Abstract
Five representative soil profiles occurring on four different physiography under subtropical environment of Damodar command area, India, were studied for soil pedological variability. Two way approaches were taken to evaluate the extent of profile development. Firstly different extractants were used to determine various forms of Fe and Al and their different ratios. Average contents of Fe and Al, extracted by different extracting reagents were found to be in descending order, as follows: Aldith > Aloxa > Alpyr and Fedith > Feoxa > Fepyr. Analysis of pyrophosphate (pyr), oxalate (oxa), and dithionate (dith) extractable Fe and Al fractions indicated that with increasing soil age, the content of crystalline Fe and Al oxides increased at the expense of the poorly crystalline forms. The mean content of amorphous Fe and Al, crystalline Fe and Al, and their ratios estimated the degree of soil development. In the second part, elemental analysis was done, silica to sesquioxide ratio as well as ratio of alkali cations was measured and weathering index of each horizon was determined. The ratios and weathering indices indicated that except Madhpur soil series, all other soils were young and pedological development was still in progress in Damodar command area.
Public Interest Statement
In the research article, an attempt was made to evaluate the soil formation pattern in Damodar Command area of West Bengal, India. The area is known for its high crop productivity and diversified cropping pattern. So an in depth study of its soil formation process might be useful to find the basic reason behind its inherent high soil productivity.
1. Introduction
Soil properties that change with duration and intensity of weathering provide vital clue toward the pedogenesis of the studied soil. Mineral weathering during pedogenesis results in translocation and/or accumulation of major or trace elements in soils (Kabata-Pendias & Pendias, Citation1992). The translocation or accumulation of the elements occured via chemical and biological processes such as leaching, podzolization, and oxidation–reduction (Mausbach & Richardson, Citation1994; Wilson, Burt, Sobecki, Engel, & Hipple, Citation1996). Determination of the different soil processes involved elemental evaluation of horizons within and between the solum (Burt, Wilson, Mays, & Lee, Citation2003). Pedogenic evaluations using elemental analysis include silica to sesquioxide ratio representing soil weathering index (Jackson, Citation1973) and ratios of alkali cations (e.g. Ca and K) as measures of pedogenic changes and geological uniformity (Muhs, Bettis, Been, & McGeehin, Citation2001). Comparative movement of silica and sesquioxide down the profile as well as their ratios calculated from the total composition of soils provide basis for understanding profile development in relation to parent material. On the other hand, profile distribution of different forms of Fe and Al oxides particularly dithionite and oxalate extractable Fe and Al serve as useful indicators to identify the horizon of accumulation of secondary oxides (McKeague & Day, Citation1966). The Fe and Al released during the weathering of Fe and Al bearing parent materials are reprecipitated in the soils as oxides or hydroxides and oxyhydroxides of iron and aluminum. The quantities of these alternation products generally increase with soil age (Dolui & Bera, Citation2001) and their distribution in soil profiles indicate the stage and degree of soil development (Mahaney & Fahey, Citation1988). However, very meager information is available on the degree of soil development and the direction of pedogenic processes in the soils of Damodar command area. Hence, in the present study these two approaches were used for pedogenic evaluation and intensity of weathering in the soils occurring under different physiographic positions in the study area.
2. Materials and methods
The study area lies between 23°11′46″ to 23°29′ 21″ N latitude and 87°28′49″ to 88°0′ 21″ E longitude in Vindyan plain of Barddhaman, West Bengal, India. It represents a transition zone of tropical dry subhumid type of climate area and belongs to hot moist subhumid ecological subregion (15.1). The mean summer (March–June) and winter (November–February) temperature are 30.0 and 21.1°C, respectively with a mean annual temperature of 26.5°C. The difference between mean summer and mean winter temperature is more than 5°C, hence the area qualifies for “hyperthermic” temperature regime. The mean annual precipitation is 1,400 mm of which approximately 74.7% is received during June–September.
