?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pulp from locally grown Apple variety was given different treatments (chemical and thermal) and its various quality parameters were studied during a storage period of 90 days. All the quality parameters viz. pH, titrable acidity, total soluble solids, reducing sugars and non-reducing sugars, color and antioxidants activity were significantly affected by treatments employed as well as storage period. As a result of chemical and thermal treatments, antioxidant activity and total phenolic content of samples decreased, while color and consumer acceptability significantly improved. Color and antioxidant activity were highly correlated and the type of preservation method used affected the correlation coefficient between color and antioxidant activity. Among different treatments, a combination of pasteurization and chemical preservation was found to be most effective in increasing the shelf life of apple pulp.
Public Interest Statement
Browning of apple pulp is one of the major obstacles during processing and storage of apple pulp. Pasteurization and chemical treatments are preferred methods in fruit preservation and processing in Asian countries that strongly affect its consumer acceptability. The results of this work reveal the individual and combined effects of various preservation techniques (thermal and chemical treatment) on color, antioxidant properties, shelf life, and consumer acceptability of apple pulp, hence establishing the comparative effectiveness of each preservation technique.
Competing interests
The authors declare no competing interest.
1. Introduction
Apple (Malus domesticus) is one of the most widely cultivated tree fruit (Harris, Yada, & Mitcham, Citation2007) and is generally believed that to have originated somewhere in Central Asia. It is the fourth most widely produced fruit in the world after banana, orange, and grapes (Ali, Raza, Khan, & Hussain, Citation2004). Apple is consumed not only because of their flavor, but also because of their nutritional importance due to vitamins, dietary fibers, and rich phenolic content in comparison to other fruits (Sun, Chu, Wu, & Liu, Citation2002). Apple contains carbohydrates (13.9 g/100 g), fiber (0.8 g/100 g), proteins (0.4 g/100 g), lipid (0.3 g/100 g), ash (0.3 g/100 g), vitamin C (8 mg/100 g), sodium (0.3 mg/100 g), potassium (145 mg/100 g), calcium (7 mg/100 g), magnesium (6 mg/100 g), iron (480 μg/100 g), phosphorus (12 mg), and iodine (2 μg/100 m) (Hussain, Citation2001). Apples have very strong anti-oxidant activity mainly due to the polyphenols, such as quercetin, catechin, phloridzin, and chlorogenic acid present in them (Boyer & Liu, Citation2004; Kähkönen et al., Citation1999; Lu & Yeap Foo, Citation2000). Due to high levels of phytochemcials present, consumption of apple is associated with reduction in cardiovascular diseases (Knekt, Jarvinen, Hakkinen, Reunanen, & Maatela, Citation1996; Sesso, Gaziano, Liu, & Buring, Citation2003), anti-asthamatic effects (Knekt et al., Citation2002), anti-cholesterolemic effects (Leontowicz et al., Citation2002), and anti-diabetic effects (Knekt et al., Citation2002; Mueller et al., Citation2009). Apple consumption is also effective in treating infant intestinal disorders, such as diarrhea and dysentery (Considine, Citation1982) and helps in weight reduction in adults (Conceição de Oliveira, Sichieri, & Sanchez Moura, Citation2003).
Apple is mostly consumed as fresh fruit, but due to its perishable nature, its quality deteriorates and cannot be stored for a long time. Quality of apple changes rapidly during storage that substantially affects the consumer acceptability (Vieira et al., Citation2009). In order to preserve the fruit for longer periods, it is processed to different products, such as juices, jams, and jellies and even stored as pulp. The preservation techniques are aimed to slow down the changes that caused by foods deterioration, due to large number of physical, chemical, enzymatic, or biological reactions (Gould, Citation2000). Chemical preservation is the most commonly and widely used method among several methods of preservation because it is simple and economical. The chemical preservatives are used to prevent microbial contamination and thus are effectively used in combinations with other preservative techniques for increasing the shelf life. No single chemical preservative is completely effective against all micro-organisms (Chiply, Citation1983). Sodium benzoate (SB) and potassium metabisulphite (KMS) are commonly used as preservatives for long-term storage of fruit pulp because of their better antimicrobial activity and prevention of browning (Lueck, Citation1990; Manganelli & Casolari, Citation1983; Sofos & Busta, Citation1981).
Although effect of storage on nutritional parameters has already been studied, combined effect of storage and different pretreatments on nutritional and antioxidant activity of apple pulp have not been reported so far. Hence, the present study was carried out to study the effect of different preservation techniques on quality and nutraceutical (antioxidant) parameters of apple pulp during storage.
2. Materials and methods
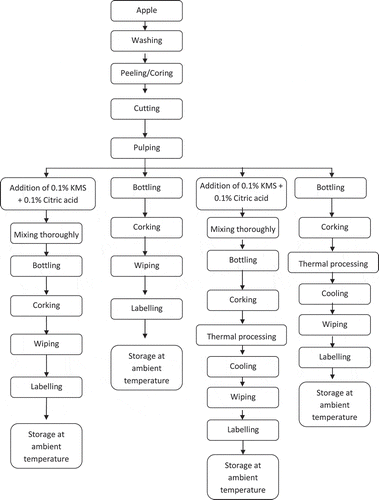
Chemicals used were of analytical grade and were provided by HiMedia. Red Delicious variety of apples was used for production of pulp. Apple was thoroughly washed to remove dirt, dust, pesticide residues, and microflora on the surface of the fruit. It was washed, peeled, cored, and sliced. Apple was crushed with the help of home-scale mixer-cum-juicer (Sujata Powermatic +) for obtaining homogenized pulp and the pulp was given different pretreatments that include:
| (1) | Treatment 1 (T1) = storage under ambient conditions (25°C) (no preservative, no pasteurization). | ||||
| (2) | Treatment 2 (T)2 = 0.1% KMS + 0.1% citric acid. | ||||
| (3) | Treatment 3 (T)3 = Pasteurization at 65°C for 30 min. | ||||
| (4) | Treatment 4 (T)4 = Pasteurization at 65°C for 30 min + preservative (0.1% KMS + 0.1% citric acid). | ||||
The treated pulp was transferred to sterilized glass bottles that were stored under ambient conditions (25°C) for a period of 90 days and analysis were carried out after every 15 days (Figure ).
