carbon in the cartridge and with the type of chemical treatment that the activated carbon is submitted to.
When comparing the average efficiency of chemical cartridges with the efficiency of other types of respiratory protection used by the population during the COVID-19 pandemic (Table ), it is clear that chemical cartridges can increase the level of protection of the population in general compared to the SARS-CoV-2, as the filtering efficiency of the cartridges is superior to the efficiency of all handmade masks of various fabrics and surgical masks.
Table 3. Comparison of the filtering efficiency of different types of respiratory protection against aerodispersoids
The advantage of using chemical cartridges is not only limited to the excellent filtration efficiency proven in this research against other alternative respiratory protection measures, but also to the fact that reusable respirators, which are attached to chemical cartridges, can be sanitized and reused. In addition, chemical cartridges can also be reused differently from PFF2 respirators and their equivalents that are disposable; however, it is important to emphasize that this study did not assess for how long chemical cartridges can offer the levels of protection shown in this research.
Considering the persistence of the COVID-19 pandemic and the shortage of PFF2, N95, KN95 and FFP2 respirators in several countries, the use of chemical cartridges associated with a reusable half-face or full-face respirator presents itself as an innovative, effective and viable alternative for protection respiratory against SARS-CoV-2 virus.
5. Conclusion
The use of PFF2 respirators and their equivalents in several countries has been adopted as the main individual protection measure for the prevention and control of COVID-19. These respirators are effective against the exposure of individuals to solid and liquid aerodispersoids, including those of biological origin. However, the COVID-19 pandemic generated great demand for PPE in several countries, which consequently led to shortage of the product, and it forced public and private health managers and scientists to seek alternatives for the respiratory protection of the population.
Given this scenario, the results of the efficiency of chemical cartridges presented in this research demonstrate that despite the filtering efficiency not being the same as that of PFF2 respirators and their equivalents, they provide satisfactory levels of efficiency, superior to various types of handmade masks and even masks surgical procedures used by the general population. Therefore, it is understood that the use of chemical cartridges associated with semifacial or facial reusable respirators is feasible, on an emergency basis, when it is not possible to use PFF2 or equivalent respirators, or other alternative protection measure that offers greater efficiency in the protection against aerodispersoids.
The results obtained in this research can support the decision-making by health area managers to recommend or not the use of chemical cartridges associated with reusable semifacial or facial respirators, to the detriment of the shortage of PFF2 and equivalent respirators, experienced recently with the emergence of the COVID-19 pandemic in several countries.
Acknowledgements
The authors are especially grateful to the government agencies of Brazil: Ministry of Science, Technology, Innovation and Communications, Ministry of Health of Brazil - MoH and National Council for Scientific and Technological Development - CNPq.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
José D. Paiva
Dr. José Paiva is a Chemical Engineer and Professor at the Department of Occupational Safety at the Instituto Federal de Pernambuco, Abreu e Lima, Pernambuco, Brazil. Acted as Judicial Expert for courts of law in Brazil. Develops research in the field of respiratory protection. His main research interests are: risk management, work safety programs and respiratory protection equipment against COVID-19.
References
- Asadi, S., Cappa, C. D., Barreda, S., Wexler, A. S., Bouvier, N. M., & Ristenpart, W. D. (2020). Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Scientific Reports, 10(1), e15665. https://doi.org/10.1038/s41598-020-72798-7
- Cai, C., & Floyd, E. L. (2020). Effects of sterilization with hydrogen peroxide and chlorine dioxide on the filtration efficiency of N95, KN95, and surgical face masks. JAMA Network Open, 3(6), e2012099. https://doi.org/10.1001/jamanetworkopen.2020.12099
- Clapp, P. W., Sickbert-Bennett, E. E., Samet, J. M., Berntsen, J., Zeman, K. L., Anderson, D. J., Weber, D. J., & Bennett, W. D. (2021). Evaluation of cloth masks and modified procedure masks as personal protective equipment for the public during the COVID-19 pandemic. JAMA Internal Medicine, 181(4), 463–13. https://doi.org/10.1001/jamainternmed.2020.8168
