?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The Syzygium caryophyllatum and Syzygium zeylanicum fruits were analysed for their nutritional potential and pharmacological property. The antibacterial activity was carried out by disc diffusion method followed by microdilution method for minimum inhibitory concentration. The antioxidant activity was assessed by 2, 2-diphenyl-1-picrylhydrazyl, free radical scavenging method. The fruits were also fermented to produce wine, and were then analysed for their physical and chemical properties. The fatty acid analysis of the seeds and pulp was carried out separately by gas chromatography. From the study, it was found that the wild fruits were nutritionally rich in comparison to the cultivated fruits. The percentage of unsaturated fatty acid was found to be higher than saturated fatty acid. The major fatty acids present were oleic acid, linoleic acid and palmitic acid. The fruit methanol extract of the S. caryophyllatum was independent of gram reaction inhibiting the growth of both gram-positive and gram-negative bacteria. The minimum inhibitory values ranged from 8 to 10 mg/ml. The calorific value of both the fruits increased upon fermentation, and the alcohol content was 4 and 5% in S. zeylanicum and S. caryophyllatum, respectively. The results highlight the importance of wild fruit species as an affordable nutrient source and as an antibacterial agent.
Public Interest Statement
The fruits of the species studied here are edible and highly nutritious in terms of carbohydrates, proteins and energy level. The nutritive quality of these fruits when compared with the commercially available ones exhibits better properties. Wine can be prepared from the fruits which do not require much effort.
1. Introduction
Many of the wild fruits have been reported to possess better nutritional properties than well-known cultivated fruits (Eromosele, Eromosele, & Kuzhkuzha, Citation1991). Fruits rich in vitamins, minerals and secondary metabolites form an important part of human diet (Delgado – Vargas & Paredes – López, Citation2002). As a result, the nutritional features of the wild edible plants have been evaluated in the recent years (Glew et al., Citation2005). Studies also demonstrate the feasibility of using wild fruits such as S. cumini (Shashikant, Rahul, & Rajasekaran, Citation2012), Syzygium malaccensis and Eugenia owariensis (Enidiok & Attah, Citation2010) in wine production. Several studies have shown correlation between consumption of phenol-rich beverages and reduced coronary heart disease mortality (Balentine, Wiseman, & Bouwens, Citation1997). The epidemiological studies have confirmed that increased consumption of fruit and vegetables reduces the risk of cancer by 15%, cardiovascular disease by 30% and mortality by 20% (Steimez & Potter, Citation1996). This is attributed to various phytochemicals present in them (Prior & Cao, Citation2000). The pharmacological industries have produced a large number of newer antibiotics in the last three decades, but the resistance to these drugs by micro-organisms has increased (Cohen, Citation1992). Treatment to the infections produced by the resistant bacteria is much more complicated (Schmidt, Citation2003). In addition to this, antibiotics are sometimes associated with adverse effects on the host (Iwu, Duncan, & Okungi, Citation1999). Contrary to the synthetic drug, antimicrobials of plant origin are not associated with any side effects (Ahmad, Mehmood, & Mohammad, Citation1998). Hence, there is a need to establish the pharmacological activities for identifying the various crude drugs for potency.
Syzygium zeylanicum (L.) DC is a medium-sized tree. It is distributed in the Indo-Malaysian regions. The fruits have thick pulp when ripe, which are sweet to taste. Plant is a stimulant and used against rheumatism and syphilis (Shetty, Kaveriappa, & Bhat, Citation2002). The fresh leaf of this plant is being consumed as food in Vietnam. A macrocyclic ellagitannin, zeylaniin isolated from the leaves exhibited potent antioxidant activities in the assays of DPPH, oxygen radical absorbance capacity and malonaldehyde/gas chromatography (Nomi et al., Citation2012).
