Abstract
This work has compared the effects of the biofertilizer Bacillus amyloliquefaciens FZB42 with that of compost for cotton production. The population dynamics of pests and predators have been studied in order to check whether the use of both fertilization materials can contribute to pest management in cotton. Three treatments were considered: (i) dressing of seeds in rhizobacteria suspension, (ii) introduction of rhizobacterial suspension directly in the pocket, same time with the seeds, and (iii) fertilization with compost. The study was carried out in northwest Benin (West Africa). Results showed that cotton aphids, Aphis gossypii, pink bollworm, Pectinophora gossypiella, leaf roller, Sylepta derogata, and cotton bugs, Dysdercus sp. are the major insect pests encountered in the experimental plots. Cotton bollworm, Helicoverpa armigera, was present but under the economic threshold. The coccinellid predators, Cheilomenes spp., occurred in the experimental plots and almost suppressed aphid proliferation. Other natural enemies such as chrysopids and ant species also occurred and probably contributed to maintain the cotton bollworm under the economic threshold. The treatment with seeds dressed with the rhizobacteria suspension yielded 39% more cotton compared to the compost fertilization. The use of both fertilization materials without application of chemicals can contribute to pest management in cotton.
Public Interest Statement
Intensive use of mineral fertilizers provokes soil erosion, water pollution as well as plant susceptibility to insect pests. Cotton production loss attributable to pests in West Africa averages about 40% of the potential yield and may reach 70% in some areas. It is therefore essential to look for an improved cotton production system that favors the activity of beneficial insects and limits the proliferation of insect pests. The present article has evaluated whether the use of compost combined with the plant growth-promoting rhizobacterium (PGPR) Bacillus amyloliquefaciens FZB42 can improve the cotton yield and simultaneously contribute to insect pest management in cotton.
Competing interests
The authors declare no competing interest.
1. Introduction
In West Africa, plants are cultivated on tropical soils that are very poor in nutritious mineral elements such as nitrogen, potassium, and phosphorus. Growers should use up to 250-kg/hectare mineral fertilizers every year. Despite intensive mineral fertilization, crops’ yield remains very low (Bationo et al., Citation2006). Moreover, this intensive use of mineral fertilizers provokes soil erosion, water pollution as well as plants’ susceptibility to insect pests (Maltais, Citation1951; Strong, Lawton, & Southwood, Citation1984). Cotton production loss attributable to pests in West Africa averages about 40% of the potential yield and may reach 70% in some areas. Four groups of pests are found on cotton in West Africa: bollworms, leaf-eating caterpillars, mites, and sucking insects (Silvie, Deguine, Nibouche, Michel, & Vaissayre, Citation2001; Vaissayre & Cauquil, Citation2000; Vaissayre & Deguine, Citation1996).
Management guidelines for the cotton pest complex in West Africa have been designed to provide growers with simple and inexpensive calendar-based intensive chemical spraying recommendations. This intensive application of chemical insecticides has led to the development of resistance to the major chemical families of insecticides (Martin, Ochou, Hala-N’Klo, Vassal, & Vaissayre, Citation2000). It is therefore essential to look for an improved cotton production system that favors the activity of natural enemies and limits the proliferation of insect pest populations.
Bacteria are important components of biological plant protection. Intensive research resulted in the formulation of effective strains such as Bacillus subtilis FZB 24 and Bacillus amyloliquefaciens FZB 42 (Bochow, El-Sayed, Junge, Stavropoulou, & Schmiedeknecht, Citation2001; Borriss, Citation2011; Grosch, Junge, Krebs, & Bochow, Citation1999; Krebs et al., Citation1998; Schmiedeknecht, Bochow, & Junge, Citation1998; Schmiedeknecht, Issoufou, Junge, & Bochow, Citation2001), which are registered and marketed for seed dressing in potatoes, corn, and vegetables. The liquid formulation of FZB 42 was identified as the most effective plant growth-promoting rhizobacterium (PGPR) (Idris, Bochow, Ross, & Borriss, Citation2004).
The influence of the PGPR FZB42 seed dressing combined with a low and full mineral nitrogen plant fertilization on the cotton growth and yield was tested in field experiments. Results showed that the cotton yield in the combined application of PGPR and low nitrogen amount increased by 75% (Monir, Bochow, & Junge, Citation2012).
The present investigation has compared the effects of PGPR B. amyloliquefaciens FZB42 with that of compost for organic cotton production. The population dynamics of insect pests and predators have been studied to determine whether the use of both fertilization materials can contribute to pest management in cotton.