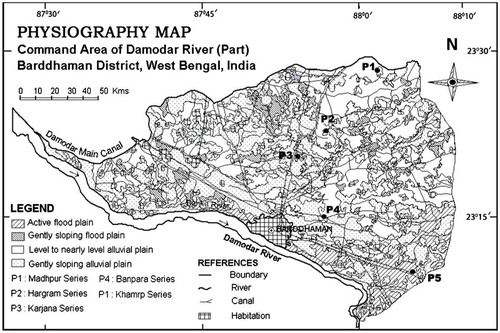
Based on reconnaissance soil survey, five soil series representing four different physiography were selected to study the soil pedological variability in Damodar command area (part). Among the selected soil series, Khamrp soil series belonged to active floodplain, Karjana soil series belonged to gently sloping floodplain, Banpara soil series belonged to nearly level alluvial plain while Hargram and Madhpur soil series represented gently sloping alluvial plain (). Soils of each horizon were processed (<2 mm) and used for analyzing the physical and chemical characteristics of the soil samples viz. particle size distribution, pH, organic carbon, CEC, and base saturation, following standard procedures (Jackson, Citation1973). The soils were classified as per the Keys to Soil Taxonomy.
Fraction of Fe and Al was determined in the soil by separate extractions with (i) sodium pyrophosphate, overnight extraction at pH 10 (Agriculture Canada, Citation1984), (ii) acid ammonium oxalate (0.2), four hour extraction at pH 3 (Agriculture Canada, Citation1984), and (iii) dithionate-citrate-bicarbonate, 20 min extraction twice (CSSC Subcommittee on methods of analysis, Citation1978). Among the extractants used dithionite-citrate-bicarbonate (DCB) reagent was the most powerful, which dissolved with the extraction capacity of both non-crystalline and crystalline Fe and Al oxides, small amount of water soluble, exchangeable, and organically bound Fe and Al along with limited amount of Fe and Al bearing silicates (Borggard, Citation1988). This was followed by acid ammonium oxalate, which dissolved both “amorphous” and “organically bound” forms of Fe and Al, but not the crystalline forms (Parfitt & Childs, Citation1988). In comparison pyrophosphate extractant dissolved only the fraction of iron (Loveland & Digby, Citation1984) and Al (Driscoll, van Breemen, & Mulder, Citation1985) that are bound with organic matter. Fe and Al in the extract were determined using atomic absorption spectrophotometer. Total elemental composition of soil samples was carried out following the procedure of Jackson (Citation1973). Weathering index of each horizon was calculated by the formula given by Evans and Cameron (Citation1979).
[(CaO + MgO + K2O + Na2O)/(CaO + MgO + K2O + Na2O + Si2O3 + Al2O3 + Fe2O3)] × 100
3. Result and discussion
Physicochemical properties of the soils from the selected soil series are represented in Table . In all the soil series, surface soils exhibited slightly acidic pH (5.4–6.3) which gradually shifted to neutral range in the subsurface and lower horizons except in Hargram soil series. In all the soil series, the lowest clay percentage was observed in the surface horizon and the values increased with increase in soil depth. However, in Hargram and Banpara soil series clay percentage decreased abruptly in the lowest horizon, while no consistent pattern was observed in the soils of Karjana and Khamrp soil series. Such abrupt changes in soil property were in close conformity to the general characteristics of alluvial soils. Cation exchange capacity of the soils also followed a similar trend in the different soil series.
Table 1. Physicochemical properties of five representative soil series
The data on various forms of Fe and Al as extracted by different extractants, their ratios, relationship with soil properties and interrelationship among themselves are presented in Tables , respectively.
Table 2. Fe and Al (gkg−1) extracted by different extractants in selected soil pedons
Table 3. Comparison of different forms of Fe and Al and their ratio
Table 4. Correlation matrix for different forms of iron and aluminum and physicochemical properties of selected soil series
3.1. Pyrophosphate extractable Fe and Al
Pyrophosphate extractant dissolved the fraction of iron (Loveland & Digby, Citation1984) and Al (Driscoll et al., Citation1985) bound with organic matter. The fraction of Fe (0.18–0.54 gkg−1) generally decreased with depth with few exceptions. The fraction of Al followed the same trend varying from 0.49 to 1.78 gkg−1. Comparison of the amount of Fe and Al extracted by pyrophosphate in different soil series indicated the largest extraction from Madhpur series while the lowest extraction from Banpara series. In comparison to Fe more Al was extracted, which might be due to the Al rich materials or because pyrophosphate is not very specific for organic complexed Al, dissolving inorganic Al compounds (like allophone and imogolite) as well (Bera, Seal, Banerjee, & Dolui, Citation2005).