Syzygium caryophyllatum (L.) Alston is a low-altitude evergreen tree. The species is endemic to Sri Lanka and also distributed in the Western Ghats of India. The fruits are oval, juicy, sweet in taste and purple in colour. They are produced towards the end of summer maturing during the rainy season. The flower buds possess antioxidant activity (Gupta & Sharma, Citation2006). The stem bark is traditionally used in the treatment of diabetes mellitus (Ediriweera & Ratnasooriya, Citation2009). The leaf and bark extracts have been reported to possess antibacterial and antioxidant properties (Shilpa & Krishnakumar, Citation2012), whereas the fruits of both the species have not been studied. Hence, the present work reports on the nutrition, fermentation and pharmacological characteristics of the fruit of S. zeylanicum and S. caryophyllatum.
2. Materials and methods
2.1. Collection of plant material
The undamaged mature fruits were harvested in between April and July 2011 around the Mangalore University Campus, since the fruits are seasonal and are available only between April and July. The fruits were washed, the pulp was separated from the seed, oven dried (45°C) and was pulverised. The pulverised samples were subjected to further analysis. The analyses were carried out in triplicates.
2.2. Nutritional analyses
The following are the various parameters used in the nutritional analysis: moisture content, soluble solids, pH, crude fibre, ash value, phenols, tannins, phytic acid, total and reducing sugars, total monomeric anthocyanin and minerals. The moisture content was determined by drying the sample in hot air oven at 100°C to a constant weight. The soluble solids was analysed in a hand-held refractometer and was represented as °Brix; pH was verified with a pH metre. Crude fibre was determined by digestion in dilute acid H2SO4 and alkali NaOH (Maynard, Citation1970). Ash content was determined by incineration of 2 g of sample at 550°C for 5 h. Soxhlet extraction method with petroleum ether as a solvent was used for the determination of crude fat. Phenolic content was determined by Follin ciocalteu method and expressed as gallic acid equivalent (Taga, Miller, & Pratt, Citation1984). Phytic acid was extracted and measured colourimetrically (Wheeler & Ferrel, Citation1971). Tannin content in the sample was estimated by Vanillin hydrochloride method (Robert, Citation1971). Total and reducing sugars were determined by anthrone (Hedge & Hofreiter, Citation1962) and dinitrosalicylic acid method (Miller, Citation1972), respectively. The total monomeric anthocyanin pigment content was determined following (AOAC Official Method, Citation2005). Nitrogen was determined by atomic absorption spectroscopy and crude protein was calculated by multiplying the percentage nitrogen content by the conversion factor 6.25. Atomic absorption spectrometer equipped with hollow-cathode lamps was used for mineral analyses. Sodium was analysed by flame photometer. Samples were prepared by triple-acid digestion. The calorific value was calculated in kilocalories (kcal) multiplying by physiological energy factor composition (4, 4 and 9) of percentage proteins, fats and carbohydrates, respectively (FAO, Citation1968; USDA, Citation1999).
2.3. Fatty acid composition
The sample was accurately weighed into a flat bottom flask. Fifty ml of methanol with five drops of concentrated sulphuric acid was then added and placed on the water bath with reflux condenser until the fat got methylated. Gas chromatography (GC) analysis was carried out using a Column: DB-wax (30 m); carrier gas, Nitrogen; split ratio, 90:1; and a flame ionisation detector. The column temperature was programmed at 100°C with increase for every 5 min up to 210°C.
2.4. Wine preparation
The fruits were harvested between April and June 2011 from around the Mangalore University campus. One kilogram of ripe and healthy fruits were selected, washed in tap water and immersed in 5% salt (NaCl) solution for 2 h after which the fruit was depulped. The pulp was soaked in water in 1:1 ratio. Cane sugar was added to adjust the sugar content to 21° brix. Sodium metabisulphite was added up to a concentration of 100 mg/l to inhibit bacterial growth. The must was then inoculated with starter culture and fermentation was carried out at room temperature (32°C). The wine after racking was clarified with the addition of 0.04% bentonite and analysed.
2.5. Preparation of the extract
The samples were soxhlet extracted with methanol (30°C) and petroleum ether (35°C). Aqueous extract was prepared by soaking 20 g of the powdered material in distilled water for seven days which was placed in water bath at 37°C. The defatted sample obtained after extraction with petroleum ether was used for nutrient analysis. The methanolic and aqueous extracts obtained were evaporated to dryness and stored at 4°C for further use.