2.1. Physicochemical analysis of the pulp
2.1.1. Determination of pH
The pH of the apple pulp was determined using digital pH meter (Inolab WTW Series 720). The pH meter was first calibrated using buffer of pH 4.0 and 7.0 at room temperature. The sample was then taken in a 100 mL beaker, stirred, and electrode of pH meter put in it and direct reading from pH meter was taken when the reading stabilized.
2.1.2. Determination of titrable acidity
Titrable acidity was done using the method of (Association of Official Analytical Chemists, Citation2000). Ten mL of sample was taken and homogenized with distilled water in a home-scale blender. The whole mass was then transferred to a 100 mL volumetric flask and the volume was made up to the mark with distilled water. Ten mL of this sample solution was taken in a conical flask. Two to three drops of phenolphthalein indicator were added and then the flask was shaken vigorously. It was then titrated immediately with 0.1 N NaOH solutions from a burette till pink color appeared. The volume of NaOH solution required for titration was noted and percent of titrable acidity was calculated as below:
2.1.3. Determination of total solid soluble
Total soluble solids (TSS) was done as per the method of Ahmad, Thompson, Hafiz, and Asi (Citation2001). A hand-held refractometer (N-1E, °Brix 0–32%, ATAGO Co., Ltd, Tokyo, Japan) was used to determine the total soluble solid by calibrating with distilled water followed by dropping 1–2 drops of sample solution onto the clean surface of the refractometer.
2.1.4. Determination of sugars
Reducing and non-reducing sugar content of apple pulp was determined using the method of Lane and Eynon (1923) as described in Ranganna (Citation1991).
2.1.5. Determination of color
The Color of the apple pulp was measured using a Hunter’s Lab color analyzer (Hunter lab scan XE, Reston VA, USA). The equipment was calibrated using white and black standard ceramic tiles. In the Hunter’s lab colorimeter, the color of a sample is denoted by the three dimensions, L*, a* and b*. L*, refers to lightness of the color of the diets and ranges from black = 0 to white = 100. A negative value of a* indicates a green color, whereas the positive value indicates red-purple color. A positive value of b* indicates a yellow color and the negative value a blue color. The ΔE* is a single value which takes into account the differences between L*, a*, and b* of the apple pulp. It was calculated using the equation (Caivano, Citation2012):
where ΔL* = (L₁*−L0 *); Δa* = (a₁*−a0 *); and Δb* = (b1 *−b0 *).
2.2 Antioxidant analysis
The pulp was stored in the laboratory for 90 days in glass bottles at ambient temperature (28–32°C). The antioxidant analysis of pulp was conducted on 1, 15, 30, 45, 60, 75, 90 days of storage intervals. Five gm of each sample was dissolved in 100 mL of 80:20 methanol/water solution followed by stirring for one hour. The solution was centrifuged at 800 g for 15 min. Supernatant was filtered through Whatman filter paper No. 4 and stored at −18°C for further analysis.
2.2.1. DPPH
DPPH was done as per the method of Giomaro et al. (Citation2014) with slight modifications. The DPPH assay was conducted as follows: a 100 μM methanolic solution of DPPH was prepared. Sample solutions of varying volumes were added to DPPH solution and the final volume was made to 3.5 mL using 80% methanol. Each solution was vortexed for 30 s and incubated for 30 min at room temperature (22°C). The decreasing absorbance at 517 nm was recorded and the percent decrease (corrected for the control, without addition of antioxidant agents) was taken as an index of the antioxidant capacity. Tests were carried out in triplicate. The IC50 values, defined as the amount of antioxidant necessary to decrease the initial DPPH (radical form) concentration by 50%, were calculated from the results.
2.2.2. TPC
Total phenolic content (TPC) of the samples was determined using the Folin–Ciocalteu (F–C) method as described by Carbone, Giannini, Picchi, Scalzo, and Cecchini (Citation2011) with slight modification. Deionized water (2.5 mL) and 500 μL of a known dilution of the extract were added to a test tube. F–C reagent (500 μL) was added to the solution and allowed to react for 3–5 min. Then, 2 mL of 10% sodium carbonate solution was aliquoted into the test tubes, and the mixture was diluted up to 20 mL with deionized water. The color developed for 90 min, and the absorbance was read at 760 nm against a blank (substituting the phenol solution in the reaction mix with water), using a UV–visible Spectrophotometer (Model U-2900 2JI-0003, Hitachi, Japan). Quercetin was used as standard to construct a calibration curve.
