?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Corrosion is one of the major issues faced by marine industries. Two of the major metals used in these industries include copper and AISI 5140 steel. This iterates the importance of understanding the microstructure and its influence on the corrosion behavior of these metals in 3.5 wt% NaCl that is studied here. Annealing treatment was performed for both the metals, and the microstructure before and after the annealing treatment was performed using an optical microscope and SEM. X-ray diffraction (XRD) of the metals before and after heat treatment was performed, and it was found that the annealing treatment caused an increase in the crystallite size irrespective of the metal. The samples were subjected to Vicker’s microhardness testing, and a decrease in the hardness was achieved post-annealing. The electrochemical studies further proved that there is an improvement in corrosion resistance post-annealing. The kinetic and thermodynamic parameters are described using Arrhenius and transition state theories.
1. Introduction
Corrosion is the process of degradation of metal due to the physicochemical interaction with its environment. The economic loss due to corrosion is high and is a major concern since the repair of the structures is found to be rather expensive (Ahmed et al., Citation2021). Chloride ions, which are present at about 80% volume percentage of seawater, are considered to be the most abundant medium for corrosion (Rodríguez-Gómez et al., Citation2019). Aqueous corrosion affects a wide range of applications including building the body of the ship’s hull, petroleum installations near the marine atmosphere, and various materials in contact with seawater (Coulibaly et al., Citation2018). Thus, it is important to develop newer techniques and modifications in metals to reduce the effect of corrosion.
A widely used alloy steel used in the marine engineering industry, characterized for its high machinability and tenacity, is AISI 5140 steel. AISI 5140 steel or EN18 steel is a type of dual-phase steel consisting of a pearlite phase embedded in the ferrite matrix. The pearlite phase of the steel consists of lamellar structures of cementite and ferrite phases. It is widely used in the production of gears, mechanical linkages, and transmission shafts after surface engineering. The cementite phase provides the metal with high strength and the ferrite phase provides characteristic ductility to the materials (Murase et al., Citation2021). One of the most common non-ferrous materials used in various industries is copper and its alloys. The malleable, ductile, and heat conductivity properties make it suitable for various applications such as the production of water-cooled copper cubicles, heat exchangers, and plumbing (Chang et al., Citation2007; Huang & Guo, Citation2020; Wang et al., Citation2016). Due to these specific characteristics of copper, it is termed a good candidate for usage in oil and petroleum industries, production of pipelines and wires in electronic industries, desalination plants, and marine industries (Gong et al., Citation2015).
Each metal differs in its chemical composition resulting in a difference in the microstructure. This difference in the microstructure plays an important role in the corrosion property exhibited by the metal. The development of a difference in the potentials can be observed within a metal as a result of the creation of small areas (microgalvanic cells) due to heterogeneities on the surface of the metal (Ao et al., Citation2014). These heterogeneities arise due to the differences in the volume percentage of the phases present, defects in the crystal lattice, or inclusions of various compounds (P. R. Prabhu et al., Citation2021). Mechanical properties like hardness, ductility, and corrosion property can be improved by considerable modification of the grain size (Palumbo et al., Citation2021; D. Prabhu et al., Citation2022a). By understanding the relationship between the deformation and the grain size, the corrosion behavior of the metal in different environments can be analyzed.
Heat treatment is an important technique used for the alteration of the microstructure and grain refinement for inculcating the corrosion resistance for a particular metal (Aung & Zhou, Citation2002, Amezhnov et al., Citation2019). Annealing is a type of heat treatment in which a particular metal is heated to a specific temperature and cooled down to obtain the desired properties. The creation of newer grains and an incline in grain growth can be obtained due to recrystallization accompanied by this process (Lei et al., Citation2021; Tan et al., Citation2009). This results in the improvement in the ductility and corrosion resistance properties of the metal (Al-Rubaiey et al., Citation2013).
Few researchers have investigated the relationship between the microstructure and the corrosion property of dual-phase steel and copper (P. R. Prabhu et al., Citation2020; D. Prabhu et al., Citation2022b). Lakshmana Rao Bhagavathi et al. have analyzed the relationship between the corrosion property and microstructure of dual-phase carbon steel and found that subsequent heat-treated ferrite—pearlite steel showed greater corrosion resistance than as-bought dual-phase steel (Bhagavathi et al., Citation2011). Sami et al. analyzed the influence of microstructural changes on the corrosion property of the metal. It was found that the coarse ferrite-pearlite microstructure of carbon steel showed higher corrosion resistance than the banded ferrite-pearlite microstructure (Al-Rubaiey et al., Citation2013). Yoshiharu et al. investigated the influence of coarser and finer ferrite-pearlite microstructures of metal with the corrosion property. A reduction in corrosion resistance was observed for coarse-grained ferrite-pearlite microstructures (Murase et al., Citation2021). The role of annealing twins on the microstructure of copper was analyzed by D.P. Field et al. A significant grain growth was observed in the twinning process which aids the recrystallization process (Field et al., Citation2007). Gaetano et al. investigated the role of grain size on the corrosion behavior of steel and found that the decrease in the corrosion resistance in fine-grained steel is due to the higher density of grain boundaries formed during grain refrainment (Palumbo et al., Citation2021). The need to understand and establish a relationship between microstructure and corrosion behavior is essential as the nature of the problem and derivation of the mechanism of the corrosion process is complex. For the application of these metals in industries, it is very important to establish the relation between the microstructure and corrosion resistance properties of annealed AISI 5140 steel and copper (Dobkowska et al., Citation2021). Since there is a lack of literature on the above-mentioned materials in an applicable medium, herein the effect of annealing on the microstructure and corrosion behavior of AISI 5140 steel and copper in a 3.5 wt% NaCl medium is presented.
2. Methodology
2.1. Material preparation and annealing process
AISI 5140 steel and copper with chemical composition as given in Table were selected for the corrosion studies. Before heat treatment, the as-cast test specimen of cylindrical rod shape with dimension (diameter 19 mm and height 8 mm) was prepared.