2.6. Antibacterial assay
The microbial strains viz., Staphylococcus aureus (NCIM 2079), Bacillus subtilis (ATCC 6633), Escherichia coli (NCIM 2931), Pseudomonas aeruginosa (NCIM 2200), Klebsiella pneumoniae (NCIM 2957) and Proteus vulgaris (NCIM 2813), were used for the study. The microbial isolates were procured from National Chemical Laboratory, Pune, India. The antibacterial assay was carried out by following the disc diffusion method (Vardar-Unlu, Candan, Sökmen, Daferera, & Polissiou, Citation2003). The MIC (Minimum Inhibitory Concentration) was determined by microdilution method (National Committee for Clinical Laboratory Standards, Citation2000).
2.7. Total phenolic, flavonoids and monomeric anthocyanin pigment content determination
Total phenolic content was estimated following Folin–Ciocalteau (FC) method (Taga et al., Citation1984) and expressed as Gallic acid equivalent (mg/g) and total flavonoid content by colorimetric assay (Zhishen, Mengcheng, & Jianming, Citation1999) and expressed as mg quercetin equivalent (mg/g). The total monomeric anthocyanin content was determined by pH differential method (FAO, Citation1968) and expressed as cyanidin-3-glucoside equivalents (mg/l).
2.8. Antioxidant assay
The antioxidant efficiency of the extracts and the wine was assessed based on its ability to scavenge DPPH free radicals (Liyana – Pathiranan & Shahidi, Citation2005). A solution of DPPH (0.135 mM) in methanol was prepared and 1 ml of this solution was mixed with 1 ml of the sample. The reaction mixture was vortexed thoroughly and left in dark at room temperature for 30 min. The absorbance of the mixture was measured at 517 nm using ascorbic acid as reference standard. The ability to scavenge DPPH radical was calculated as:
2.9. Physical and chemical parameters of wine
Brix value was measured using a refractometer, pH value with pH metre. Total and reducing sugars were determined by Anthrone (Hedge & Hofreiter, Citation1962) and dinitrosalicylic acid method (Miller, Citation1972), respectively. Alcohol was measured using alcoholometer. Absorbance was measured using spectrophotometer. The calorific value was calculated following (Vine, Harkness, Browning, & Wagner, Citation1997). Calorific value (kcal) = 6.9 × 0.794 × alcohol (%) + (4 × reducing sugar) + (2.4 × total sugar).
3. Statistical analysis
One-way analysis of variance was performed by Excel (Microsoft Inc. 2007) and Graph pad prism (version 5.01), and means were compared by Bonferroni post-test (p < 0.05).
4. Results and discussion
4.1. Nutritional analyses
The nutritional status of the wild fruits (Table ) has been compared with some cultivated fruits (Anonymous, Citation2010; Rathore, Citation2009). The percentage of water in the food has a greater impact on its energy content as it adds to the weight of the food thereby decreasing the energy content without any increase in its calories (Grunwald, Citation2001) and also the presence of moderate moisture content in the fruit is an indication of long-time storage without mould development (Umar, Hassan, & Ado, Citation2007). But it is obvious from the results that the fruits of S. zeylanicum registered high moisture content (92.22%) in comparison to S. caryophyllatum (63.17%) and also than the commercial fruits which ranged from 74 to 86%. The flavour of the fruits was acidic and sweet as represented by its low pH (S. caryophyllatum −4.04 and S. zeylanicum −3.75, respectively), and high soluble solids (S. caryophyllatum −7° Brix and S. zeylanicum −8.5° Brix). The pulp of S. zeylanicum (2.89%) and S. caryophyllatum (0.95%) contained lower amounts of fat but slightly higher than that of commercial fruits. Fats are known to hinder the onset of hunger and also serve as a transport medium for the fat soluble vitamins. They are a source