2.3. Sensory analysis
Various apple pulp samples were subjected to sensory analysis, i.e. color, odor, texture, and taste by using a 9-point hedonic scale. Before the sensory evaluation was conducted, the panelists were trained by using commercial apple pulp to get familiar with the use of rating method, terminology for each attribute, and sensory characteristics. The judges rated the quality characteristics of each sample on a nine-point hedonic rating scale, where 9 = like extremely, 8 = like very much, 7 = like moderately, 6 = like slightly, 5 = neither like nor dislike, 4 = dislike slightly, 3 = dislike moderately, 2 = dislike very much, 1 = dislike extremely. The judges evaluated randomly coded apple pulp samples in terms of color, odour, texture, and taste. Assessors were instructed to cleanse their palate with cold, filtered tap water before tasting each sample. Product characterization was carried out under daylight illumination and in isolated booths within a sensory laboratory.
2.4. Statistical design
Results were expressed as mean of triplicate analyses. A two-way analysis of variance and Duncan’s test were used to establish the significance of differences among the mean values at the 0.05 significance level. The statistical analyses were performed using SPSS software (Systat statistical program version 21 (SPSS Inc., USA).
3. Results and discussion
The samples were studied for Titrable acidity, TSS, pH, reducing sugars, non-reducing sugars, sensory analysis, color analysis, and anti-oxidant analysis.
3.1. Physicochemical properties
3.1.1. Titrable acidity/pH
Acidity and pH are interdependent, and lower the pH, higher is the acidity during the storage period at room temperature. Flavor promotion and preservation are mainly affected by pH and acidity of fruits, and every fruit has a specific range of pH and acidity over which consumers prefer it. A significant (p ≤ 0.05) increase in acidity and simultaneous decrease in pH were seen in untreated as well as treated samples over 90 days of storage (Table ). A similar trend of slight increase in titrable acidity with storage has been reported by Durrani, Ayub, Muhammad, and Ali (Citation2010) in apple pulp during storage up to 90 days at ambient temperature, Akhtar, Riaz, Ahmad, and Nisar (Citation2010) in mango pulp, Zewter, Woldetsadik, and Workneh (Citation2012) in banana fruit, and Sabina, Miyan, and Hoque (Citation2011) in strawberry juice. Acidity of fruits is attributed to the production of acids by degradation of polysaccharides, pectic substances, and uronic acid (Durrani et al., Citation2010; Yadav, Tripathi, & Jha, Citation2013). Oxidation of reducing sugars can also contribute to increase in the acidity of fruits (Hussain, Zeb, Shakir, & Sattar Shah, Citation2008). Among different treatments, highest value of titrable acidity was seen in sample T2 (0.26–0.41%), while lowest value of titrable acidity was seen in sample T3 (0.20–0.25%). A decrease in titrable acidity as a result of heat treatment was also seen by Li, Li, Wang, Jiang, and Ban (Citation2012) in two varieties of apple fruit. Highest value of titrable acidity in sample T2 (0.26–0.41%) may be due to the addition of citric acid. Although titrable acidity increased with storage, lesser increase was seen in heat-treated samples T3 (0.20–0.25%). This might be due to deactivation of metabolic enzymes involved in the degradation process of polysaccharides for production of acids (Kumhar, Pareek, & Ameta, Citation2014). The combined effect of treatment and storage significantly (p ≤ 0.05) affected pH of apple pulp (Table ). From Table , it is observed that initial pH of apple pulp with different treatments was in range of 3.79–4.41 that varied significantly with the treatments used and after 90 days of storage, the pH decreased significantly. Highest value of pH was seen in sample T3 (4.41), while lowest was seen in sample T2 (3.79) in an antagonistic manner to acidity. Similar decrease in pH with storage period were reported by Muhammad et al. (Citation2011) in apple pulp, Wisal, Ullah, Zeb, and Khan (Citation2013) in strawberry juice, and Durrani et al. (Citation2010) in apple pulp. Decrease in pH value and increase in total titrable acidity during the storage period of 90 days may also be due to activity of some acid-producing bacteria such as Alicyclobacillus acidoterrestris as suggested by Hussain, Zeb, & Ayub, Citation2011 in apple apricot juice blends.
Table 1. Effect of storage period and treatments on titrable acidity (%) and pH of apple pulp
3.1.2. Total soluble solids
Storage period and treatment had significant (p ≤ 0.05) effect on TSS of apple pulp (Table ). TSS represent the content of soluble sugars, organic acid, and other minor constituents. TSS increase with increase in storage in both treated as well as untreated sample. This increase in TSS with storage can be due to solubilization of fruit constituents during storage (Shah, Sufi, & Zafar, Citation1975). Also, an increase in TSS with storage might be due to hydrolysis of polysaccharides into monosaccharide and oligosaccharides (Rathod, Shakya, & Ade, Citation2014). Similar results were also reported by Kumhar et al. (Citation2014) in custard apple pulp, Sharma, Vaidya, and Rana (Citation2013) in kiwi apple juice, and Muhammad et al. (Citation2011) in apple pulp. Treatments however affected the increase in TSS during storage period of 90 days. TSS values in T4 increased to 14.5 (°Brix), which was highest as compared to other treatments. This might be due to the combined effect of heat treatment and addition of KMS and citric acid that increases the TSS content of apple pulp. Application of heat results in disruption of cell structure, solubilization of cell constituents, and evaporation of part of water. Kaushik, Kaur, Rao, and Mishra (Citation2014) reported that pasteurization results in an increase in TSS content of mango pulp. Abd-Elhady (Citation2014) reported that citric acid increases the TSS in strawberries. Increased TSS concentration with storage period (of 60 days) is in consent with the previous findings of Sirohi, Patel, Choudhary, and Sahu (Citation2005) who observed increased TSS in fruit during storage, and attributed it to the solubilization of the insoluble portion of product.