3.2. Acid ammonium oxalate extractable Fe and Al
Oxalate dissolved both “amorphous” and “organically bound” forms of Fe and Al, but not the crystalline forms (Parfitt & Childs, Citation1988). Amorphous forms of Fe and Al like hydroxides and oxyhydroxides were dissolved from soil using this extractant (McKeague, Brydon, & Miles, Citation1971). Al compounds associated with organic matter were also extracted as amorphous alumino-silicates including allophane and imogolite (Kodama & Ross, Citation1991). In the study area, oxalate extractable of Fe (0.84–2.24 gkg−1) was more in illuvial horizon of all the soil series indicating translocation of Fe. In these horizons, the fraction of Al (1.61–7.19 gkg−1) also followed the same trend probably due to the deposition of translocated Al-fulvate complex, protoimogolite, and hydroxy polymers of the element (Farmer, Russell, & Berrow, Citation1980). Hence, the difference between oxalate and pyrophosphate extractable Fe and Al gave a measure of amorphous inorganic Fe and Al (Dolui & Bera, Citation2001; Dolui & Chakraborty, Citation1998) in soils.
Data (Table ) revealed that amorphous Fe (Feoxa–Fepyr) in the soil ranged between 0.44 and 1.82 gkg−1 with a wide variation among the different soil series. Amorphous Al (Aloxa–Alpyr) of the soils varied from 0.92 to 5.73 gkg−1. The data presented provided evidence that an approximate differentiation can be made between organically complexed Fe and Al and amorphous inorganic Fe and Al by selective extraction of soils with pyrophosphate and oxalate. The large apparent increase in the quantities of Feoxa–Fepyr forms and Aloxa–Alpyr forms suggested a shift toward inorganic pedogenic phases at the expense of organically bound phase (Jersak, McColl, & Hetzel, Citation1992). Though non-crystalline Fe and Al do not have a definite composition or structure and are only poorly defined, there is probably no precise differentiation between crystalline and non-crystalline material. The microprobe investigation of Norrish and Rossier (Citation1983) indicated that amorphous or microcrystalline Fe and Al oxides were unlikely to exist as separate entities in soils.
3.3. Dithionite-citrate-bicarbonate (DCB) extractable Fe and Al
Two dissolution reagents in particular are common (i) acid ammonium oxalate (pH 3) for extraction of non-crystalline Fe and Al and (ii) DCB for extraction of non-crystalline plus crystalline Fe and Al. In the DCB procedure (Mehra & Jackson, Citation1960), dithionite is a powerful reductant, bicarbonate buffers the system (pH 7–9) and sodium citrate prevents the reprecipitation of dissolved Fe and Al (Borggard, Citation1988). The procedure dissolved both non-crystalline and crystalline Fe and Al oxides and the extracts may also include small amount of water soluble, exchangeable, and organically bound Fe and Al along with limited amount of Fe and Al bearing silicates (Borggard, Citation1988). The difference between the values obtained by the two methods represented the amount of Fe and Al present in definite crystalline forms (Dolui & Chakraborty, Citation1998; Dolui, Chandran, & Nayek, Citation1988). The Fedith of the soils showed a variation of 1.84–5.43 gkg−1 whereas Aldith of the soils showed a variation of 4.01–17.22 gkg−1 among the different soil series.