2. Materials and methods
2.1. Application of the PGPR B. amyloliquefaciens FZB42 and compost
The experiment was carried out in the district of Tanguiéta, near National Pendjari Parc, northwest Benin, where only organic cotton is produced.
Cotton seed variety H279-1 was used. Three treatments were tested: T1—seed dressing with rhizobacteria suspension (without compost), T2—pouring rhizobacteria suspension directly into the pocket, simultaneously with the seeds (without compost), and T3—fertilization with compost alone (according to the current recommendation of organic cotton fertilization). Each treatment was replicated five times on plots measuring 115 m2.
The liquid formulation of PGPR FZB42 (RhizoVital®, ABiTEP GmbH, Germany) was used for cotton seed dressing as follows: 20-ml product (bacterial concentration of about 1010 cells per ml) was diluted with 10-L water to get a bacterial suspension with 2 × 107 cells/ml in which 2-kg cotton seeds were dipped and stirred for 15 min. Thereafter, treated seeds were removed, spread in a thin layer on paper, air dried, and sown (Treatment T1). For Treatment T2, 1.5-ml bacterial suspension (2 × 107 cells/ml) was poured immediately into the pocket where non-treated cotton seeds were sown. Regarding treatment T3, compost was applied, twice during the experiment (on day 30 and 76 after sowing), respectively, 0.4 and 0.2-kg compost was applied per m² (corresponding to four and two tons of compost per hectare, respectively). The compost has been applied according to the organic cotton production guidelines for West Africa (Ouédraogo, Yombi, Doumbia, Eyhorn, & Dischl, Citation2008).
2.2. Assessment of insect population dynamics and pest damages to cotton bolls in tested treatments
The diversity and populations dynamics of all cotton insect pests and predators were studied. We counted the number of insects on 10 plants chosen in the 2 diagonals of each plot, every 3 days. The observations started on day 30 after sowing and continued until the end of the season.
Damage of cotton bollworms and other pests was assessed by counting injured as well as safe bolls on 20 plants chosen in the 2 diagonals of each plot, every week.
2.3. Impact of the fertilization materials on cotton yield
To assess the impact of tested treatments on the cotton yield, we harvested cotton on two lines located in the center of each experimental plot and the yield per hectare was calculated.
2.4. Statistical analyses
We performed analysis of variance (one-way ANOVA) for data that met the ANOVA hypotheses and non-parametric tests (Mann–Whitney test) for the remaining data. SPSS, Statistics Package version 16.0, was used for these analyses.
3. Results
Cotton aphid, Aphis gossypii (Aphididae), pink bollworm, Pectinophora gossypiella (Gelechiidae), leaf roller, Sylepta derogata (Pyralidae), and cotton bug, Dysdercus sp. (Pyrrhocoridae) are the major insect pests encountered in the experimental plots.
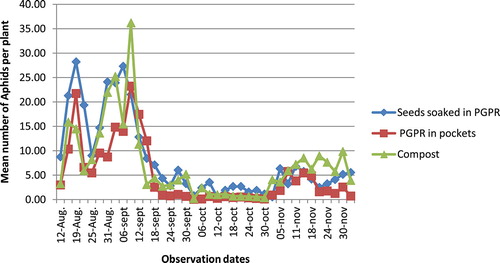
At beginning of the season, from 15 August to 12 September, aphid populations were abundant in all experimental plots. After this time period, aphid population density significantly decreased and was about only one individual per plant by 2 November. A slight increase of population density was, however, noticed in November, but this increase did not reach the initial density level (Figure ). There was no significant difference between the tested treatments (p > 0.05).
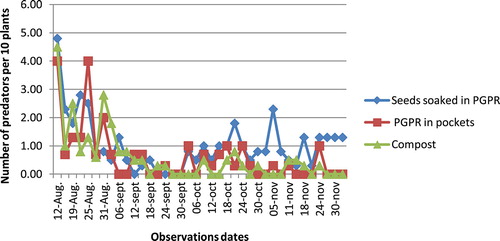
The aphid predator, Cheilomenes spp., has been encountered in all experimental plots; their population density was also abundant at the beginning of the season. At this time, we counted about 4 individuals of coccinellid predators for 10 plants in all experimental plots. These beneficial organisms maintained themselves in all plots until the end of the season, but their density was less abundant compared to the beginning of the season (Figure ). There was no significant difference between the tested treatments (p > 0.05).