Table 1. Chemical composition of AISI 5140 steel and copper
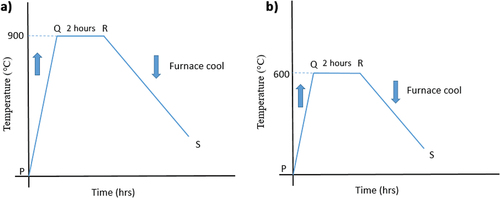
The metal specimens were made to undergo annealing by heating them to a specified temperature (AISI 5140 steel-900 ℃ and copper-600 ℃) for 2 h and followed by furnace cooling (Figure ). The annealing process is illustrated in Figure .
2.2. Surface and medium preparation
The corrosive medium of 3.5 wt% NaCl solution is prepared by dissolving extra pure sodium chloride (99.5%) from Loba Chemie PVT. LTD. The specimen was cleaned using emery sheets (80–800) and then disc polished using diamond paste (3 microns to 0.25 microns), followed by washing using double distilled water and acetone and dried.
2.3. Corrosion studies using EIS and PDP method
The potentiostat CH600E was used for electrochemical impedance and polarisation studies. The electrochemical cell comprised a three-electrode system consisting of a calomel electrode as the counter electrode, platinum as the reference electrode, and molded AISI 5140 steel and copper specimen of the exposed area of 0.8 cm2 and 1.2 cm2 as the working electrode. Before electrochemical measurements, a pseudo-steady state open-circuit potential (OCP) was established by immersing the working electrode in the medium. For impedance measurements, a frequency of 0.1 Hz to 100 kHz with an amplitude of 10 mV was set up. ZSimpWin 3.2 was used for the analysis of the impedance curve. For polarisation measurement, scanning of the working electrode (scan rate 1 mVs−1) from −250 mV of cathodic potential and +250 mV anodic potential with respect to OCP. From the obtained data, the corrosion rate was calculated by extrapolation of the tafel curves. For ensuring the accuracy of the values, the electrochemical measurements were carried out three times.
2.4. Scanning electron microscopy, X-Ray Diffraction analysis (XRD), and microhardness testing
Pre-treatment of the prepared AISI 5140 steel samples was performed by emery sheet polishing followed by 2% NITAL etching. For copper metals, the etching was performed according to ASTM26 standard. The composition of copper etchant is as follows: 5 g of FeCl3 +10 ml HCl +50 ml glycerine +30 ml deionized water. The elemental composition and the surface morphology of the prepared samples before and post-annealing were determined using optical microscopy and SEM. A Taylor Hobson Surtronic 3+ optical microscope was used for surface analysis at a micro-scale. The average grain size was determined by the planimetric method using OLYMPUS Stream Image Analysis Software 2.5.
For the SEM analysis, EVO MA18 was utilized and the magnification selected was 5000X and 1000X for AISI 5140 steel and copper, respectively. The microhardness of the samples was analyzed using the Matsuzawa Micro Vicker’s hardness tester. The indenter was loaded with a load of 200 gf for a time duration of 15 s. Three successive values were obtained, and the average microhardness was taken. The XRD of AISI 5140 steel and copper before post-annealing was carried out using Minifex 600 model instruments. The crystallite size was calculated from XRD. The Debye-Scherrer equation is used to calculate the average crystallite size given (Zeng et al., Citation2022):
Where; D - crystallite size, - shape factor constant,
- X-ray wavelength (1.54 A°),
- full-width half maxima (radian),
- diffraction angle.
3. Result and discussion
3.1. Microstructure of commercially available and annealed AISI 5140 steel and copper
The microstructures (optical and SEM) of AISI 5140 steel prior to and post-annealing treatment are shown in Figure . The microstructure (optical) of as-bought and annealed AISI 5140 steel is shown in Figure respectively. The pearlite region is represented by the yellow circles, and the proeutectoid ferrite phases are represented by the red circles. The proeutectoid ferrite phase is uniformly distributed in both conditions. The proeutectoid ferrite and the pearlite phases before heat treatment are displayed in Figure . A fine pearlitic colony with well-dispersed proeutectoid ferrite is observed in both images. The pearlitic colony shows finer ferrite and cementite particles, known as fine pearlite. The distance between both phases (interlamellar spaces) is observed to be smaller in the microstructure of AISI 5140 steel post-annealing. This is the well-defined pattern observed in normalized or as-cast (as-bought) conditions. Figure represent SEM images of annealed structures of AISI 5140 steel at two different random locations. The interlamellar distance between two consecutive cementite phases (needles) of the pearlitic colony seems to be larger compared to that of as-bought AISI 5140 steel. Between the needles, the dark spaces are present in the ferrite phase. Hence, pearlite is the lamellar mixture of ferrite and cementite. It is seen that pearlitic colonies are oriented in different directions (randomly). The cooling rate of steel under normalizing conditions is due to air cooling, which is faster than annealing (furnace cooling). In annealing, enough time is provided by furnace cooling (slow) to decompose austenite into ferrite and cementite by the eutectoid reaction. Since phase transformation depends on the rate of nucleation and the rate of crystal growth, in annealing when the degree of supercooling is less, the growth rate dominates the rate of nucleation to form coarser grains. In normalization, due to a greater supercooling rate compared to annealing, finer grains are yielded as the nucleation rate overshadows the growth rate (Al-Rubaiey et al., Citation2013).
Figure 3. Optical microstructure of AISI 5140 steel (a) prior to and (b) post-annealing at 900 °C. SEM micrograph of AISI 5140 steel (c) and (d) before annealing at 900 °C, (e) and (f) after annealing at 900 °C.