Table 2. Effect of storage period and treatments on TSS (°Brix) of apple pulp
3.1.3. Sugars
Reducing sugar is very important component for a processed product with respect to quality, shelf life, taste, and discoloration during storage. The storage period and treatments had significant (p ≤ 0.05) effect on the reducing sugar of the apple pulp (Table ). During the storage period, reducing sugar content gradually increased. These results were in agreement with the earlier reports for apple fruit (Ali et al., Citation2004), mango juice (Khan et al., Citation2012), and cherry tomatoes (Gharezi, Joshi, & Sadeghian, Citation2012). According to Hakim et al. (Citation2013) increase in reducing sugar with the progress of ripening as well as storage time was due to the degradation of starches to glucose and fructose by the activities of amylase and maltase. Ewaidah (Citation1992) also reported that reducing sugars increased due to the hydrolysis of sucrose present in fruit pulp. The minimum value for reducing sugar was observed in untreated sample. All the treatments showed an increase in reducing sugar content during storage. The treatments T3 (6.91–7.14%) and T4 (6.93–7.62%) resulted in an increase in reducing sugar values than T1 (6.83–6.94%) during storage (Table ). This might be due to the heat treatment given to these samples that lead to an increase in starch hydrolysis (Barwal & Shrera, Citation2009). However, T4 showed even higher values of reducing sugar than T3, which can be due to increased hydrolysis of cellular polysaccharides under acidic conditions due to addition of citric acid (Bal, Ahmad, Senapati, & Pandit, Citation2014).
Table 3. Effect of storage period and treatments on reducing sugar (%) and non-reducing sugars (%) of apple pulp
Results showed that non-reducing sugar content decreased in all samples significantly (p ≤ 0.05) during 90 days of storage (Table ). Similar results were reported by Ali et al. (Citation2004) in apple fruit, Khan et al. (Citation2012) in mango-sea buckthorn blended juice, Chowdhury et al. (Citation2013) in strawberry pulp during storage period of 120 days at ambient temperature. Hussain, Zeb, and Ayub (Citation2010) also found a decrease in non-reducing sugar content during storage at low temperature in apple and apricot-blended juice preserved at refrigeration temperature during storage. This decrease is due to the conversion of non-reducing sugar to reducing sugar through the process of glucogenesis. Different treatments showed a significant (p < 0.05) effect on non-reducing sugar content of apple pulp during 90 days of storage. Non-reducing sugar of pulp treated with T1 (2.11) varied non-significantly with T2 treated samples (2.10), while values of T3 and T4 decreased significantly with respect to T1 (Table ). Hameed (Citation1996) also reported that the levels of non-reducing sugar decreased and reducing sugar increased in all the preservation treatments.
3.1.4. Color
Change in color of apple pulp during storage is primarily attributed to enzymatic browning, in which polyphenols are converted into brown compounds by the action of polyphenol oxidase (Wickramarachchi & Ranamukhaarachchi, Citation2005). Storage period and treatment had a significant effect on total color change (ΔE), which indicates the magnitude of the color difference (Table ). During storage, TCD increased significantly in all treatments. The increase in ΔE values can be due to Maillard condensation, and destruction of pigments. Eissa, Hassanane, and Sharaf (Citation2014) have also reported a similar trend in commercial fruit drinks during storage up to 6 months at refrigeration temperature (Ibarz, Pagan, & Garza, Citation2000). Similar results were reported by Landl, Abadias, Sárraga, Viñas, and Picouet (Citation2010) in apple puree product and Kunitake, Ditchfield, Silva, and Petrus (Citation2014) in sugarcane beverage. Treatments also significantly affected the TCD of apple pulp. TCD decreased with treatment and followed the decreasing order T1 > T3 > T2 > T4. The ΔE values of sample treated with T3 and T4 were significantly lower than those of T₁ during the entire period of storage. This might be due to inactivation of polyphenol oxidase (Abd-Elhady, Citation2014). Since both heat and chemical preservatives lead to inactivation of polyphenol oxidase (Aguilar-Rosas, Ballinas-Casarrubias, Nevarez-Moorillon, Martin-Belloso, & Ortega-Rivas, Citation2007), hence maximum decrease in ΔE with treatments was seen in T4 where both heat as well as chemical preservatives was added.
Table 4. Effect of storage and pretreatment on ΔE of apple pulp
3.2. Antioxidant activity
3.2.1. TPC
TPC of pulp was significantly affected by storage as well as pretreatments (Table ). TPC of treated samples was higher in comparison to TPC of untreated samples on day 1 in an increasing order of (T2 > T4≈T3 > T1). KMS and citric acid prevent polyphenol degradation by inactivation of enzyme polyphenol oxidase (Saengnil, Lueangprasert, & Uthaibutra, Citation2006). On day 1, pretreatment with 0.1% KMS and 0.1% citric acid showed an increase in TPC of treated sample (T2) than untreated samples. Citric acid lowers the pH below 3 that renders polyphenol oxidase inactive (Altunkaya & Gokmen, Citation2011; He & Luo, Citation2007). It also acts as a chelating agent for the copper, which is a cofactor for browning reaction. Hence, binding of citric acid to copper prevents degradation of polyphenols in comparison to untreated samples. Pasteurization leads to an increase in TPC of treated sample (T3) than untreated samples on day 1. A possible explanation for this can be that heating leads to deactivation of polyphenol oxidase (Mizobutsi et al., Citation2010). However, increase in TPC due to treatment T3 (≈11%) is lesser as compared to T2 (34.01%) that can be because of degradation of polyphenols due to pasteurization. It has already been reported that polyphenols are stable only over a limited range of temperature. Similar results reported heat treatments decreases the concentration of polyphenols in apple juice (Aguilar-Rosas et al., Citation2007). There was a non-significant difference in the values of TPC between T3 and T4 treated samples, but was higher (≈11%) than untreated sample. Storage of pulp at ambient temperature resulted in decrease in value of total phenols in all the samples. Antioxidant activity of orange juices decreased by 45 percent after 6 months of storage at 28°C and the decrease in antioxidant activity may be linked to a decrease in TPC during storage (Klimczak, Małecka, Szlachta, & Gliszczyńska-Świgło, Citation2007). However, percentage decrease in TPC was more in untreated sample than rest of the samples during storage. This is because of greater activity of polyphenol oxidase in untreated samples that resulted in conversion of greater percentage of polyphenols to brown pigments, hence decreasing the content of phenols in apple pulp. Maximum antioxidant activity was retained in samples that were treated with KMS and citric acid only.