of antioxidant and also enrich the flavour of the food (Wardlaw & Kessel, Citation2002). The ash content in the fruits, which is the total mineral content, was compared well with that of commercial fruits which ranged from 0.3 to 0.7%. Protein content of the fruits was 3.37 and 3.68% for S. caryophyllatum and S. zeylanicum, respectively, which is higher in comparison to the cultivar counterparts. The crude fibre percentage of S. caryophyllatum (4.12) was compared well with the crude fibre content of guava (5.2%) and pomegranate (5.1%) whereas the fruit of S. zeylanicum contained slight higher percentage (12.67). S. caryophyllatum contained 50 mg/100 g of vitamin C. Even though this value is quite low when compared to guava (212 mg/100 g), it is appreciably high in comparison to the vitamin C content in apple (1 mg/100 g), mango (16 mg/100 g) and pomegranate (16 mg/100 g). S. zeylanicum contained about 6 mg/100 g. The total and reducing sugar were 58.71 and 48.08% in S. zeylanicum and 37.70 and 30.405% in S. caryophyllatum, respectively. The energy value of the two fruits was also high (p < 0.05) in comparison to the commercial fruits. The phenolic and tannin content was quite low i.e. 12.56 and 1.19% in S. zeylanicum 47.51 and 16.77% in S. caryophyllatum, respectively. The purple colour of the fruits is mainly due to anthocyanin and since the S. caryophyllatum fruits are purple in colour, they were analysed for the anthocyanin pigments. The pigment was found to be in the range of 240.36 mg/l. The values of mineral composition have been depicted in Table . The macronutrients such as phosphorus, calcium and magnesium in S. caryophyllatum and S. zeylanicum were superior in comparison to the cultivated fruits except for sodium, whereas the micronutrients such as copper, iron and zinc were low and lower than that of the cultivated fruits. The amount of minerals in the plants chiefly depends on the abundance of minerals in the soil and also on the intensity of fertilisation (Wardlaw & Kessel, Citation2002)
Table 1. Nutritive analysis of S. caryophyllatum and S. zeylanicum fruits
Table 2. Mineral composition of S. caryophyllatum and S. zeylanicum fruits
4.2. Fatty acid composition
Fats and oils, have immense use in medicine and in diet, act as heat insulators and energy reserves (Hawk & Oser, Citation1995). Essential fatty acids in medicinal seed oils and other vegetable oils maintain the function and integrity of cellular membranes (Ackman, Citation1981). Linoleic acid, an unsaturated omega six fatty acid, maintains the integrity of integuments. It is a precursor for the prostaglandins synthesis and blocks the gastric acid production (Taylor et al., Citation1988). It has been reported that dietary fat rich in linoleic acid prevents disorders such as coronary heart diseases and atherosclerosis (Ramsden et al., Citation2013). Oleic acid is reported to be effective in lowering LDL content and LDL cholesterol content (Grundy, Citation1989).
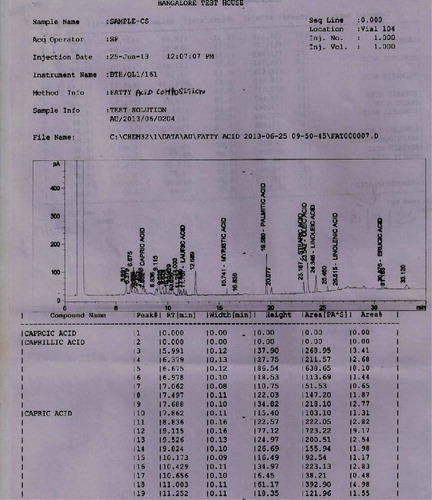
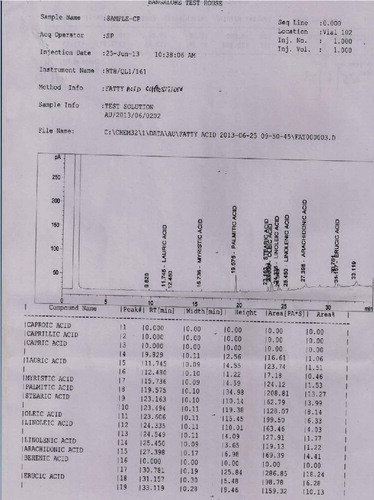
The pulp and seeds of S. zeylanicum contained palmitic acid, oleic acid, linoleic acid, linolenic acid and stearic acid along with caprillic acid which was present only in the seed. The major fatty acids detected in the seed were oleic acid (31.37%), Linoleic acid (26.04%) and Palmitic acid (25.74%). Similarly oleic acid (21.09%), Linoleic acid (24.80%) and Palmitic acid (32.18%) were also the major compounds present in the pulp (Table ; Figures and ). The pulp and seeds of S. caryophyllatum contained lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid, linoleic acid, linolenic acid and erucic acid in common. In addition to this arachidonic acid and capric acid were also present in the pulp and seed, respectively (Figures and ).