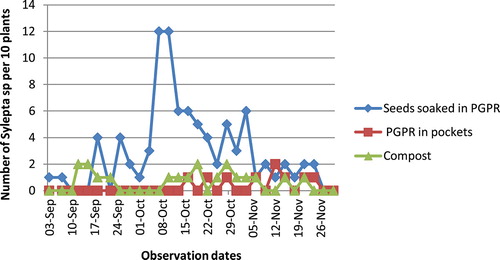
Cotton leaf roller, S. derogata, appeared in all experimental plots, but this pest was more abundant in plots that received seeds soaked in PGPR, from 24 September to 29 October. In this plot, the number of leaf roller reached 12 for 10 plants against only 2 individuals for both “compost” and “PGPR in pockets” treatments (Figure ).
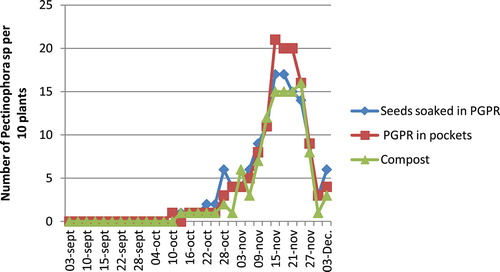
Pink bollworm, P. gossypiella, appeared in the plots by 16 October; their population density slowly increased and reached, respectively, 17, 21, and 15 individuals for 10 plants, in “seeds dressed in PGPR,” “PGPR in pockets,” and “compost” treatments. Its density decreased, however, starting from 24 November (Figure ). There was no significant difference between the tested treatments (p > 0.05).
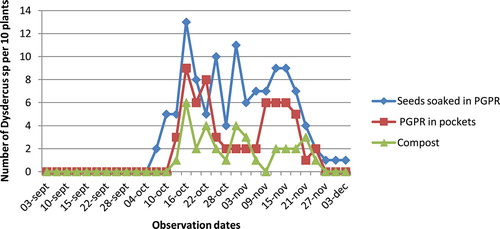
Regarding the cotton bug, Dysdercus völkeri, it appeared in the experimental plots by 13 October and was abundant until 15 November, after which its density decreased (Figure ). There was no significant difference between the tested treatments (p > 0.05).
3.1. Damage to bolls and cotton yield in the tested treatments
Percent of cotton bolls injured by different insect pests was, respectively, 18, 15, and 25%, for “seeds dressed in PGPR,” “PGPR in pockets,” and “compost” treatments. Regarding the cotton yield, we harvested, respectively, 652.77, 333.33, and 469.44 kg/ha on plots receiving “seeds dressed in PGPR,” “PGPR in pockets,” and “compost” treatments. Statistical analysis indicated significant difference between the tested treatments (p < 0.05).
4. Discussion
The cotton aphid, A. gossypii, had infested plants at the beginning of the season, from 15 August to 12 September; aphid populations were abundant in all experimental plots. After this time period, aphid population density significantly decreased and was about only one individual per plant by 2 November. The decrease in aphid populations coincided with the occurrence of the coccinellid predator Cheilomenes sulfurea, in the experimental plots. We think that this spectacular decrease in the aphid population density is attributable to the occurrence of the coccinellid species. In fact, insects belonging to Cheilomenes spp. are known as major predators of A. gossypii. Laboratory studies conducted to find out the consumption rate and biology of coccinellid predators Cheilomenes spp. revealed that their per day predation rate (number of aphids) ranged from 35 to 57 (Pandi, Bishwajeet, Shah, & Shankarganesh, Citation2012; Prabhakar & Roy, Citation2010). Numerous studies have also demonstrated the predation ability of Cheilomenes spp. against aphids (Hodek, Chakrabarti, & Rejmanek, Citation1984; Ofuya, Citation1986, Citation1995; Omkar & Bind, Citation2004). We therefore conclude that the coccinellid predators significantly contributed to the protection of cotton plants against aphids since it was not necessary to apply any other control measure. Other factors may explain the limited aphid proliferation in our experimental plots. In general, nitrogen concentration in plant tissues increases with mineral N-fertilization (Carrow & Betts, Citation1973). Nitrogen is the principal component of plant tissues which is vital for phytophagous insects such as aphids (Kytö, Niemelä, & Larsson, Citation1996); their development and proliferation is strictly correlated to nitrogen concentration in plant tissues (Mattson, Citation1980). Therefore, the fact that no mineral fertilizer has been used in the present study may also contribute to the reduced abundance of aphids in the experimental plots.