Table 5. Effect of storage and pretreatment on antioxidant properties of apple pulp
3.2.2. DPPH
DPPH is a free radical generator to which sample is added. Antioxidants present in the sample scavenge the free radicals generated by DPPH. Scavenging of free radicals is accompanied by change in color from blue to golden yellow that is detected spectrophotometrically. Samples were analyzed for their antioxidant activity using DPPH antioxidant assay (Table ) and then antioxidant capacity was compared using IC50 values. IC50 value refers to the quantity of the sample that is required for reducing the concentration of free radicals by 50% of initial concentration. Hence, lesser the value of IC50, greater is the antioxidant activity. Untreated samples (highest IC50) showed lowest antioxidant activity, while treated samples (lower IC50 values) showed higher antioxidant activity following an increasing order of T2 < T3 ≈T4. Although the TPC of chemically treated samples was higher than T3 and T4 treated samples, the IC50 value of T2 samples was higher suggesting lesser antioxidant activity. Increase in antioxidant activity of T3 and T4 can be attributed to generation of browning compounds (melanoidins) by maillard reaction due to heat treatment that shows potent antioxidant activity (Baba et al., Citation2014).
A high correlation coefficient was seen between TPC and DPPH in T2 (r 2 = 0.968**) and T4 (r 2 = 0.963**) treated samples. However, a lower correlation coefficient was seen in T1 and T3 samples. In untreated samples, TPC value decreased by ≈36% from day 1 to day 15. However, antioxidant activity (DPPH) showed a lesser decrease (≈23.5%) that is depicted by a lesser change in IC50 value from day 1 to day 15. This lesser change in IC50 value can be due to formation of brown-colored compounds that are reported to show antioxidant activity (Baba et al., Citation2014). A similar irregular trend can be seen in T3 treated samples. In T4, T3, T2 treated samples, a high correlation coefficient of 0.997**, 0.999**, 0.998** was seen between color change and TPC that further confirms that loss in antioxidant activity of fruit pulp is primarily due conversion of polyphenols to brown pigments.
3.3. Sensory analysis
The analysis of our data showed that storage period and treatment had a significant effect on sensory attributes (Table ). From Table , it is observed that the initial color score of apple pulp was in range of 4.02–8.33. The maximum color score of apple pulp was found in treatment T2 (8.33) followed by T4 (7.22) that were treated with chemical preservatives and pasteurization, respectively. The minimum color score was reported in T1 (4.02), which is untreated sample. The sample T1 showed mold growth at the end of 15 days due to the absence of chemical preservatives. The treatment T4 was the best in terms of microbial stability followed by T2 and is attributed to the combined effect of chemical and thermal treatments. The color score showed decreasing trend during storage that might be due to the action of acidity that enhances the hydrolytic reaction causes browning, and acid also enhances the Millard reaction which causes more browning in product. Polyphenolic compound present in fruit pulp gets oxidized under the influence of enzymes and causes discoloration. These findings were in accordance with the observation of Vidhya and Narain (Citation2011) for apple fruit, Iman, Akter, Mazumder, and Rana (Citation2011) for apple slice, Akhtar et al. (Citation2010) in mango pulp and Kumhar et al. (Citation2014) in custard apple. From Table , it is observed that the score for odor and taste decreased significantly during the storage period of 90 days. The initial taste and texture score of apple pulp, was found to be maximum in treatment T1 and T2. The sensory score for taste also decreased significantly during the storage period of 90 days. The minimum decrease was observed in sample T4, which decreased from 7.25 to 5.13. Maximum decrease was observed in sample T2, which decreased from 7.21 to 4.61. The decrease in taste score may be attributed to the development of bitterness and increase in acidity in the samples during storage (Yadav et al., Citation2013).
Table 6. Sensory analysis of apple pulp preserved by different techniques
4. Conclusion
From the present study it was concluded that different treatments had different effects on quality parameters and shelf life of apple pulp. Chemical preservatives and pasteurization method was most efficient in increasing the shelf life. Samples treated with both the treatments showed maximum antioxidant activity and could be consumed even after ninety days of storage under ambient conditions. Combination of both the treatments was also effective in preventing browning to a considerable extent, but chemical treatment was more effective in preventing browning.
Acknowledgements
Authors are thankful to the Department of Biotechnology, Govt. of India.
Additional information
Funding
Notes on contributors
Rafiya Nisar
Rafiya Nisar is a doctoral fellow at University of Kashmir in Institute of Home Science. Her research area includes development and quality evaluation of complementary foods, economical sources for production of weaning foods and nutrition and parasitic infestation in children.