Table 3. Chemical composition of the fatty acid methyl ester extracts
Figure 1. Chromatogram of chemical composition of the fatty acids methyl ester extract of S. zeylanicum seed
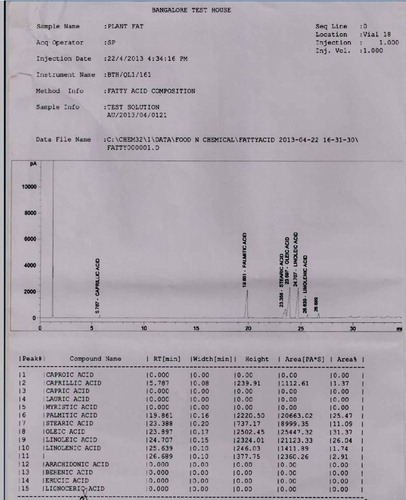
Figure 2. Chromatogram of chemical composition of the fatty acids methyl ester extract of S. zeylanicum pulp
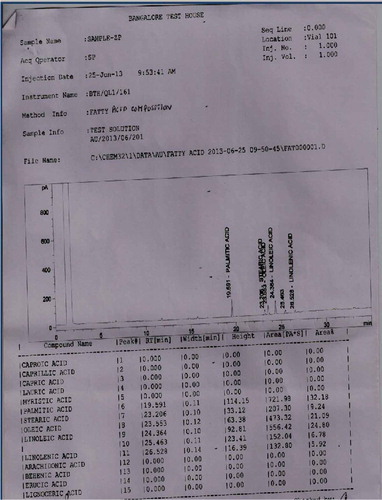
4.3. Wine analyses
Fermentation was carried out for 21 days after which, the must was filtered and analysed (Table ). The pH decreased from 5.61 to 4.88 representing between day 0 and day 21. Our results are comparable to the work carried out by Chowdhury and Ray (Citation2007), who have reported similar results in S. cumini fruits. The brix value decreased from 18.16° to 4.8° and from 19.5° to 6° for S. zeylanicum and S. caryophyllatum, respectively, indicating the conversion of insoluble feedstock to soluble form. The decrease in the amount of total sugar and the production of alcohol indicated the utilisation of sugar for the yeast growth resulting in the production of alcohol. The alcohol content was 4 and 5% on 21st day for S. zeylanicum and S. caryophyllatum, respectively. About 6 and 3% alcohol was found to be present in the 21st day wine of S. cumini and Garcinia indica fruits, respectively. Hence, the wine of these fruits are all categorised as low alcohol wine, as the fermented liquid to be considered as wine, it should contain alcohol between 7 and 12% (Pickering, Citation2000). There was an increase in the total phenol concentration. The calorific value increased in the 21st day wine. The DPPH scavenging activity was carried out only in the 21st day wine. About 81.65 ± 7.09% and 49.03 ± 0.7% inhibition was obtained per 10 μl of the wine for S. caryophyllatum and S. zeylanicum, respectively.
Table 4. Changes in the physical and chemical characteristics of wine
4.4. Percentage yield
The percentage yield of S. caryophyllatum in methanol and water was 61.16 and 45.30, respectively. The percentage yield of S. zeylanicum in methanol and water was 68.73 and 24.05, respectively.
4.5. Total phenolic and flavonoid content
The fruits registered a phenolic content of 75.16 ± 7.59 and 28.18 ± 2.7 mg/g for methanol extracts of S. caryophyllatum and S. zeylanicum, respectively, and 33.55 ± 1.44 and 18.11 mg/g for aqueous extracts of S. caryophyllatum and S. zeylanicum, respectively. Flavonoids were not detected in the aqueous extract of S. zeylanicum but the methanol extract contained 4.31 ± 0.671 mg/g. The methanol and aqueous extract of S. caryophyllatum registered 27.2 ± 1.41 and 8.25 ± 1.14 mg/g of flavonoid content.