3.4. Co-migration of clay
The co-migration of Fe and Al with clay in the different soil profiles is evidenced by the ratios of clay/Fedith and clay/Aldith. The ratios increased with increase in depth in Madhpur soil series, which can be corroborated with the observations of Dolui and Mazumdar (Citation2003) and Seal, Bera, Bhattacharyya, Mukhopadhyay, and Giri (Citation2006) in alfisols of India. According to Dolui and Bera (Citation2001) these ratios increased with depth within the profile where horizon formation was well expressed and established. However, in Hargram and Karjana soil series, the clay/Fedith and clay/Aldith ratios showed somewhat decreasing trend with few exceptions. This suggested preferential migration of clay over Fe and Al oxides, which were partially independent of clay movement (Bera et al., Citation2005). In Banpara and Khamrp soil series, the ratios did not follow any consistent pattern with relevance to the different horizons indicating that horizon formation was still in progress.
3.5. Different forms of Fe and Al and their ratios
In all the soils, DCB extracted Fe and Al values were higher than the acid ammonium oxalate extracted values indicating that a considerable fraction was present in crystalline form. Crystalline Fe (Fedith–Feoxa) in the soils ranged between 0.87 and 3.24 gkg−1 while crystalline Al (Aldith–Aloxa) in the soils varied from 2.01 to 10.16 gkg−1.
The crystalline Fe and Al oxides increased at the expense of the poorly crystalline forms with increasing soil age as indicated by the ratios of Feoxa/Fedith and Aloxa/Aldith (Mahaney & Fahey, Citation1988). According to Mahaney, Hancock, and Sanmugadas (Citation1991), soils with high ratios were younger soils, whereas low ratios indicated older soils. The mean Fe and Al ratios of Madhpur soil series were comparatively lower than other soil series, varying from 0.36 to 0.41 and 0.37 to 0.42, respectively. This suggested that Madhpur soil series could be comparatively older or weathering could have progressed relatively well to an advanced stage in comparison to other soil series. On the other hand, Khamrp soil series (Feoxa/Fedith and Aloxa/Aldith ratios varied from 0.43 to 0.58 and 0.44 to 0.58, respectively) might be comparatively younger in the process of soil development, while in the other soils pedogenic development represented an intermediary stage with respect to Madhpur and Khamrp soil series.
3.6. Interrelationship among different forms of Fe and Al
Fepyr was positively and significantly correlated with Alpyr (0.889**), Fedith (0.481**), and Aldith (0.484**) while Alpyr was positively and significantly correlated with Feoxa (0.579**), Aloxa (0.696**), Fedith (0.719**), and Aldith (0.773**). Similar relationships were reported by Dolui and Bera (Citation2001), Dolui, Chandran, and Nayek (Citation1987) while working with the tropical soils of India. Highly positive correlation of Fedith with Fepyr (0.481**) and Feoxa (0.943**) as well as Aldith with Alpyr (0.773**) and Aloxa (0.974**) suggested that their relative distribution in soils were functions of the same pedological factor and more importantly all the extractants removed essentially the same forms of Fe and Al (Dolui & Chattopadhyay, Citation1997).
3.7. Relationship with soil properties
pH of the soils was found to have significant positive correlation with oxalate extractable fractions of Fe (0.433*) and Al (0.385*). This may be correlated with the fact that increase in soil pH increased the adsorptive affinity of clay and iron oxides, therefore reducing the concentration of iron in labile pool (Dolui & Bera, Citation2001). Organic carbon was positively and significantly correlated with Fepyro (0.557**) while negatively and significantly correlated with Feoxa (−0.487**) and Aloxa (−0.406*). Similar observation was also reported by Seal et al. (Citation2006). The highly significant correlation of clay with Feoxa (0.638**), Fedith (0.741**), Aloxa (0.549**), and Aldith (0.636**) indicated that these fractions might have been present within the crystal lattice or sorbed on the surface and the interlayer of clay and iron oxides (Dolui & Chattopadhyay, Citation1997; Howkes & Webb, Citation1962). Cation exchange capacity showed significant positive correlation with pyrophosphate (0.447* and 0.438*, respectively), oxalate (0.591** and 0.455*, respectively), and dithionate (0.646** and 0.519**, respectively) extractable Fe and Al. Cation exchange capacity indicated the total negative charge of the colloid and in soil may be correlated to the availability of iron and aluminum from charge sites (Bera et al., Citation2005).