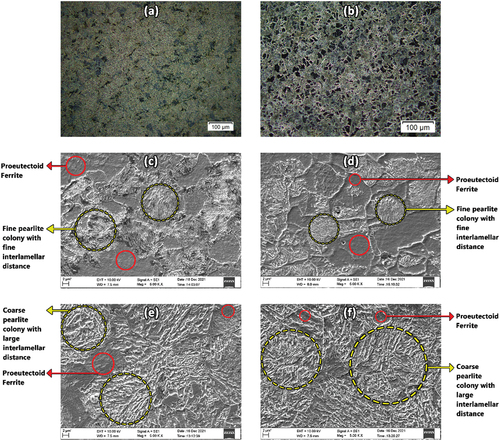
The microstructure and SEM obtained before and after the heat treatment of copper are shown in Figure . In the images of as-received samples, dark and light regions are observed within each grain as a result of the curved crystal lattice. This leads to a deformed microstructure that supports the as-cast microstructure (Cai & Bellon, Citation2019). After the annealing process, it can be observed that the color contrast (dark and light regions) is still observed but to a lower degree. The average grain sizes of as-bought and annealed copper are 1.72 µm and 2.49 µm, respectively. This increment in the grain size for the annealed sample can be due to the annealing twin formation. During annealing, recrystallization leads to the formation of newer grains. When the growth of the grains becomes stagnant, the annealing twin formation will resume the grain growth (Ghiamati Yazdi et al., Citation2017). The annealing twin will alter the misorientation arising between the recrystallized grain and deformation matrix, which will give more energy to the grain growth.
3.2. XRD analysis and microhardness testing
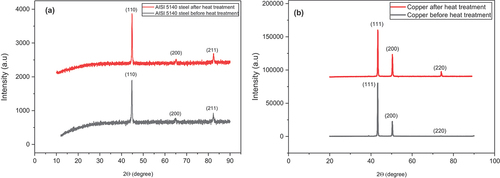
The XRD patterns of the AISI 5140 steel before and after annealing are given in Figure . The peak (110), (200), and (211) corresponds to the ferrite phase of steel and the predominant peak is (110). After the annealing process, there is no inclusion of a γ peak corresponding to the martensite phase, which indicates that there is no phase transformation. There is an increase in the average crystallite size after annealing as observed in Table . This is because of the increase in growth rate and diffusion rate during annealing, which supports the formation of coarser pearlite.
Table 2. The average crystallite size of AISI 5140 steel and copper before and after annealing
The XRD patterns on copper prior to and post-annealing are given in Figure . The peaks correspond to (111), (200), and (220) characteristic planes of pure copper with a preferential orientation at the (111) plan (Krishnaveni & Ravichandran, Citation2015). There is an increase in the average crystallite size for annealed copper, and this can be attributed to the formation of annealing twins that promotes grain growth as explained in the microstructure analysis.
There is a decrease in the microhardness value for both AISI 5140 steel and copper post-annealing as seen in Table . This phenomenon can be described by the Hall-Petch effect. During the plastic deformation of the material, dislocations in the crystal lattice can move through the material. This dislocation gets piled up near the grain boundaries, which will cause the development of repulsive stress within the grain boundary. This stress causes a decrease in the energetic barrier for diffusion across boundaries, which leads to further deformation in the material causing higher yield strength. Since smaller grains have larger grain boundaries, it will lead to higher strength of the material (Yu et al., Citation2015). From the XRD, it is clear that annealing causes an increase in the grain size, thus causing a decrease in the strength of both the metals.
Table 3. Microhardness values of AISI 5140 steel and copper
3.3. EIS studies
EIS studies were employed in determining the corrosion behavior of copper and AISI 5140 steel prior to and post-annealing treatment. A corrosion behavior study was performed using 3.5 wt% NaCl as the corrosive medium. The Nyquist diagram and the Bode plot for AISI 5140 steel and copper before and after heat treatment are given in () respectively. For both metals, the distinguishable feature of the curve is the presence of a capacitive loop at the high-frequency corresponding to the process of charge transfer (Gong et al., Citation2015). The second feature is the presence of a straight line at low frequencies which indicates the Warburg impedance indicating a diffusion-controlled mechanism arising due to the transport of dissolved oxygen from the bulk solution to the metal surface, which means that diffusion is the reason behind the anodic dissolution of the metal and cathodic reduction of the dissolved oxygen. The depressed capacitive loop at the center that has a center below the real axis indicates the inhomogeneity in the surface of the working electrode (Ghiamati Yazdi et al., Citation2017). Since the nature of the graph is different for both metals, the curves obtained from the Nyquist plot are fitted with two different circuits as given in . The elements present in the equivalent circuit are as follows: Rs is the resistance of the solution between the working electrode and the reference electrode; Rf depicts the resistance developed on the electrode due to film formation; Rct shows the charge transfer resistance. For copper, the equivalent circuit consists of one constant phase element Q1 consisting of double-layer capacitance (CPEdl) and deviation parameter (n1). For AISI 5140 steel, there is a presence of two constant phase elements Q1 and Q2 consisting of capacitances formed due to the corrosion product (CPEf) and CPEdl having deviation parameters n1 and n2. The values of these different parameters are given in Table and Table for AISI 5140 steel and copper, respectively. To explain the depressed nature of the capacitive loop, the ideal double-layer capacitance was replaced by CPE (Fernine et al., Citation2022a). The mathematical expression for finding the impedance of CPE (Khaled et al., Citation2010) is as follows:
Figure 6. Nyquist plots of AISI 5140 steel (a) prior and (b) post-annealing in 3.5 wt% NaCl solution at different temperatures. Bode plots for the corrosion of AISI 5140 steel (c) before and (d) after annealing in 3.5 wt% NaCl solution at different temperatures.