The cotton bollworm, Helicoverpa armigera, (Lepidoptera: Noctuidae), the cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae), the leaf roller S. derogata (Lepidoptera: Pyralidae), and the cotton spiny bollworm Earias spp. (Lepidoptera: Noctuidae) are major lepidopteran pests in Northern Benin (Alavo, Yarou, & Atachi, Citation2010). All these insect pests have been encountered in our experimental plots; however, only a limited number of S. littoralis, Earias spp. and H. armigera occurred. This may be explained by the presence of natural enemies of these pests in the experimental plots. For instance, Chrysopids and ant species, which are known as the most important predators of H. armigera (Alavo, Citation2006; Mansfield, Elias, & Lytton-Hitchins, Citation2003; Romeis & Shanower, Citation1996; van den Berg, Cock, & Oduor, Citation1997; van den Berg & Cock, Citation1993, Citation1995), were occasionally encountered in the plots. We considered that the presence of these beneficial organisms in the plots was due to the fact that these plots have not been treated with any chemicals. We think that the beneficial organisms maintained the population density of H. armigera (the most damaging cotton pest in the region) under the economic threshold (one individual for 40 plants).
Other lepidopteran pests and cotton bugs (whose population density was much more important) have certainly induced important crop damage. S. derogata rolls the leaves into a cone in which it stays until pupation; the cotton strainers Dysdercus spp. attacked green bolls. Nevertheless, the most important damages caused to the bolls came from the pink bollworm, P. gossypiella, which was particularly abundant in our experimental plots during the period of bolls formation. Percent of injured bolls was, respectively, 18, 25, and 15%, for plots receiving seeds dressed in PGPR, PGPR in pockets, and compost. The substantial damage to the bolls is certainly the reason why we obtained, in general, a weak cotton yield in our plots in comparison to the yield obtained usually when insecticides are applied against exocarpic and endocarpic bollworms.
On the other hand, plots receiving seeds dressed with the PGPR yielded 39% more cotton yield in comparison to the compost treatment. This yield increase may be attributed to the beneficial properties of the PGPR which is known to promote plant and root growth. The stronger root system induced by the bacterial-born auxin in bacterized plants leads to an improved uptake of water and nutrients, and hence to faster plant growth and higher stress tolerance against pathogen attacks and dryness. The PGPR Bacillus spp. produces phytase that mobilizes phytate, the organic-bound phosphorus for the plant. Because plants do not form this enzyme, only bacterized plants profit from increased phosphorus nutrition (Bochow et al., Citation2001; Idris et al., Citation2002, Citation2004; Kilian et al., Citation2000; Kloepper, Citation2004; Koumoutsi et al., Citation2004). In field experiments, the influence of the PGPR FZB42 (B. amyloliquefaciens) seed dressing combined with different levels of mineral nitrogen plant fertilization on the cotton growth and yield was tested in Egypt. Results showed that the PGPR promoted the plant growth and the cotton yield (in the combined application of PGPR and low nitrogen amount) increased by 75% (Monir et al., Citation2012). These data confirm that the yield increase obtained in our experimental plot is really the effect of seed dressing with the PGPR B. amyloliquefaciens FZB42.
Acknowledgments
The authors thank Mr. Barnabé N’DA and technicians of U-AVIGREV-BENIN for their assistance. Professor E. G. Platzer (University of California, Riverside, USA) critically reviewed the manuscript.
Additional information
Funding
Notes on contributors
Thiery B. Charles Alavo
Thiery B. Charles Alavo is an associate professor, Laboratory of Applied Entomology, University of Abomey-Calavi, Benin. He holds a PhD in Agricultural Sciences from Humboldt-University, Berlin (Germany). His research interest emphasizes the use of non-chemical methods for crop protection as well as for insect pests and vectors control.
Helmut Bochow
Helmut Bochow is an Emeritus Professor, Institute of Agriculture and Horticulture Science, Humboldt-University, Berlin, Germany. He has more than 50 years experience as a university professor. His research focuses on the use of plant growth-promoting rhizobacteria (PGPR) for plant fertilization as well as for the biological control of plant diseases.