4. Elemental composition
The SiO2 content of soils ranged between 49.20 and 61.39%, comprising more than half of the total mineral matter (Table ). These variations in SiO2 content were mainly associated with the nature of parent material and variation in topography (Sidhu, Ghosh, & Manjaiah, Citation2000). The SiO2 content generally tend to decrease down the profile in all the soil series with some exceptions. On the contrary, the chemical composition of the soils indicated higher accumulation of Al2O3 and Fe2O3 in the subsurface horizons, which generally increased down the profile in all the soil series with some exceptions. The accumulation of Al2O3 (23.20–32.16%) and Fe2O3 (7.47–14.87%) in the cambic/argillic horizons of the soils indicated the mobilization of these elements within the soil profile due to active soil forming processes (Sidhu et al., Citation2000). The movement of manganese oxide and its distribution in the soil profiles were significant criteria in pedogenic study as it behaved more or less like free iron oxides (Elahi, Hossin, & Kamal, Citation1996). Mn2O3 content in the soils decreased down the profile with few exceptions while the content of CaO (0.04–0.25%) and MgO (0.16–0.37%) varied within a narrow range. There were also not much variations in ZnO (0.020–0.204%) and CuO (0.001–0.005%) content throughout the profile in all the soil series. The total base percentage (CaO + MgO + Na2O + K2O) in Madhpur soil series (mean 2.38%) was lower than that of the other soil series. This indicated advanced stage of weathering in Madhpur soil series when compared with other soil series.
Table 5. Chemical composition (ignition basis) of the soils
4.1. Molar ratios
Chemical changes occurring in the selected soil series as a result of weathering were determined from changes in the molar ratios of mobile to resistant oxides. These ratios were calculated on the assumption that the resistant oxides of Fe and Al were immobile in the weathering environment (Colman, Citation1982). Therefore, any changes in the ratios that did occur reflected the extent to which the more mobile oxides of Ca, Mg, Na, and K were removed. In general as weathering progressed, oxide molar ratios decreased as greater quantities of mobile oxides were removed from the weathering profile (Birkeland, Citation1984). The molar ratios SiO2:R2O3 (R2O3 = Fe2O3, + Al2O3), SiO2:Al2O3, CaO:MgO, Al2O3:Fe2O3, CaO + MgO:R2O3, Na2O + K2O:R2O3, total bases (CaO + MgO + Na2O + K2O):R2O3, (Table ) were determined to evaluate most comprehensively the extent of chemical changes occurring in the Vindyan alluvial soils in subtropical environment. According to Sharma, Totawat, and Shyampura (Citation1996) relative accumulation of Fe was considered as one of the most important index for understanding the stage of weathering and the impact of topography on the weatherability of soil. Decrease in molar ratios of SiO2/Al2O3 and SiO2/R2O3 were noticed down the profile in all the soil series excluding few anomalies in Hargram and Khamrp soil series. The trend exhibited similarities with the observations of Walia and Rao (Citation1996), Kudrat, Manchanda, Prasad, Saxena, and Tayal (Citation1995) and Patil and Dasog (Citation1999). The data also pointed out that SiO2/Al2O3 and SiO2/R2O3 molar ratios in Madhpur soil series (2.60–3.26 with mean 2.88 and 2.00–2.48, mean 2.22, respectively) were comparatively lower than all other soil series (3.06–4.49 and 2.41–3.72, respectively) indicating that weathering process was at a more advanced stage in Madhpur soil series as compared to the other soil series (Burt et al., Citation2003; Msanya, Kaaya, Araki, Otsuka, & Nyadzi, Citation2003).
Table 6. Different ratios and weathering index of selected soil series
Alkali earth:resistate (CaO + MgO:R2O3) ratio in Madhpur soil series increased down the profile suggesting loss of calcium and magnesium from the upper horizon. However, change of ratios in other soil series did not follow any specific trend. Alkali:resistate (Na2O + K2O:R2O3) ratios ranged between 0.33 and 0.60, while total bases (CaO + MgO + Na2O + K2O):resistate (R2O3) ratios varied from 0.08 to 0.34, respectively, in the different soil series.