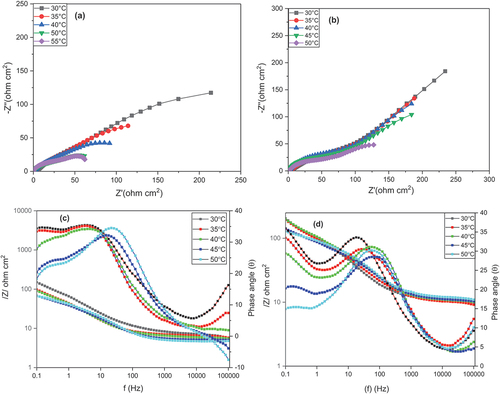
Figure 7. Nyquist plots for the corrosion of copper (a) before and (b) after annealing in 3.5 wt% NaCl solution at different temperatures. Bode plot for the corrosion of copper (c) before and (d) after annealing in 3.5 wt% NaCl solution at different temperatures.
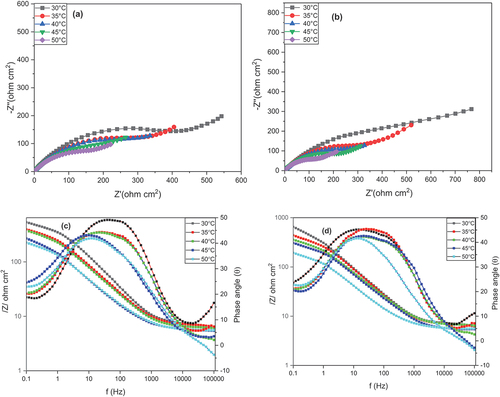
Figure 8. Equivalent circuit for Nyquist plots for the corrosion of (a) AISI 5140 steel and (b) copper in 3.5wt% NaCl medium.
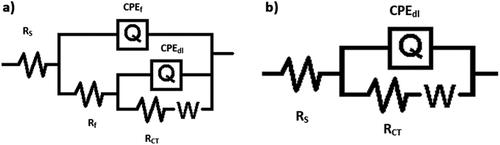
Table 4. Results of EIS measurements for the corrosion of AISI 5140 steel before and after heat treatment in 3.5 wt% NaCl solution
Table 5. Results of EIS measurements for the corrosion of copper before and after heat treatment in 3.5 wt% NaCl solution
where is the factor of proportionality, ω is the angular frequency, i is the imaginary root, and n is the deviation factor ranging between 0 and 1. The curves are fitted with an accuracy of <0.005 with less than 5% error. The values of the capacitance CPEdl can be calculated using the formula
:
where Y2 and n2 are constant phase elements Q2 parameters.
The Bode plot between log(/Z/) and log(f) shows that there is a decrease in the impedance significantly with an increase in temperature. The Bode plot for AISI 5140 steel shows two relaxation time constants, while for copper only one relaxation time constant is observed. This led to the fitting of two different circuits for the metals. With an increase in temperature, the diameter of the capacitance loop decreases. This indicates that the corrosion rate increases with an increase in temperature due to the increase in the diffusion of ions and metal dissolution rate (M. Wu et al., Citation2021).
For AISI 5140 steel, the polarisation resistance Rp = Rf + Rct, and for copper Rp = Rct. A decrease in the Rp value is observed with the rise in the temperature as a result of oxide layer dissolution with temperature. This, in turn, increases the corrosion rate (Deepa & Padmalatha, Citation2017).
Post-annealing the Rp value is found to be increased irrespective of the temperature conditions, thus a decline in the corrosion rate post-annealing is observed. As the grain size increases, the length of grain boundaries decreases due to grain coarsening. This phenomenon is observed in annealed metals, whereas in normalizing (as bought), the particles become finer and the grain boundary length increases, which reflects as finer grains, this process is known as grain refinement. Since the grain boundary is in the weaker region. With the increase in grain boundary length, the tendency to get corroded increases. Thus, the metal after annealing treatment exhibits greater corrosion resistance (Palumbo et al., Citation2021).
3.4. PDP studies
The Tafel plot of AISI 5140 steel and copper in 3.5 wt% NaCl solution is given in Figure . The PDP parameters such as corrosion current density (icorr), corrosion potential (Ecorr), Tafel polarisation cathodic (-bc), and anodic slopes (ba) are obtained for AISI 5140 steel and copper (Khaled, Citation2011). The values of these parameters are tabulated in Table and Table respectively. From the Tafel curve obtained, it is seen that the cathodic polarization curve exhibits a typical Tafel behavior. In the anodic region, instead of a typical Tafel behavior, a curvature is observed due to the formation of anodic passivation oxide film followed by the dissolution of metal with increasing anodic potential (Amin et al., Citation2016). This curvature leads to inaccuracy in calculating icorr from the anodic curve. From the extrapolation of the cathodic curve, the corrosion current (icorr) and the cathodic slope (-ba) were determined. The corrosion rate was calculated using the formula:
Figure 9. Tafel plots for the corrosion of AISI 5140 steel (a) before and (b) after annealing in 3.5 wt% NaCl solution. Tafel plots for the corrosion of Copper (c) before and (d) after annealing in 3.5 wt% NaCl solution.
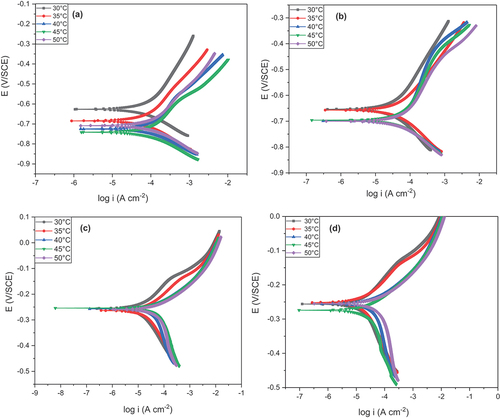
Table 6. Results of PDP measurements for the corrosion of AISI 5140 steel before and after heat treatment in 3.5 wt% NaCl solution
Table 7. Results of PDP measurements for the corrosion of copper before and after heat treatment in 3.5 wt% NaCl solution
Where E.W. is the equivalent weight of the corroding species and D is the density of the corroding species in gcm−2.