References
- Alavo, T. B. C. (2006). Biological control agents and environmentally-friendly compounds for the integrated management of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) on cotton: Perspectives for pyrethroid resistance management in West Africa? Archives of Phytopathology and Plant Protection , 39 , 105–111.10.1080/03235400500181576
- Alavo, T. B. C. , Yarou, B. B. , & Atachi, P. (2010). Field effects of kaolin particle film formulation against major cotton lepidopteran pests in North Benin, West Africa. International Journal of Pest Management , 56 , 287–290.10.1080/09670871003628389
- Bationo, A. , Hartemink, A. , Lungu, O. , Naimi, M. , Okoth, P. , Smaling, E. , & Thiombiano, L. (2006, June 9–13). African soils: Their productivity and profitability of fertilizer use . Background Paper Prepared for the African Fertilizer Summit , Abuja.
- Bochow, H. , El-Sayed, S. F. , Junge, H. , Stavropoulou, A. , & Schmiedeknecht, G. (2001). Use of Bacillus subtilis as biocontrol agent. IV. Salt-stress tolerance induction by Bacillus subtilis FZB24 seed treatment in tropical vegetable field crops, and its mode of action. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz , 108 , 21–30.
- Borriss, R. (2011). Use of plant-associated Bacillus strains as biofertilizers and biocontrol agents in agriculture. Detailed summary of international publications. In D. K. Maheshwari (Ed.), Bacteria in agrobiology: Plant growth responses (pp. 41–76). Berlin-Heidelberg: Springer Verlag.10.1007/978-3-642-20332-9
- Carrow, J. R. , & Betts, R. E. (1973). Effects of different foliar-applied nitrogen fertilizers on balsam woolly aphid. Canadian Journal of Forest Research , 3 , 122–139.10.1139/x73-017
- Grosch, R. , Junge, H. , Krebs, B. , & Bochow, H. (1999). Use of Bacillus subtilis as biocontrol agent. III. Influence of Bacillus subtilis on fungal root diseases and on yield in soilless culture. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 106 , 568–580.
- Hodek, I. , Chakrabarti, S. , & Rejmanek, M. (1984). The effect of prey density on food intake by adult Cheilomenes sulphurea [Col.: Coccinellidae]. Entomophaga , 29 , 179–184.10.1007/BF02372107
- Idris, E. E. , Bochow, H. , Ross, H. , & Borriss, R. (2004). Use of Bacillus subtilis as biocontrol agent. VI. Phytohormone-like action of culture filtrates, prepared from plant growth-promoting Bacillus amyloliquefaciens FZB24, FZB42, FZB45 and Bacillus subtilis FZB37. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz , 111 , 583–597.
- Idris, E. E. , Makarewicz, O. , Farouk, A. , Rosner, K. , Greiner, R. , Bochow, H. , … Borriss, R. (2002). Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology , 148 , 2097–2109.
- Kilian, M. , Steiner, U. , Krebs, B. , Junge, H. , Schmiedeknecht, G. , & Hain, R. (2000). FZB24® Bacillus subtilis—Mode of action of a microbial agent enhancing plant vitality. Pflanzenschutz-Nachrichten Bayer , 1 , 72–93.
- Kloepper, J. W. (2004, September 12–17). Progress towards implementation of Bacillus spp. for plant growth promotion and biological control. In Rhizosphere Congress . Munich.
- Koumoutsi, A. , Chen, X.-H. , Henne, A. , Liesegang, H. , Hitzeroth, G. , Franke, P. , … Borriss, R. (2004). Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. Journal of Bacteriology , 186 , 1084–1096.10.1128/JB.186.4.1084-1096.2004
- Krebs, B. , Höding, B. , Kübart, S. , Alemayehu, M. , Junge, H. , Schmiedknecht, G. , … Hevesi, M. (1998). Use of Bacillus subtilis as biocontrol agent. I. Activities and characterization of Bacillus subtilis strains. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz , 105 , 181–197.