4.6. Total monomeric anthocyanin pigment content
A positive correlation was obtained between the anthocyanins and antioxidant activity in a study conducted on 28 different fruits suggesting their role towards the activity (Vlachos, Critchely, & Von Holy, Citation1996). However, the fruit of S. caryophyllatum with an anthocyanin content of 240.36 ± 1.21 mg/l exhibited poor antioxidant activity.
4.7. Antibacterial activity
The in vitro antibacterial property of the fruit extracts and their MIC values are depicted in Tables and , respectively. Among the four extracts, the methanol extracts of S. caryophyllatum exhibited the best activity with a MIC value of 8–10 mg/ml being active against all the bacterial strains used. The methanol extract of S. zeylanicum with a MIC value of 5 mg/ml inhibited the growth of gram negative bacteria alone. In comparison, the methanol extracts of both the fruits were effective in inhibiting the bacterial growth. Similar results have been reported from the methanol extracts of S. alternifolium and S. samarangense, and the fruits being active against both gram positive and gram negative bacteria (Venkataratnam & Venkataraju, Citation2008). This may be due to the solvent extract containing different constituents having antimicrobial activity and also methanol has been proved to be the most efficient solvent for extracting a broad spectrum of antimicrobial compounds from plants (Gracia-Alonso, Pascual-Teresa, Santos-Buelga, & Rivas-Gonzalo, Citation2004).
Table 5. Antibacterial activity of the methanol and aqueous extracts of S. caryophyllatum and S. zeylanicum
Table 6. Minimum inhibitory concentration of the methanol and aqueous extracts of S. caryophyllatum and S. zeylanicum
5. Conclusion
The results highlight the importance of wild fruit species as an affordable nutrient source and as an antibacterial agent. A comparison was made between S. caryophyllatum, S. zeylanicum the wild edible fruit species and the popular edible cultivars such as mango, papaya, apple, orange and pomegranate in terms of vitamin, minerals protein, sugar content, gross energy, etc. The fruits were fermented to form wine. The rich nutrient composition provides scope for the utilisation of these fruits as an alternate for bionutrition. Although the wine contained low alcohol the energy tend to increase after fermentation. Among the four extracts used the methanol extract of S. caryophyllatum was effective in inhibiting the growth of all the bacteria studied.
Cover image
Source: Author.
Acknowledgement
One of the authors (Shilpa K.J.) is thankful to DST-PURSE for the financial support.
Additional information
Funding
Notes on contributors
K.J. Shilpa
We are mainly concentrating on the following research activities: the phytochemical and pharmacological studies on some of the Syzygium species of Western Ghats; compound isolation, extraction and characterisation of essential oil from various plant parts like seeds, leaves, etc.; the biochemical and ecology-related studies in some important ferns; and structural and floristic studies on Myristica swamp.
References
- Ackman, R. G. (1981). New sources of fats and oils. In E. H. Pryde , L. H. Princer , & K. D. Mukergee (Eds.), Journal of American oil Chemists Society (pp. 189–220). Champaign, IL.
- Ahmad, I. , Mehmood, Z. , & Mohammad, F. (1998). Screening of some Indian medicinal plants for their antimicrobial properties. Journal of Ethnopharmacology , 62 , 183–193.10.1016/S0378-8741(98)00055-5
- Anonymous . (2010). USDA national nutrition database for standard reference . Release 22.
- AOAC Official Method . (2005). Total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines—pH differential method. Journal of AOAC International , 88 , 1269–1278.
- Balentine, D. A. , Wiseman, S. A. , & Bouwens, L. C. M. (1997). The chemistry of tea flavonoids. Critical Reviews in Food Science and Nutrition , 37 , 693–704.10.1080/10408399709527797
- Chowdhury, P. , & Ray, R. C. (2007). Fermentation of jamun (Syzgium cumini) fruits to form red wine. ASEAN Food Journal , 14 , 15–23.