4.2. Weathering index
The average weathering index was the highest (7.13) in Khamrp soil series and the lowest (2.48) in Madhpur soil series. This indicated that extent of weathering was comparatively more in Madhpur soil series since lower value of weathering index indicated more exposure to intense weathering (Walia & Rao, Citation1999). The depth-wise pattern of weathering index showed high values down the profile, which indicated the fall in weathering intensity with depth (Evans & Cameron, Citation1979). In these alluvial-derived soils, the increase in weathering index was noticed to be erratic in most of the pedons with no specific trend of profile development, which manifested their juvenile stage of pedogenesis (Walia & Rao, Citation1999). This was expected because their source of materials was mostly transported sediments, which were deposited in different cycles. Similar observations were also reported by Bhowmick, Bera, Seal and Mukhopadhyay (Citation2004) and Raghu Mohan and Rao (Citation1981) in some alluvial-derived soils of India.
5. Conclusion
To ascertain the degree of soil development, two-way approaches were taken up under the present investigation i.e. assessment of different forms of Fe, Al, and their ratios as well as elemental composition of soil. Assessment revealed relative higher content of crystalline Fe and Al oxides vis-a-vis their low ratio values only in Madhpur soil series, which indicated that the series could be comparatively older or weathering could have progressed relatively well to an advanced stage here when compared with other soil series. Elemental composition, sesquioxide ratio as well as weathering indices only corroborated the above findings, revealing the juvenile stage of pedogenesis in all the other pedons and also demonstrated that weathering was still active in these soils.
Cover image
Source: Authors.
Additional information
Funding
Notes on contributors
Ranjan Bera
The authors of the research article are working in the arena of soil science. The study was basically PhD work of Dr. Ranjan Bera, corresponding author and other authors attached with two leading Institute of India viz. National Bureau of Soil Survey and Land Use Planning and Department of Soil Science, Viswa-Bharati University joined hands to fulfill the objectivity.
References
- Agriculture Canada . (1984). Analytical methods manual. In B. H. Sheldrick (Ed.), LLRI contribution (pp. 84–90). Ottawa: Land Resource Research Institute.
- Bera, R. , Seal, A. , Banerjee, M. , & Dolui, A. K. (2005). Nature and profile distribution of iron and aluminum in relation to pedogenic processes in some soils developed under tropical environment in India. Environmental Geology , 47 , 241–245.10.1007/s00254-004-1149-2
- Bhowmick, A. K. , Bera, R. , Seal, A. , & Mukhopadhyay, K. (2004). Pedological variability and classification of some acid soils of Bihar. Indian Journal of Landscape Systems and Ecological Studies , 27 , 186–192.
- Birkeland, P. W. (1984). Soils and geomorphology . New York, NY: Oxford.
- Borggard, O. K. (1988). Phase identification by selective dissolution technique. In S. W. Stucki , B. A. Goodman , & U. Schwertmann (Eds.), Iron in soils and clay minerals (pp. 93–98). Boston, MA: D. Reidel.
- Burt, R. , Wilson, M. A. , Mays, M. D. , & Lee, C. W. (2003). Major and trace elements of selected pedons in the USA. Journal of Environment Quality , 32 , 2109–2121.10.2134/jeq2003.2109
- Canada Soil Survey Committee (CSSC) subcommittee on methods of analysis . (1978). In J. A. McKeague (Ed.), Manual of soil sampling and methods of analysis (pp. 98–106). Ottawa: Canadian Society of Soil Science.
- Colman, S. M. (1982). Chemical weathering of basalts and andesites: Evidence from weathering rinds (Professional Paper, p. 1246). US Geological Survey (p. 52). USGS Publications.
- Dolui, A. K. , Bera, R. (2001). Relation between iron forms and pedogenic processes in some alfisols of Orissa, India. Agrochimica , XLV , 161–170.