- Kytö, M. , Niemelä, P. , & Larsson, S. (1996). Insects on trees: Population and individual response to fertilization. Oikos , 75 , 148–159.10.2307/3546238
- Maltais, J. B. (1951). The nitrogen content of different varieties of peas as a factor affecting infestations by Macrosiphum pisi (Kltb.) (Homoptera: Aphididae). A preliminary report. The Canadian Entomologist , 83 , 29–33.10.4039/Ent8329-2
- Mansfield, S. , Elias, N. V. , & Lytton-Hitchins, J. A. (2003). Ants as egg predators of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) in Australian cotton crops. Australian Journal of Entomology , 42 , 349–351.10.1046/j.1440-6055.2003.00367.x
- Martin, T. , Ochou, G. O. , Hala-N’Klo, F. , Vassal, J. M. , & Vaissayre, M. (2000). Pyrethroid resistance in the cotton bollworm, Helicoverpa armigera (Hubner), in West Africa. Pest Management Science , 56 , 549–554.10.1002/(ISSN)1526-4998
- Mattson, W. J. (1980). Herbivory in relation to plant nitrogen content. Annual Review of Ecology and Systematics , 11 , 119–161.10.1146/annurev.es.11.110180.001003
- Monir, M. E. H. , Bochow, H. , & Junge, H. (2012). The biofertilising effect of seed dressing with PGPR Bacillus amyloliquefaciens FZB 42 combined with two levels of mineral fertilising in African cotton production. Archives of Phytopathology and Plant Protection . doi:10.1080/03235408.2012.673259
- Ofuya, T. I. (1986). Predation by Cheilomenes vicina [Coleoptera: Coccinellidae] on the cowpea aphid, Aphis craccivora [Homoptera: Aphididae]: Effect of prey stage and density. BioControl , 31 , 331–335.
- Ofuya, T. I. (1995). Studies on the capability of Cheilomenes lunata (Fabricius) (Coleoptera: Coccinellidae) to prey on the cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae) in Nigeria. Agriculture, Ecosystems & Environment , 52 , 35–38.
- Omkar, S. S. K. , & Bind, R. B. (2004). Prey quality dependent growth, development and reproduction of a biocontrol agent, Cheilomenes sexmaculata (Fabricius) (Coleoptera: Coccinellidae). Biocontrol Science and Technology , 14 , 665–673.10.1080/091583150410001682359
- Ouédraogo, A. , Yombi, L. , Doumbia, S. , Eyhorn, F. , & Dischl, R. (2008). Guide de production du coton biologique et équitable [Organic cotton production guideline] (47 p.). Ouagadougou: Helveta Azur Conseil.
- Pandi, G. G. P. , Bishwajeet, P. , Shah, V. , & Shankarganesh, K. (2012). Feeding potential and biology of coccinellid predator Cheilomenes sexmaculata (Fabricius) (Coleoptera) on aphid hosts. Indian Journal of Entomology , 74 , 388–393.
- Prabhakar, A. K. , & Roy, S. P. (2010). Evaluation of the consumption rates of dominant coccinellid predators on aphids in North-East Bihar. The Bioscan , 5 , 491–493.
- Romeis, J. , & Shanower, T. G. (1996). Arthropod natural enemies of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in India. Biocontrol Science and Technology , 6 , 481–508.10.1080/09583159631136
- Schmiedeknecht, G. , Bochow, H. , & Junge, H. (1998). Use of Bacillus subtilis as biocontrol agent II. Biological control of potato disease. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz , 105 , 376–386.
- Schmiedeknecht, G. , Issoufou, I. , Junge, H. , & Bochow, H. (2001). Use of Bacillus subtilis as biocontrol agent V. Biological control of diseases on maize and sunflowers. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz , 108 , 500–512.
- Silvie, P. , Deguine, J. P. , Nibouche, S. , Michel, B. , & Vaissayre, M. (2001). Potential of threshold-based interventions for cotton pest control by small farmers in West Africa. Crop Protection , 20 , 297–301.10.1016/S0261-2194(00)00146-0
- Strong, D. R. , Lawton, J. H. , & Southwood, R. (1984). Insect of plant community pattern and mechanisms ( 313 p.). Oxford: Blackwell scientific publications.
- Vaissayre, M. , & Cauquil, J. (2000). Main pests and diseases of cotton in sub-saharan Africa (60 p.). Montpellier: CIRAD Service des éditions. ISBN 2-87614-416-6.
- Vaissayre, M. , & Deguine, J. P. (1996). Cotton protection programmes in francophone Africa. Phytoma , 489 , 26–29.
- van den Berg, H. , & Cock, M. J. W. (1993). Exclusion cage studies on the impact of predation on Helicoverpa armigera in cotton. Biocontrol Science and Technology , 3 , 491–497.10.1080/09583159309355304
- van den Berg, H. , & Cock, M. J. W. (1995). Natural control of Helicoverpa armigera in cotton: Assessment of the role of predation. Biocontrol Science and Technology , 5 , 453–464.10.1080/09583159550039648
- van den Berg, H. , Cock, M. J. W. , & Oduor, G. I. (1997). Natural control of Helicoverpa armigera in sunflower: Assessment of the role of predation. Biocontrol Science and Technology , 7 , 613–630.10.1080/09583159730668