It is observed that there is an increase in icorr with temperature for both metals irrespective of the conditions. This is due to the improvement in electrochemical kinetics (greater diffusion of ions) that leads to an increase in the corrosion rate (Fernine et al., Citation2022b; Y. Wu et al., Citation2020). From Table and Table , it is observed that there is a significant amount of reduction in the corrosion rate value for heat-treated samples of both metals for all temperatures. In the case of AISI 5140 steel, post-annealing grain size increases, which will decrease the lattice strain and dislocation density and decreases the surface potential. Thus, with annealing the corrosion rate decreases (Bahmani et al., Citation2020). In the case of copper, the annealed metal specimen shows greater corrosion resistance than the as-cast metal due to a rise in the crystallite size post-annealing. This result is in line with the results obtained from EIS studies.
3.5. Effect of kinetic and thermodynamic factors
The effect of temperature on the corrosion rate is given by the Arrhenius equation:
where CR is the corrosion rate of the reacting species, Ea is the activation energy, A is the pre-exponential factor and T is the absolute temperature. From the slope of the Arrhenius plot (log(CR) vs 1/T) (Figure ), the activation energy was obtained and given in Table . Transition state theory is used to find the enthalpy of activation (ΔH#) and entropy of activation (ΔS#) given by (Zhang et al., Citation2020):
Figure 10. Arrhenius plots of (a) AISI 5140 steel (b) copper in 3.5 wt% NaCl solution for the corrosion in as-bought and annealed condition.
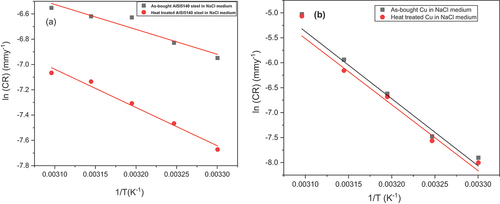
Table 8. Activation parameters for the corrosion of AISI 5140 steel and copper in 3.5 wt% NaCl in as-bought and annealed conditions
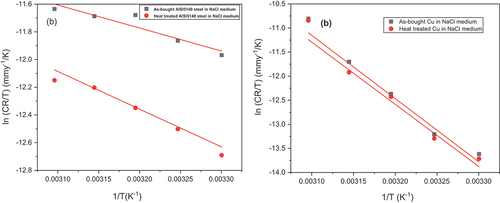
where h is the planks constant, and N is the Avogadro's number. ΔH# and values are obtained from the slope and intercept of the plot (log (CR/T) vs 1/T) (Figure ) and are represented in Table .
Figure 11. Plots of ln (CR/T) versus 1/T of (a) AISI 5140 steel (b) copper in 3.5 wt% NaCl solution for the corrosion in as-bought and annealed condition.
The graph between log (CR) vs 1/T is found to be linear, which explains that icorr value increases with an increase in temperature. The Ea value of both metals irrespective of the heat treatment is greater than 20 KJ/mol which indicates that the process of corrosion was surface controlled (Y. Wu et al., Citation2020). For AISI 5140 steel, the value of Ea is higher in the case of annealing when compared to as-cast metal, but the corrosion rate determined using electrochemical studies revealed that there is a decrease in corrosion rate with annealing. This indicates that the activation energy is dependent on the concentration polarisation. Activation energy indicates the energy required for anodic polarisation for passive product formation (Slemnik, Citation2016). Thus, here the rate-determining step is the formation of the passive layer.
For copper, the Ea value is less in the case of annealed copper than for as-cast copper, but the CR is lesser for annealed copper. This indicates that the activation energy is due to activation polarization (Slemnik, Citation2016). As Ea is decreased, the diffusion rate is lowered, and less transported through the electrolyte. The rate-determining step is the dissolution of metal by the attack of the Cl− ions.
The ΔH# is found to be positive for both metals irrespective of the heat treatment due to the endothermic nature of the metal dissolution process (Loto & Loto, Citation2018). In this case, it has a direct relationship with Ea values. The large and negative value of ΔS# for AISI 5140 steel implies that the rate-determining step represents association rather than dissociation (Slemnik, Citation2016). The positive value of ΔS# for copper metal substrate indicates that a disorderliness has taken place going from reactant to activated complex.
3.6. SEM analysis
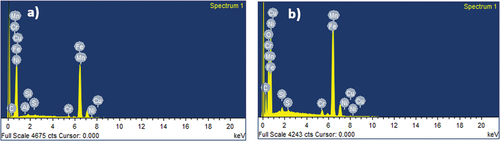
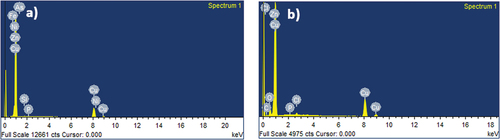
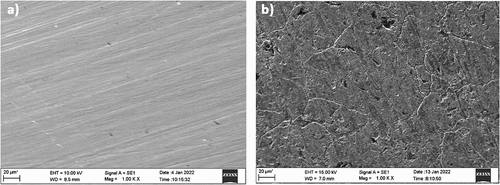
To evaluate the morphology of the surface of annealed copper and AISI 5140 steel, SEM images of the corroded and non-corroded samples were compared. To analyze the composition of the metal specimen EDAX was performed. () represent the SEM micrographic image of the freshly polished annealed AISI 5140 steel and copper after immersion of 3.5 wt% NaCl for 24 NITA, respectively. Degradation of the metal immersed in NaCl is visible, and a uniform attack is observed for both AISI 5140 steel and copper. A few scratches were observed for the freshly prepared samples due to polishing. In the EDAX spectra of the annealed AISI 5140 steel immersed in NaCl (Figure ), an oxygen peak is observed. This is due to the formation of a corrosion product (iron oxide). The EDAX spectra of annealed copper immersed in NaCl (Figure ) chloride peaks are visible which indicates the formation of corrosion product (cupric chloride).