- Dolui, A. K. , & Chakraborty, N. (1998). Differentiation of forms of extractable aluminium in relation to pedogenic processes in two alfisols and an entisols. International Journal of Tropical Agricultural , 16 , 257–266.
- Dolui, A. K. , Chandran, P. , & Nayek, A. K. (1987). Comparative studies on different forms of aluminium using various extractants in relation to pedogenic processes in soil profiles. Journal of the Indian Society of Soil Science , 35 , 103–108.
- Dolui, A. K. , Chandran, P. , & Nayek, A. K. (1988). Nature and profile distribution of iron in some soil series of Jalpaiguri district, West Bengal. The Proceedings of the National Academy of Sciences India , 58 ( A), 1–6.
- Dolui, A. K. , & Chattopadhyay, P. P. (1997). Extraction of forms of iron from some soil series of West Bengal and Bihar. Agropedology , 7 , 44–47.
- Dolui, A. K. , & Mazumdar, A. (2003). Relation between forms of aluminium and pedogenic processes in some alfisols of Bihar and West Bengal. Crop Research Hisar , 25 , 69–77.
- Driscoll, C. J. , van Breemen, N. , & Mulder, J. (1985). Aluminum chemistry in a forested spodosol1. Soil Science Society of America Journal , 49 , 437–444.10.2136/sssaj1985.03615995004900020033x
- Elahi, S. F. , Hossin, M. F. , & Kamal, A. S. M. M. (1996). Characteristics of some soils developed on Madhupur clay in Bangladesh. Journal of the Indian Society of Soil Science , 44 , 482–488.
- Evans, L. J. , & Cameron, B. H. (1979). A chronosequence of soils developed from granitic morainal material, Baffin Island, N.W.T. Canadian Journal of Soil Science , 59 , 203–210.10.4141/cjss79-020
- Farmer, V. C. , Russell, J. D. , & Berrow, M. L. (1980). Imogolite and proto-imogolite allophane in spodic horizons: Evidence for a mobile aluminium silicate complex in podzol formation. Journal of Soil Science , 31 , 673–684.10.1111/ejs.1980.31.issue-4
- Howkes, H. E. , Webb, J. (1962). Geochemistry of mineral exploration (p. 9). New York, NY: Harper and Row.
- Jackson, M. L. (1973). Soil chemical analysis . New Delhi: Prentice Hall of India.
- Jersak, J. M. , McColl, J. G. , & Hetzel, J. F. (1992). Changes in extractability of iron, aluminium and silicon and dispersibility by storage of Carlifornia forest soils. Communications in Soil Science and Plant Analysis , 23 , 993–1018.10.1080/00103629209368645
- Kabata-Pendias, A. , & Pendias, H. (1992). Trace elements in soils and plants (2nd ed.). Boca Rotan, FL: CRC Press.
- Kodama, H. , & Ross, G. J. (1991). Tiron dissolution method used to remove and characterize inorganic components in soils. Soil Science Society of America Journal , 55 , 1180–1187.10.2136/sssaj1991.03615995005500040047x
- Kudrat, M. , Manchanda, M. L. , Prasad, J. , Saxena, P. D. , & Tayal, H. D. (1995). Morphology, physico-chemical characteristics and classification of lateritic soils of a part of Ajoy catchment in West Bengal. Agropedology , 5 , 9–14.
- Loveland, P. J. , & Digby, P. (1984). The extraction of Fe and Al by 0.1 M pyrophosphate solutions: A comparison of some techniques. Journal of Soil Science , 35 , 243–250.10.1111/ejs.1984.35.issue-2
- Mahaney, W. C. , & Fabey, B. D. (1988). Extractable Fe and Al in late Pleistocene and Holocene paleosols on Niwot Ridge, Colorado Front Range. Catena , 15 , 17–26.10.1016/0341-8162(88)90013-6
- Mahaney, W. C. , Hancock, R. G. V. , & Sanmugadas, K. (1991). Extractable Fe-Al and geochemistry of late Pleistocene Paleosol in the Dalijia Shan, Western China. Journal of Southeast Asian Earth Sciences , 6 , 75–82.10.1016/0743-9547(91)90098-I
- Mausbach, M. , Richardson J. L. (1994). Biogeochemical processes in hydric soil formation. In Current topics in wetland biogeochemistry (Vol. 1, pp. 68–127). Baton Rouge, LA: Wetland Biogeochem. Inst., Louisiana State Univ.