- Dharmarajan, V., Lingg, R. D., & Myer, H. E. (2001). Evaluation of organic-vapor respirator cartridge efficiency for hexamethylene diisocyanate vapor in the presence of organic solvents. Applied Occupational and Environmental Hygiene, 16(3), 397–404. https://doi.org/10.1080/10473220117074
- Duff, J. H., Liu, A., Saavedra, J., Batycki, J. N., Morancy, K., Stocking, B., Gostin, L. O., Galea, S., Bertozzi, S., Zuniga, J. M., Alberto-Banatin, C., Dansua, A. S., Del Rio, C., Kulzhanov, M., Lee, K., Scaglia, G., Shahpar, C., Ullmann, A. J., Hoffman, S. J., … Szapocznik, J. (2021). A global public health convention for the 21st century. The Lancet Public Health, 6(6), 428–433. https://doi.org/10.1016/S2468-2667(21)00070-0
- Fischer, R. J., Morris, D. H., Van Doremalen, N., Sarchette, S., Matson, M. J., Bushmaker, T., Yinda, C. K., Seifert, S. N., Gamble, A., Williamson, B. N., Judson, S. D., Wit, E., Lloyd-Smith, J. O., & Munster, V. J. (2020). Effectiveness of N95 respirator decontamination and reuse against SARS-CoV-2 virus. Emerging Infectious Diseases, 26(9), 2253–2255. https://doi.org/10.3201/eid2609.201524
- Gallo, O., Locatello, L. G., Mazzoni, A., Novelli, L., & Annunziato, F. (2021). The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunology, 14(2), 305–316. https://doi.org/10.1038/s41385-020-00359-2
- Howard, J., Huang, A., Li, Z., Tufekci, Z., Zdimal, V., Westhuizen, H., Delft, A., Price, A., Fridman, L., Tang, L., Tang, V., Watson, G. L., Bax, C. E., Shaikh, R., Questier, F., Hernandez, D., Chu, L. F., Ramirez, C. M., & Rimoin, A. W. (2021). An evidence review of face masks against COVID-19. Proceedings of the National Academy of Sciences, 118(4), e2014564118. https://doi.org/10.1073/pnas.2014564118
- Ibn-Mohammeda, T., Mustaphab, K. B., Godsella, J., Adamuc, Z., Babatunded, K. A., Akintade, D. D., Acquayeg, A., Fujii, H., Ndiaye, M., Yamoahj, F. A., & Koh, S. C. L. (2021). A critical analysis of the impacts of COVID-19 on the global economy and ecosystems and opportunities for circular economy strategies. Resources, Conservation and Recycling, 164, 105169. https://doi.org/10.1016/j.resconrec.2020.105169
- Ishikawa, K. (2012). Introduction to Quality Control (3rd ed.). Chapman & Hall.
- Kirubarajan, A., Khan, S., Got, T., Yau, M., Bryan, J. M., & Friedman, S. M. (2020). Mask shortage during epidemics and pandemics: A scoping review of interventions to overcome limited supply. BMJ Open, 10(11), e040547. https://doi.org/10.1136/bmjopen-2020-040547
- Matuck, B. F., Dolhnikoff, M., Maia, G. V. A., Sendyk, D. I., Zarpellon, A., Gomes, C. S., Duarte-Neto, A. N., Rebello Pinho, J. R., Gomes-Gouvêa, M. S., Sousa, S. C. O. M., Mauad, T., Saldiva, H. D. N., Braz-Silva, P. H., & da Silva, L. F. F. (2021). Periodontal tissues are targets for Sars-Cov-2: A post-mortem study. Journal of Oral Microbiology, 13(1), 1848135. https://doi.org/10.1080/20002297.2020.1848135
- Morais, F. G., Sakano, V. K., Lima, L. N., Franco, M. A., Reis, D. C., Zanchetta, L. M., Jorge, F., Landulfo, E., Catalani, L. H., Barbosa, H. M. J., John, V. M., & Artaxo, P. (2021). Filtration efficiency of a large set of COVID-19 face masks commonly used in Brazil. Aerosol Science and Technology, 55(9), 1028–1041. https://doi.org/10.1080/02786826.2021.1915466
- NBR ABNT 13698. (2011). Respirator y protective devices — Filtering half mask to protect against particles.
- Niu, X., Guinot, B., Cao, J., Xu, H., & Sun, J. (2014). Particle size distribution and air pollution patterns in three urban environments in Xi’an, China. Environmental Geochemistry and Health, 37(5), 801–812. https://doi.org/10.1007/s10653-014-9661-0
- Pan, M., Lednicky, J. A., & Wu, C. Y. (2019). Collection, particle sizing and detection of airborne viruses. Journal of Applied Microbiology, 127(6), 1596–1611. https://doi.org/10.1111/jam.14278
- Plana, D., Tian, E., Cramer, A. K., Yang, H., Carmack, M. M., Sinha, M. S., Bourgeois, F. T., Yu, S. H., Masse, P., Boyer, J., Kim, M., Mo, J., LeBoeuf, N. R., Li, J., & Sorger, P. K. (2021). Assessing the filtration efficiency and regulatory status of N95s and nontraditional filtering face-piece respirators available during the COVID-19 pandemic. BMC Infectious Diseases, 21(1), 712–725. https://doi.org/10.1186/s12879-021-06008-8
- Wang, C. C., Prather, K. A., Sznitman, J., Jimenez, J. L., Lakdawala, S. S., Tufekci, Z., & Marr, L. C. (2021). Airborne transmission of respiratory viruses. Science, 373(6558), 981–993. https://doi.org/10.1126/science.abd9149
- Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., Zhao, X., Huang, B., Shi, W., Lu, R., Niu, P., Zhan, F., Ma, X., Wang, D., Xu, W., Wu, G., Gao, G. F., Phil, D., & Tan, W. (2020). A Novel Coronavirus from Patients with Pneumonia in China, 2019. The New England Journal of Medicine, 382, 727–733. https://doi.org/10.1056/NEJMoa2001017