Waqas N. Baba
Waqas N. Baba is working as a lab analyst in the Department of Food Science and Technology, University of Kashmir. Baba has several publications in product development and antioxidant analysis.
Farooq Ahmad Masoodi
Farooq Ahmad Masoodi is a professor in the department of Food Science and Technology, with a number of publications in fruit, vegetable and cereal processing.
References
- Abd-Elhady, M. (2014). Effect of citric acid, calcium lactate and low temperature prefreezing treatment on the quality of frozen strawberry. Annals of Agricultural Sciences , 59 , 69–75.10.1016/j.aoas.2014.06.010
- Aguilar-Rosas, S. F. , Ballinas-Casarrubias, M. L. , Nevarez-Moorillon, G. V. , Martin-Belloso, O. , & Ortega-Rivas, E. (2007). Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. Journal of Food Engineering , 83 , 41–46.10.1016/j.jfoodeng.2006.12.011
- Ahmad, S. , Thompson, A. K. , Hafiz, I. A. , & Asi, A. A. (2001). Effect of temperature on the ripening behaviour and quality of banana fruit. International Journal of Agriculture and Biology , 3 , 224–227.
- Akhtar, S. , Riaz, M. , Ahmad, A. , & Nisar, A. (2010). Physico-chemical, microbiological and sensory stability of chemically preserved mango pulp. Pakistan Journal of Botany , 42 , 853–862.
- Ali, M. S. , Raza, H. , Khan, M. A. , & Hussain, M. (2004). Effect of different periods of ambient storage on chemical composition of apple fruit. International Journal of Agriculture & Biology , 6 , 568–571.
- Altunkaya, A. , & Gokmen, V. (2011). Purification and characterization of polyphenol oxidase, peroxidase and lipoxygenase from freshly cut lettuce. Food Technology Biotechnology , 49 , 249–256.
- Association of Official Analytical Chemists . (2000). Official methods of analysis of the AOAC international (17th ed.). Washington, DC: Author.
- Baba, W. N. , Rashid, I. , Shah, A. , Ahamd, M. , Gani, A. , Masoodi, F. A. , … Wani, S. M. (2014). Effect of microwave roasting on antioxidant and aticancerous activities of barley flour. Journal of the Saudi Society of Agricultural Sciences . doi:10.1016/j.jssas.2014.06.003
- Bal, L. M. , Ahmad, T. , Senapati, A. K. , & Pandit, P. S. (2014). Evaluation of quality attributes during storage of guava nectar Cv. Lalit from different pulp and TSS ratio. Journal of Food Processing Technology , 5 , 1–5.
- Barwal, V. S. , & Shrera, S. K. (2009). Standardization of extraction methods and preservation techniques of hill lemon juice. Journal of Scientific and Industrial Research , 68 , 608–610.
- Boyer, J. , & Liu, R. H. (2004). Apple phytochemicals and their health benefits. Nutrition Journal , 3 , 1–15.
- Caivano, J. (2012). Whiteness, yellowness, and browning in food colorimetry. In J. L. Caivano & M. P. Buera (Eds.), Color in food (pp. 93–104). Boca Raton, FL: CRC Press.10.1201/b11878
- Carbone, K. , Giannini, B. , Picchi, V. , Scalzo, R. L. , & Cecchini, F. (2011). Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type and storage. Food Chemistry , 127 , 493–500.10.1016/j.foodchem.2011.01.030
- Chiply, J. R. (1983). Sodium benzoate and benzoic acid. In A. L. Baren & P. M. Davidson (Eds.), Antimicrobials in foods (pp. 11–35). New York, NY: M.Decker.
- Chowdhury, A. N. , Nargis, A. , Rahman, M. Z. , Alam, A. K. M. S. , Ibrahim, M. , & Akhter, S. (2013). Freezing adaptability and chemical composition of Strawberry (Fragaria X ananassa Duch.) in Bangladesh. IOSR Journal of Environmental Science, Toxicology and Food Technology , 7 , 50–54.10.9790/2402
- Conceição de Oliveira, M. , Sichieri, R. , & Sanchez Moura, A. (2003). Weight loss associated with a daily intake of three apples or three pears among overweight women. Nutrition , 19 , 253–256.10.1016/S0899-9007(02)00850-X
- Considine, M. (1982). Food and food production. Encyclopedia. Van-Nostrand , 24 , 203–205.
- Durrani, Y. , Ayub, M. , Muhammad, A. , & Ali, A. (2010). Physicochemical response of apple pulp to chemical preservatives and antioxidant during storage. International Journal of Food Safety , 12 , 20–28.
- Eissa, H. A. , Hassanane, M. M. , & Sharaf, H. A. (2014). Effect of colorant on cytogenetic, biochemical and histochemical parameters and quality changes during storage of some commercial fruit drinks. Journal Nutrition and Food Science , 4 , 1–11.
- Ewaidah, E. H. (1992). Studies on commercially canned juices produced locally in Saudi Arabia: Part 3—Physicochemical, organoleptic and microbiological assessment. Food Chemistry , 44 , 103–111.10.1016/0308-8146(92)90320-2
- Gharezi, M. , Joshi, N. , & Sadeghian, E. (2012). Effect of post-harvest treatment on stored cherry tomatoes. Journal of Nutrition and Food Science , 2 , 157–167.