- Cohen, M. L. (1992). Epidemiology of drug resistance: Implications for a post-antimicrobial era. Science , 257 , 1050–1055.10.1126/science.257.5073.1050
- Delgado – Vargas, F. , & Paredes – López, O. (2002). Natural colorants for food & nutraceutical uses (1st ed.). Boca Raton, FL: CRC Press.
- Ediriweera, E. R. H. S. S. , & Ratnasooriya, W. D. (2009). A review on herbs used in treatment of diabetes mellitus by Sri Lankan ayurvedic and traditional physicians. Ayu , 30 , 373–391.
- Enidiok, S. E. . & Attah, L. E. (2010). Chemical composition in relation to the quality of wines produced from Nigerian Syzygium malaccensis and Eugenia owariensis apples. The African Journal of Food Agriculture and Development , 10 , 2124–2138.
- Eromosele, I. C. , Eromosele, C. O. , & Kuzhkuzha, D. M. (1991). Evaluation of mineral elements and ascorbic acid contents in fruits of some wild plants. Plant Foods for Human Nutrition , 41 , 151–154.10.1007/BF02194083
- FAO . (1968). Food composition table for use in Africa. Energy factor (FAO Document Repository, p. 226).
- García-Alonso, M. , Pascual-Teresa, S. , Santos-Buelga, C. , & Rivas-Gonzalo, J. C. (2004). Evaluation of the antioxidant properties of fruits. Food Chemistry , 84 , 13–18.10.1016/S0308-8146(03)00160-2
- Glew, R. S. , VanderJagt, D. J. , Bosse, R. , Huang, Y. S. , Chuang, L. T. , & Glew, R. H. (2005). The nutrient content of three edible plants of the Republic of Niger. Journal of Food Composition and Analysis , 18 , 15–27.10.1016/j.jfca.2003.12.002
- Grundy, S. M. (1989). Monounsaturated fatty acids and cholesterol metabolism. Implication of dietary recommendation. Journal of Nutrition , 119 , 529–533.
- Grunwald, G. K. (2001). Quantifying and separating the effects of macronutrient composition and non-macronutrients on energy density. British Journal of Nutrition , 86 , 265–276.10.1079/BJN2001404
- Gupta, V. K. , & Sharma, S. K. (2006). Plants as natural antioxidants. Natural Product Radiance , 5 , 326–334.
- Hawk, P. B. , & Oser, B. (1995). Hawk’s physiological chemistry (pp. 112–218). Bombay: Tata McGraw-Hill.
- Hedge, J. E. , & Hofreiter, B. T. (1962). Carbohydrate chemistry (Vol. 17). ( R. L. Whistler & J. N. Be Miller , Eds.). New York, NY: Academic Press.
- Iwu, M. M. , Duncan, A. R. , & Okungi, C. O. (1999). New antimicrobials of plant origin. In J. Janik (Ed.), Perspectives on new crops and new uses (pp. 457–462). Alexandria, VA: ASHS Press.
- Liyana - Pathiranan, C. M. , & Shahidi, F. (2005). Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L) as affected by gastric pH conditions. Journal of Agricultural Food Chemistry , 53 , 2433–2440.
- Maynard, A. J. (Ed.). (1970). Methods in food analysis (p. 176). New York, NY: Academic Press.
- Miller, G. L. (1972). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Chemistry , 31 , 426–428.
- National Committee for Clinical Laboratory Standards . (2000). Performance standard for antimicrobial disk susceptibility test (Approved standard NCCLS document M2-A7). Wayne, PA.
- Nomi, Y. , Shimizu, S. , Sone, Y. , Tuyet, M. T. , Gia, T. P. , Kamiyama, M. , … Otsuka, Y. (2012). Isolation and antioxidant activity of zeylaniin A, a new macrocyclic ellagitannin from Syzygium zeylanicum leaves. Journal of Agricultural and Food Chemistry , 60 , 10263–10269.10.1021/jf302977n
- Pickering, G. J. (2000). Low and reduced-alcohol wine: A review. Journal of Wine Research , 11 , 129–144.10.1080/09571260020001575
- Prior, R. L. , & Cao, G. (2000). Antioxidant phytochemicals in fruits and vegetables - diet and health implications. Journal of Horticultural Science , 35 , 588–592.