Figure 12. SEM micrographs of (a) AISI 5140 steel without any treatment (b) AISI 5140 steel after immersion in 3.5 wt% NaCl solution.
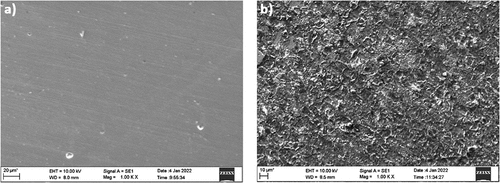
Figure 13. SEM images of (a) copper (without treatment) (b) AISI 5140 steel after immersion in 3.5 wt% NaCl solution.
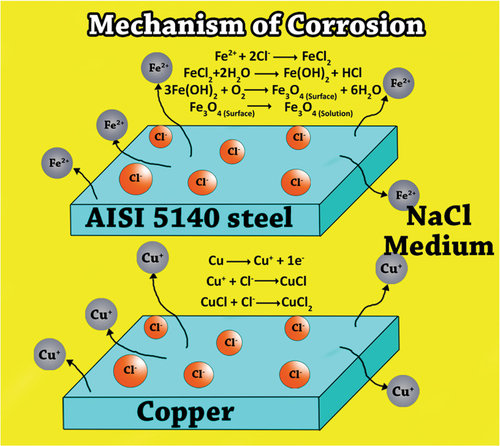
3.7. Corrosion behavior mechanism of copper and AISI 5140 steel in 3.5 wt% NaCl medium
The corrosion reaction mechanism of AISI 5140 and copper has been discussed in this section as per the literature and is illustrated in Figure .
Cathodic corrosion reaction of both AISI 5140 steel and copper is the reduction of the oxygen which is given by:
The anodic corrosion mechanism of AISI 5140 steel in sodium chloride medium involves the dissolution of metal to ferrous cation which will further get converted to ferric cations. Further Fe reacts with oxygen to form different oxides which act as a passive layer (Sherif et al., Citation2013). The reactions involved are given below:
The anodic mechanism for copper in sodium chloride medium mainly involves the following steps (Liu et al., Citation2007):
The is a soluble complex formed which further accelerates metal dissolution.
4. Conclusion
This study presents the effect of annealing treatment on the microstructural and corrosion behavior changes in copper and AISI 5140 steel in 3.5 wt% NaCl medium. The microstructure of the AISI 5140 steel revealed that the annealing treatment has resulted in the formation of a coarser pearlite phase in the pro-eutectoid ferrite matrix. In annealed AISI 5140 steel, since the degree of supercooling is less, the growth rate dominates the nucleation rate, which leads to the formation of coarser grain. An increase in the grain size was observed in the microstructure of copper as a result of annealing twin formation. The XRD analysis showed that there is a rise in the average crystalline size post-annealing for both metals. This result is in accordance with the results observed from the microstructures. The microhardness value of both AISI 5140 steel and copper decreases with annealing. Since smaller grains have larger grain boundaries and with annealing grain size increases, higher strength will be exhibited by as-bought samples. An increase in the corrosion resistance post-annealing was confirmed by EIS and PDP studies for both metals. SEM and EDAX analysis showed that uniform corrosion is observed when immersed in 3.5 wt% NaCl medium.
Acknowledgments
The authors extend their acknowledgment to the Department of Chemistry, Department of Mechanical & Manufacturing Engineering, Central Instrumentation Facility, Manipal Institute of Technology (MIT), Manipal, and Manipal Academy of Higher Education (MAHE) for the characterization facilities. The first author extends her appreciation to MAHE, Manipal for providing the Dr. TMA Pai scholarship.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Inquiries about data availability should be directed to the corresponding author.
Additional information
Funding
References
- Ahmed, A. H., Sherif, E. S. M., Abdo, H. S., & Gad, E. S. (2021). Ethanedihydrazide as a corrosion inhibitor for Iron in 3.5% NaCl solutions. ACS Omega, 6(22), 14525–19. https://doi.org/10.1021/acsomega.1c01422
- Al-Rubaiey, S. I., Anoon, E. A., & Hanoon, M. M. (2013). The influence of microstructure on the corrosion rate of carbon steels. Engineering & Technology Journal, 31(10), 1825–1836.
- Amezhnov, A. V., Rodionova, I. G., Kuznetsov, D. V., Komissarov, A. A., & Sidorova, E. P. (2019). Effect of heat treatment on corrosion activity of nonmetallic inclusions and steel corrosion resistance in aqueous media. Metallurgist, 62(11), 1232–1239. https://doi.org/10.1007/s11015-019-00779-x
- Amin, M. A., Saracoglu, M., El-Bagoury, N., Sharshar, T., Ibrahim, M. M., Wysocka, J., Krakowiak, S., & Ryl, J. (2016). Microstructure and corrosion behaviour of carbon steel and ferritic and austenitic stainless steels in NaCl solutions and the effect of p-nitrophenyl phosphate disodium salt. International Journal of Electrochemical Science, 11(12), 10029–10052. https://doi.org/10.20964/2016.12.17
- Ao, S.-I., Douglas, C., Grundfest, W. S., & Burgstone, J. (2014). Microstructural features and mechanical behavior of unalloyed medium carbon steel after subsequent heat treatment. Proceedings of the World Congress on Engineering and Computer Science (p. 1107).