- Giomaro, G. , Karioti, A. , Bilia, A. R. , Bucchini, A. , Giamperi, L. , Ricci, D. , & Fraternale, D. (2014). Polyphenols profile and antioxidant activity of skin and pulp of a rare apple from Marche region (Italy). Chemistry Central Journal , 8 , 1–10.10.1186/1752-153X-8-45
- Gould, G. W. (2000). Preservation: Past, present, future. British Medical Bulletin , 56 , 84–96.10.1258/0007142001902996
- Hakim, K. A. , Sarkar, M. A. R. , Khan, M. Z. H. , Rahman, S. M. , Ibrahim, M. , & Islam, M. K. (2013). Effect of post-harvest treatments on physiochemical characters during storage of two bananas (Musa spp.L.) cv. Sabri and Amritsagar. International Journal of Biosciences , 3 , 168–179.
- Hameed (1996). Storage stability of carrot pulp at various condition and treatment. Indian Food Packer , 45 , 48–53.
- Harris, L. J. , Yada, S. , & Mitcham, E. (2007). Apples: Safe methods to store, preserve, and enjoy . Oakland, CA: Division of Agriculture and Natural Resources. University of California.
- He, Q. , & Luo, Y. (2007). Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Review , 3 (6), 1–7.10.2212/spr.2007.6.3
- Hussain, I. , Zeb, A. , & Ayub, M. (2010). Quality attributes of apple and apricot blend juice preserved with potassium sorbate during storage at low temperature. Internet Journal of Food Safety , 12 , 80–86.
- Hussain, I. , Zeb, A. , & Ayub, M. (2011). Evaluation of apple and apricot blend juice preserved with sodium benzoate at refrigeration temperature. World Journal of Agricultural Sciences , 7 , 136–142.
- Hussain, I. , Zeb, A. , Shakir, I. , & Sattar Shah, A. (2008). Combine effect of potassium sorbate and sodium benzoate on individual and blended juices of apricot and apple fruits grown in Azad Jammu and Kashmir. Pakistan Journal of Nutrition , 7 , 181–185.10.3923/pjn.2008.181.185
- Hussain, T. (2001). Food composition table for Pakistan . Islamabad: Govt. of Pakistan, Ministry of P & D.
- Ibarz, A. , Pagan, J. , & Garza, S. (2000). Kinetic models of non-enzymatic browning in apple puree. Journal of the Science of Food and Agriculture , 80 , 1162–1168.10.1002/(ISSN)1097-0010
- Iman, M. Z. , Akter, S. , Mazumder, M. E. H. , & Rana, M. S. (2011). Antimicrobial and cytotoxic properties of different extracts of Musa sapientum L. subsp. sylvestris. International Research Journal of Pharmacy , 2 , 62–65.
- Kähkönen, M. P. , Hopia, A. I. , Vuorela, H. J. , Rauha, J. P. , Pihlaja, K. , Kujala, T. S. , & Heinonen, M. (1999). Antioxidant activity of plant extracts containing phenolic compounds. Journal of Agricultural and Food Chemistry , 47 , 3954–3962.10.1021/jf990146l
- Kaushik, N. , Kaur, B. P. , Rao, P. S. , & Mishra, H. N. (2014). Effect of high pressure processing on color, biochemical and microbiological characteristics of mango pulp (Mangifera indica cv. Amrapali). Innovative Food Science and Emerging Technologies , 22 , 40–50.10.1016/j.ifset.2013.12.011
- Khan, R. U. , Afridi, S. R. , Ilyas, M. , Abid, H. , Sohail, M. , & Khan, S. A. (2012). Effect of different chemical preservatives on the storage stability of mango-sea buckthorn blended juice. Pakistan Journal of Biochemistry and Molecular Biology , 45 , 6–10.
- Klimczak, I. , Małecka, M. , Szlachta, M. , & Gliszczyńska-Świgło, A. (2007). Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. Journal of Food Composition and Analysis , 20 , 313–322.10.1016/j.jfca.2006.02.012
- Knekt, P. , Jarvinen, R. , Hakkinen, R. , Reunanen, A. , & Maatela, J. (1996). Flavonoid intake and coronary mortality in Finland: A cohort study. BMJ , 312 , 478–481.10.1136/bmj.312.7029.478
- Knekt, P. , Kumpulainen, J. , Jarvinen, R. , Rissanen, H. , Heliovaara, M. , Reunanen, A. , … Aromaa, A. (2002). Flavonoid intake and risk of chronic diseases. American Journal of Clinical Nutrition , 76 , 560–568.
- Kumhar, D. S. , Pareek, S. , & Ameta, K. D. (2014). Effect of antioxidants and storage temperatures on browning and quality of custard apple (Annona squamosal L.) pulp. Journal of Scientific and Industrial Research , 73 , 622–626.
- Kunitake, M. , Ditchfield, C. , Silva, C. , & Petrus, R. (2014). Effect of pasteurization temperature on stability of an acidified sugarcane juice beverage. Ciência e Agrotecnologia , 38 , 554–561.10.1590/S1413-70542014000600004
- Landl, A. , Abadias, M. , Sárraga, C. , Viñas, I. , & Picouet, P. A. (2010). Effect of high pressure processing on the quality of acidified Granny Smith apple purée product. Innovative Food Science and Emerging Technologies , 11 , 557–564.10.1016/j.ifset.2010.09.001
- Leontowicz, H. , Gorinstein, S. , Lojek, A. , Leontowicz, M. , Čı́ž, M. , Soliva-Fortuny, R. , … Martin-Belloso, O. (2002). Comparative content of some bioactive compounds in apples, peaches, and pears and their influence on lipids and antioxidant capacity in rats. The Journal of Nutritional Biochemistry , 13 , 603–610.10.1016/S0955-2863(02)00206-1
- Li, L. , Li., X , Wang, A. , Jiang, Y. , & Ban, Z. , (2012). Effect of heat treatment on physiochemical, color, antioxidant and microstructural characteristics of apples during storage. International Journal of Food Science and Technology , 48 , 727–734.