- McKeague, J. A. , Brydon, J. E. , & Miles, N. M. (1971). Differentiation of forms of extractable iron and aluminum in soils. Soil Science Society of America Journal , 35 , 33–38.10.2136/sssaj1971.03615995003500010016x
- McKeague, J. A. , & Day, J. H. (1966). Dithionite and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Canadian Journal of Soil Science , 46 , 13–22.10.4141/cjss66-003
- Mehra, O. P. , & Jackson, M. L. (1960). Iron oxide removal from soils and clays by a dithionite citrate system buffered with sodium bicarbonate. Clays and Clay Miner , 7 , 317–327.
- Msanya, B. M. , Kaaya, A. K. , Araki, S. , Otsuka, H. , & Nyadzi, G. I. (2003). Pedological characteristics, general fertility and classification of some benchmark soils of Morogoro District, Tanzania. African Journal of Science and Technology , 4 , 101–112.
- Muhs, D. R. , Bettis, E. A. , Been, J. , & McGeehin, J. P. (2001). Impact of climate and parent material on chemical weathering in loess-derived soils of the Mississippi river valley. Soil Science Society of America Journal , 65 , 1761–1777.10.2136/sssaj2001.1761
- Norrish, K. , & Rossier, H. (1983). Mineral phosphate. In Soils: An Australian viewpoint (pp. 335–361). Sponsored by the Division of Soils, Commonwealth Scientific and Industrial Research Organization. Melbourne: Academic Press. CSIRO/London, UK/Australia.
- Parfitt, R. L. , & Childs, C. W. (1988). Estimation of forms of Fe and Al—A review, and analysis of contrasting soils by dissolution and Mossbauer methods. Australian Journal of Soil Research , 26 , 121–144.10.1071/SR9880121
- Patil, P. L. , & Dasog, G. S. (1999). Genesis and classification of ferruginous soils in Western Ghat and Coastal region of North Karnataka. Agropedology , 9 , 1–5.
- Raghu Mohan, N. G. , & Rao, A. E. V. (1981). Morphogenesis and classification of ferruginous soils of Goa II. Physico-chemical properties and mineralogy of epipedons and endopedons of Goa. Mysore journal of Agricultural Science , 15 , 29–39.
- Seal, A. , Bera, R. , Bhattacharyya, P. , Mukhopadhyay, K. , & Giri, R. (2006). Degree of soil development in some alfisols of subtropical India with special reference to the nature and distribution of Fe and Al. International Journal of Agricultural Research , 1 (4), 1–7.
- Sharma, S. S. , Totawat, K. L. , & Shyampura, R. L. (1996). Charecterization and classification of soils in a toposequence over basaltic terrain. Journal of the Indian Society of Soil Science , 44 , 470–475.
- Sidhu, G. S. , Ghosh, S. K. , & Manjaiah, K. M. (2000). Pedological variabilities and classification of some dominant soils of Aravallies-Yamuna river transect in semi-arid tract of Haryana. Agropedology , 10 , 80–87.
- Walia, C. S. , & Rao, Y. S. (1996). Genesis, characteristics and taxonomic classification of some soils in Bundelkhand region of Uttar Pradesh. Journal of the Indian Society of Soil Science , 44 , 476–481.
- Walia, C. S. , & Rao, Y. S. (1999). Forms of iron and weathering index of some typical pedons of Bundelkhand region in relation to physiography and parent material. Agropedology , 9 , 82–87.
- Wilson, M. A. , Burt, R. , Sobecki, T. M. , Engel, R. J. , & Hipple, K. (1996). Soil properties and genesis of pans in till-derived Andisols, Olympic Peninsula, Washington. Soil Science Society of America Journal , 60 , 206–218.10.2136/sssaj1996.03615995006000010033x