- Aung, N. N., & Zhou, W. (2002). Effect of heat treatment on corrosion and electrochemical behaviour of AZ91D magnesium alloy. Journal of Applied Electrochemistry, 32(12), 1397–1401. https://doi.org/10.1023/A:1022698916817
- Bahmani, A., Arthanari, S., & Shin, K. S. Formulation of corrosion rate of magnesium alloys using microstructural parameters. (2020). Journal of Magnesium and Alloys, 8(1), 134–149. National Engg. Reaserch Center for Magnesium Alloys. https://doi.org/10.1016/j.jma.2019.12.001
- Bhagavathi, L. R., Chaudhari, G. P., & Nath, S. K. (2011). Mechanical and corrosion behavior of plain low carbon dual-phase steels. Materials and Design, 32(1), 433–440. https://doi.org/10.1016/j.matdes.2010.06.025
- Cai, W., & Bellon, P. (2019). Effect of annealing treatment on the dry sliding wear behavior of copper. Wear, 426–427, 1187–1194. https://doi.org/10.1016/j.wear.2019.01.014
- Chang, J.-W., Guo, X.-W., Fu, P.-H., Peng, L.-M., & Ding, W.-J. (2007). Effect of heat treatment on corrosion and electrochemical behaviour of Mg–3Nd–0.2Zn–0.4Zr (wt.%) alloy. Electrochimica Acta, 52(9), 3160–3167. https://doi.org/10.1016/j.electacta.2006.09.069
- Coulibaly, N. H., Brou, Y. S., Diomande, G. D., Creus, J., & Trokourey, A. (2018). Nicotinic acid as green inhibitor for copper corrosion in 3.5 wt % NaCl solution: Experimental and quantum chemical calculations. International Journal of Biological and Chemical Sciences, 12(2), 1008. https://doi.org/10.4314/ijbcs.v12i2.30
- Deepa, P., & Padmalatha, R. (2017). Corrosion behaviour of 6063 aluminium alloy in acidic and in alkaline media. Arabian Journal Chemistry, 10, S2234–S2244. https://doi.org/10.1016/J.ARABJC.2013.07.059
- Dobkowska, A., Adamczyk – Cieślak, B., Kubásek, J., Vojtěch, D., Kuc, D., Hadasik, E., & Mizera, J. (2021). Microstructure and corrosion resistance of a duplex structured Mg–7.5Li–3Al–1Zn. Journal of Magnesium and Alloys, 9(2), 467–477. https://doi.org/10.1016/j.jma.2020.07.007
- Fernine, Y., Arrousse, N., Haldhar, R., Raorane, C. J., Kim, S.-C., Hajjaji, F. E., Touhami, M. E., Beniken, M., Haboubi, K., & Taleb, M. (2022a). Synthesis and characterization of phenolphthalein derivatives, detailed theoretical DFT computation/molecular simulation, and prevention of AA2024-T3 corrosion in medium 3.5% NaCl. Journal of the Taiwan Institute of Chemical Engineers, 140, 104556. https://doi.org/10.1016/j.jtice.2022.104556
- Fernine, Y., Arrousse, N., Haldhar, R., Raorane, C. J., Kim, S.-C., Hajjaji, F. E., Touhami, M. E., Beniken, M., Haboubi, K., & Taleb, M. (2022b). Synthesis and characterization of phenolphthalein derivatives, detailed theoretical DFT computation/molecular simulation, and prevention of AA2024-T3 corrosion in medium 3.5% NaCl. Journal of the Taiwan Institute of Chemical Engineers, 140, 104556. https://doi.org/10.1016/j.jtice.2022.104556/
- Field, D. P., Bradford, L. T., Nowell, M. M., & Lillo, T. M. (2007). The role of annealing twins during recrystallization of Cu. Acta Materialia, 55(12), 4233–4241. https://doi.org/10.1016/j.actamat.2007.03.021
- Ghiamati Yazdi, E., Ghahfarokhi, Z. S., & Bagherzadeh, M. (2017). Protection of carbon steel corrosion in 3.5% NaCl medium by aryldiazonium grafted graphene coatings. New Journal of Chemistry, 41(21), 12470–12480. https://doi.org/10.1039/c7nj01655g
- Gong, Y., Wang, Z., Gao, F., Zhang, S., & Li, H. (2015). Synthesis of new benzotriazole derivatives containing carbon chains as the corrosion inhibitors for copper in sodium chloride solution. Industrial and Engineering Chemistry Research, 54(49), 12242–12253. https://doi.org/10.1021/acs.iecr.5b02988
- Huang, H., & Guo, X. (2020). The relationship between the inhibition performances of three benzo derivatives and their structures on the corrosion of copper in 3.5 wt.% NaCl solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 598, 598. https://doi.org/10.1016/j.colsurfa.2020.124809
- Khaled, K. F. (2011). Studies of the corrosion inhibition of copper in sodium chloride solutions using chemical and electrochemical measurements. Materials Chemistry and Physics, 125(3), 427–433. https://doi.org/10.1016/j.matchemphys.2010.10.037
- Khaled, K. F., Amin, M. A., & Al-Mobarak, N. A. (2010). On the corrosion inhibition and adsorption behaviour of some benzotriazole derivatives during copper corrosion in nitric acid solutions: A combined experimental and theoretical study. Journal of Applied Electrochemistry, 40(3), 601–613. https://doi.org/10.1007/s10800-009-0035-8
- Krishnaveni, K., & Ravichandran, J. (2015). A study on the inhibition of copper corrosion in sulphuric acid by aqueous extract of leaves of Morinda tinctoria. Journal of Failure Analysis & Prevention, 15(5), 711–721. https://doi.org/10.1007/s11668-015-0002-0
- Lei, Y. B., Wang, Z. B., Zhang, B., Luo, Z. P., Lu, J., & Lu, K. (2021). Enhanced mechanical properties and corrosion resistance of 316L stainless steel by pre-forming a gradient nanostructured surface layer and annealing. Acta Materialia, 208, 116773. https://doi.org/10.1016/j.actamat.2021.116773