- Lu, Y. , & Yeap Foo, L. (2000). Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chemistry , 68 , 81–85.10.1016/S0308-8146(99)00167-3
- Lueck, F. (1990). Food applications of sorbic acid and its salts. Food Additives & Contaminants , 7 , 711–715.
- Manganelli, E. , & Casolari, A. (1983). Sensitivity of yeasts to sorbic and benzoic acids and their salts. Indian Conservation , 58 , 23–25.
- Mizobutsi, G. P. , Finger, F. L. , Ribeiro, R. A. , Puschmann, R. , Neves, L. L. M. , & Mota, W. F. (2010). Effect of pH and temperature on peroxidase and polyphenoloxidase activities of litchi pericarp. Scientia Agricola , 67 , 213–217.10.1590/S0103-90162010000200013
- Mueller, D. B. , Koczwara, K. , Mueller, A. S. , Pallauf, J. , Ziegler, A. G. , & Bonifacio, E. (2009). Influence of early nutritional components on the development of murine autoimmune diabetes. Annals of Nutrition and Metabolism , 54 , 208–217.10.1159/000220416
- Muhammad, A. , Ayub, M. , Zeb, A. , Durrani, Y. , Ullah, J. , & Afridi, S. U. R. (2011). Physicochemical analysis of apple pulp from Mashaday variety during storage. Agriculture and Biology Journal of North America , 2 , 192–196.10.5251/abjna.2011.2.2.192.196
- Ranganna, S. (1991). Handbook of analysis and quality control for fruit and vegetable products (pp. 186–189). New Delhi: Tata McGraw-Hill.
- Rathod, A. S. , Shakya, B. A. , & Ade, K. D. (2014). Studies on effect of thermal processing on preparation of bael fruit rts blended with aonla. International Journal of Research in Engineering & Advanced Technology , 2 , 1–6.
- Sabina, R. , Miyan, S. H. , & Hoque, M. M. (2011). Studies on the effect of chemical preservatives on the quality of strawberry (Fragaria ananassa) juice in Bangladesh. International Journal of Natural sciences , 1 , 97–101.
- Saengnil, K. , Lueangprasert, K. , & Uthaibutra, J. (2006). Control of enzymatic browning of harvested ‘hong huay’ litchi fruit with hot water and oxalic acid dips. ScienceAsia , 32 , 345–350.10.2306/scienceasia1513-1874.2006.32.345
- Sesso, H. , Gaziano, J. M. , Liu, S. , & Buring, J. (2003). Flavonoid intake and risk of cardiovascular disease in women. American Journal of Clinical Nutrition , 77 , 1400–1408.
- Shah, W. H. , Sufi, N. A. , & Zafar, S. I. (1975). Studies on the storage stability of guava fruit juice. Pakistan Journal of Science Industrial Research , 18 , 179–183.
- Sharma, S. , Vaidya, D. , & Rana, N. (2013). Development and quality evaluation of kiwi-apple juice concentrate. Indian Journal of Applied Research , 3 , 229–231.
- Sirohi, D. , Patel, S. , Choudhary, P. L. , & Sahu, C. (2005). Studies on preparation and storage of whey-based mango herbal Pudina (Mentha arvensis) beverage. Journal of Food Science and Technology , 42 , 157–161.
- Sofos, J. N. , & Busta, F. F. (1981). Antimicrobial activity of sorbate. Journal of Food Protection , 44 , 614–622.
- Sun, J. , Chu, Y. , Wu, X. , & Liu, R. H. (2002). Antioxidant and antiproliferative activities of common fruits. Journal of Agricultural and Food Chemistry , 50 , 7449–7454.10.1021/jf0207530
- Vidhya, R. , & Narain, A. (2011). Formulation and evaluation of preserved products utilizing under exploited fruit, wood apple (Limonia acidissima). American- Eurasian Journal of Agriculture & Environmental Science , 10 , 112–118.
- Vieira, F. G. K. , Borges, G. D. C. , Copetti, C. , Amboni, R. D. M. C. , Denardi, F. , & Fett, R. (2009). Physico-chemical and antioxidant properties of six apple cultivars (Malus domestica Borkh) grown in southern Brazil. Scientia Horticulturae , 122 , 421–425.10.1016/j.scienta.2009.06.012
- Wickramarachchi, K. S. , & Ranamukhaarachchi, S. L. (2005). Preservation of fiber-rich banana blossom as a dehydrated vegetable. ScienceAsia , 31 , 265–271.10.2306/scienceasia1513-1874.2005.31.265
- Wisal, S. , Ullah, J. , Zeb, A. , & Khan, M. Z. (2013). Effect of refrigeration temperature, sugar concentrations and different chemicals preservatives on the storage stability of strawberry juice. International Journal of Engineering and Technology , 13 , 160–168.
- Yadav, R. , Tripathi, A. D. , & Jha, A. (2013). Effect of storage time on the physicochemical properties and sensory attributes of Aloe vera ready-to-serve (RTS) beverage. International Journal of Food, Nutrition and Public Health , 6 , 173–192.
- Zewter, A. , Woldetsadik, K. , & Workneh, T. S. (2012). Effect of 1-methylcyclopropene, potassium permanganate and packaging on quality of banana. African Journal of Agricultural Research , 7 , 2425–2437.