- Ramsden, C. M. , Zamora, D. , Leelarthaepin, B. , Sharon, F. M. , Faurot, K. R. , & Suchindran, C. M. , … Hibbeln, J. R. (2013). Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney diet heart study and updated meta-analysis. British Medical Journal , 346 , 1–18. doi:10.1136/bmj.e8707
- Rathore, M. (2009). Nutrient content of important fruit trees from arid zone of Rajasthan. Journal of Horticulture and Forestry , 1 , 103–108.
- Robert, E. B. (1971). Method of estimation of tannin in grain sorghum. Agronomy Journal , 63 , 511–512.
- Schmidt, F. R. (2003). The challenge of multidrug resistance: Actual strategies in the development of novel antibacterials. Applied Microbiology and Biotechnology , 63 , 335–335.
- Shashikant, S. P. , Rahul, M. T. , & Rajasekaran, P. (2012). Utilization of jamun fruit (Syzygium Cumini) for production of red wine. Journal of Advanced Laboratory Research in Biology , 3 , 200–203.
- Shetty, B. V. , Kaveriappa, K. M. , & Bhat, K. G. (2002). Plant resources of Western Ghats and lowlands of Dakshina Kannada and Udupi districts . Mangalore: Pilikula Nisargadhama Society.
- Shilpa, K. J. , & Krishnakumar, G. (2012). Phytochemical screening and antibacterial and antioxidant efficacy of the leaf and bark extracts of Syzygium caryophyllatum (L.) Alston. International Journal of Pharmacy and Pharmaceutical Sciences , 4 , 198–302.
- Steimez, K. A. , & Potter, J. D. (1996). Vegetables, fruits and cancer prevention: A review. Journal of American Dietetic Association , 96 , 1027–1039.
- Taga, M. S. , Miller, E. , & Pratt, D. E. (1984). Chia seeds as a source of natural lipid antioxidants. Journal of the American Oil Chemists’ Society , 61 , 928–931.10.1007/BF02542169
- Taylor, J. B. , Vidal, A. , Torpier, G. , Meyer, D. J. , Roitsch, C. , Balloul, J. M. , … Ketterer, B. (1988). The glutathione transferase activity and tissue distribution of a cloned Mr28 K protective antigen of Schistosoma mansoni . The EMBO Journal , 7 , 465–472.
- Umar, K. J. , Hassan, L. G. , & Ado, Y. (2007). Mineral composition of Detarium microcarpum grown in Kwatarkwashi, Zamfara state, Nigeria. International Journal of Pure and Applied Sciences , 1 , 43–48.
- USDA, US Department of Agriculture, Agricultural Research Service . (1999). USDA nutrient database for standard reference . Release 13. USDA Nutrient Data Laboratory.
- Vardar-Ünlü, G. , Candan, F. , Sökmen, A. , Daferera, D. , & Polissiou, M. (2003). Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae). Journal of Agricultural Food Chemistry , 51 , 63–67.10.1021/jf025753e
- Venkataratnam, K. , & Venkataraju, R. R. (2008). In vitro antimicrobial screening of the fruit extracts of two Syzygium species (Myrtaceae). Advances in Biological Research , 2 , 17–20.
- Vine, R. P. , Harkness, E. M. , Browning, T. , & Wagner, C. (1997). Winemaking (p. 439). New York, NY: Chapman and Hall.10.1007/978-1-4757-2656-5
- Vlachos, V. , Critchely, A. T. , & Von Holy, A. (1996). Establishment of a protocol for testing antimicrobial activity in Southern African macroalgae. Microbios , 88 , 115–123.
- Wardlaw, G. M. , & Kessel, M. W. (2002). Minerals: Dietary needs, absorption, transport and excretion. In Perspectives in nutrition (5th ed., pp. 418–864). New York, NY: McGraw‐Hill.
- Wheeler, E. L. , & Ferrel, R. E. (1971). A method for phytic acid determination in wheat and wheat fractions. Cereal Chemistry , 48 , 312–317.
- Zhishen, J. , Mengcheng, T. , & Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry , 64 , 555–559.10.1016/S0308-8146(98)00102-2