- Liu, T., Chen, S., Cheng, S., Tian, J., Chang, X., & Yin, Y. (2007). Corrosion behavior of super-hydrophobic surface on copper in seawater. Electrochimica Acta, 52(28), 8003–8007. https://doi.org/10.1016/j.electacta.2007.06.072
- Loto, R. T., & Loto, C. A. (2018). Corrosion behaviour of S43035 ferritic stainless steel in hot sulphate/chloride solution. Journal of Materials Research and Technology, 7(3), 231–239. https://doi.org/10.1016/j.jmrt.2017.07.004
- Murase, Y., Masuda, H., & Katayama, H. (2021). Corrosion resistance of finer/coarser pearlitic structures of carbon steel. Journal of the Electrochemical Society, 168(4), 041501. https://doi.org/10.1149/1945-7111/abf185
- Palumbo, G., Dunikowski, D., Wirecka, R., Mazur, T., Lelek-Borkowska, U., Wawer, K., & Banaś, J. (2021). Effect of grain size on the corrosion behavior of Fe-3wt.%Si-1wt.%Al electrical steels in pure water saturated with CO2. Materials, 14(17), 5084. https://doi.org/10.3390/ma14175084
- Prabhu, P. R., Hiremath, P., Prabhu, D., Gowrishankar, M. C., & Gurumurthy, B. M. (2021). Chemical, electrochemical, thermodynamic and adsorption study of EN8 dual-phase steel with ferrite-martensite structure in 0.5 M H2SO4 using pectin as inhibitor. Chemical Papers, 75(11), 6083–6099. https://doi.org/10.1007/s11696-021-01773-x
- Prabhu, P. R., Prabhu, D., Sharma, S., & Kulkarni, S. M. (2020). Surface properties and corrosion behavior of turn-assisted deep-cold-rolled AISI 4140 steel. Journal of Materials Engineering and Performance, 29(9), 5871–5885. https://doi.org/10.1007/s11665-020-05051-x
- Prabhu, D., Sharma, S., Prabhu, P. R., Jomy, J., & Sadanand, R. V. (2022a). Analysis of the inhibiting action of pectin on corrosion of AISI1040 dual-phase steel with ferrite–martensite and ferrite–bainite structure: A comparison in 0.5 M sulphuric acid. Journal of the Iranian Chemical Society, 19(4), 1109–1128. https://doi.org/10.1007/s13738-021-02368-9
- Prabhu, D., Sharma, S., Prabhu, P. R., Jomy, J., & Sadanand, R. V. (2022b). Analysis of the inhibiting action of pectin on corrosion of AISI1040 dual-phase steel with ferrite–martensite and ferrite–bainite structure: A comparison in 0.5 M sulphuric acid. Journal of the Iranian Chemical Society, 19(4), 1109–1128. https://doi.org/10.1007/s13738-021-02368-9
- Rodríguez-Gómez, F. J., Valdelamar, M. P., Vazquez, A. E., Del Valle Perez, P., Mata, R., Miralrio, A., & Castro, M. (2019). Mycophenolic acid as a corrosion inhibitor of carbon steel in 3% wt. NaCl solution. An experimental and theoretical study. Journal of Molecular Structure, 1183, 168–181. https://doi.org/10.1016/j.molstruc.2018.12.035
- Sherif, E.-S., Almajid, A., Khalil, A., Junaedi, H., & Hamdan Latief, F. (2013). Electrochemical studies on the corrosion behavior of API X65 pipeline steel in chloride solutions. International Journal of Electrochemical Science, 8(7), 9360–9370. https://doi.org/10.1016/S1452-3981(23)12975-0
- Slemnik, M. (2016). Activation energies ratio as corrosion indicator for different heat treated stainless steels. Materials and Design, 89, 795–801. https://doi.org/10.1016/j.matdes.2015.10.035
- Tan, H., Jiang, Y., Deng, B., Sun, T., Xu, J., & Li, J. (2009). Effect of annealing temperature on the pitting corrosion resistance of super duplex stainless steel UNS S32750. Materials Characterization, 60(9), 1049–1054. https://doi.org/10.1016/j.matchar.2009.04.009
- Wang, Z., Gong, Y., Jing, C., Huang, H., Li, H., Zhang, S., & Gao, F. (2016). Synthesis of dibenzotriazole derivatives bearing alkylene linkers as corrosion inhibitors for copper in sodium chloride solution: A new thought for the design of organic inhibitors. Corrosion Science, 113, 64–77. https://doi.org/10.1016/j.corsci.2016.10.005
- Wu, M., Gao, Z., Wu, S., Liu, Y., & Hu, W. (2021). Effect of temperature on corrosion behavior of X70 pipeline steel in 3.5% NaCl solution. International Journal of Electrochemical Science, 16(6), 1–14. https://doi.org/10.20964/2021.06.64
- Wu, Y., Luo, S., & Mou, Q. (2020). Influence of temperature on the corrosion behavior of X80 steel in an acidic soil environment. International Journal of Electrochemical Science, 15(1), 576–586. https://doi.org/10.20964/2020.01.03
- Yu, J., Wang, G., & Rong, Y. (2015). Experimental study on the surface integrity and chip formation in the micro cutting process. Procedia Manufacturing, 1, 655–662. https://doi.org/10.1016/j.promfg.2015.09.063
- Zeng, Y., Kang, L., Wu, Y., Wan, S., Liao, B., Li, N., & Guo, X. (2022). Melamine modified carbon dots as high effective corrosion inhibitor for Q235 carbon steel in neutral 3.5 wt% NaCl solution. Journal of Molecular Liquids, 349, 118108. https://doi.org/10.1016/j.molliq.2021.118108
- Zhang, Q. H., Hou, B. S., Li, Y. Y., Zhu, G. Y., Liu, H. F., & Zhang, G. A. (2020). Two novel chitosan derivatives as high efficient eco-friendly inhibitors for the corrosion of mild steel in acidic solution. Corrosion Science, 164, 108346. https://doi.org/10.1016/j.corsci.2019.108346