Abstract
A disease complex involving Meloidogyne incognita–Rhizoctonia solani was studied on tomato (Lycopersicon esculentum var. Pusa Ruby) under glasshouse conditions to determine their concomitant effect on plant growth variables. Biofertilizers Nerium indicum and Trichoderma harzianum were tested against both pathogens individually as well as concomitantly and found its role in minimizing disease severity. Inoculation of M. incognita and R. solani resulted a significant reduction in plant growth variables over control. The plant growth variables reduction was more pronounced by M. incognita as compared to R. solani. However, T. harzianum exhibited their potential against the disease complex but was less effective than N. indicum. A manifold improvement in plant growth parameters was observed when plants were treated with biofertilizers, N. indicum and T. harzianum simultaneously. The present work has revealed that the combined application of N. indicum and T. harzianum may be a better option for the management of disease complex M. incognita–R. solani on tomato. Application of these biofertilizers after field trials may be suitable module of organic farming.
Keywords:
Public Interest Statement
Tomato, Lycopersicon esculentum Mill., is an important vegetable crop being grown across the world including India. It is consumed in diverse way including as an ingredient in many dishes, sauces, salads, and drinks. The fruit is rich in lycopene, which may have beneficial heath effect. Present findings will indeed disseminate the paramount informations among the non-specialist readers especially the farmers/growers and business entrepreneurs who are getting hurdles in tomato cultivation across the world, especially in India. In this way, growers and related persons may be given assistance by deploying the methodology and doses of the different treatments and results obtained from this research. This will promote the organic production of vegetables globally.
Competing interests
The authors declare no competing interest.
1. Introduction
Tomato is one of the most important vegetable crop which is being cultivated worldwide. India is the second largest producer of tomato in the world after China with annual production of 17,500,000 metric tons (FAO, Citation2012). Tomato belongs to the family solanaceae, is a rich source of lycopene, used in the cancer treatment especially in prostate cancer (Giovannucci, Citation1999). According to the National Cancer Institute, there is enough data to show that people who consume large amount of tomato products have significantly decreased risk of prostate, lung, and stomach cancer. A large number of phytopathogens have been encountered in relation to productivity reduction of tomato crop. Among them, root-knot nematodes are the major problem (Keshari & Gupta, Citation2015; Singh, Rai, Singh, & Singh, Citation2011).
Meloidogyne incognita causes root-knot disease in tomato plant by abnormal expansion of root cell and forming giant cells (Singh & Patel, Citation2013). M. incognita is one of the key nematode which is supposed to be widely distributed (Siddiqui & Shahid Shaukat, Citation2003; Sikora & Fernández, Citation2005) and difficult to control (Chitwood, Citation2002) because of its high reproduction rate (Ananhirunsalee, Barker, & Beute, Citation1995). A yield loss of tomato ranging from 32 to 40% due to root-knot nematode has been reported by Anwaar and Mckenry (Citation2012).
Many soil fungi also caused innumerable diseases on tomato plants and hampered its production. Fungi are spore-forming and cause local or general necrosis as well as chlorosis of plant tissue. Among them, Rhizoctonia solani causes root rot and stem rot on tomato plants (El-Mohamedy, Jabnoun-Khiareddine, & Daami-Remadi, Citation2014).
Existence of M. incognita and/or R. solani was monitored in areas where tomato is cultivated in large scale (Aligarh, Uttar Pradesh). The interactive nature of these pathogens has already been reported (Chahal & Chhabra, Citation1984; Golden & Van Gundy, Citation1974; Goswami, Seth, Gupta, & Singh, Citation1975; Kumar & Haseeb, Citation2009; Safiuddin, Tiyagi, Rizvi, & Mahmood, Citation2014; Sagar, Rao, & Varaprasad, Citation2012). It has been advocated by several research that the pernicious effects of R. solani become more pronounced in the presence of M. incognita (Abuzar, Citation2013; Anwar & Khan, Citation2002; Bhagawati, Das, & Sinha, Citation2007; Mokbel, Ibrahim, Shehata, & El-Saedy, Citation2007).
Application of synthetic pesticides to control phytopathogens warrants immediate results. However, its application has changed the food web of natural biodiversity. Also, application of chemical pesticides causes many diseases to mankind and other vertebrates. On the other hand, natural products proved to be environmentally safe which may replace synthetic pesticides (Kim et al., Citation2005). Hence, there is a need to identify these eco-friendly plant products (Duke, Citation1990). Nerium indicum is a well-known alternative to chemical pesticides specially for the purpose of protecting crops against nematodes and also for the conservation of biodiversity (Ahmad, Karim, & Khan, Citation1990; Elbadri, Lee, Park, Yu, & Choo, Citation2008; Hameed, Citation1990; Singh & Patel, Citation2013). Large number of informations are available about botanicals which are being utilized for the eco-friendly and sustainable management of plant parasitic nematodes and soil-pathogenic fungi (Rizvi, Mahmood, Tiyagi, & Khan, Citation2012). Beneficial mycoflora are also found in the rhizosphere which protect the crops against harmful pathogens. Biomanagement of root-knot nematodes (Meloidogyne spp.) by Trichoderma spp. has been found safe to soil biota (Affokpon et al., Citation2011; AL-Shammari, Bahkali, Elgorban, El-Kahky, & Al-Sum, Citation2013; Mascarin, Junior, Filho, & de, Citation2012; Naserinasab, Sahebani, & Etebarian, Citation2011; Rao, Reddy, & Nagesh, Citation1998; Sharon, Chet, & Spiegel, Citation2011; Sharon et al., Citation2001, Citation2007; Spiegel, Sharon, & Bar-Eyal, Citation2007). Trichoderma harzianum is used frequently as an antagonistic (Montealegre et al., Citation2010). That acts as indirectly by releasing toxic metabolites, or by competing other pathogens for food and space, directly by releasing toxic antibiotic substances (Kumar, Citation2013). Large number of information on this important cash crop has generated an impetus to assess the significant role of N. indicum and T. harzianum against the disease complex involving M. incognita and R. solani in relation to some growth parameters of tomato plants.
2. Materials and method
2.1. Preparation and sterilization of soil mixture
Soil was collected from the field of the Botany Department, Aligarh Muslim University, Aligarh having the texture of sandy-loam. Before experimentation, the soil was processed to asses some important features of the soil e.g. pH 6.5, particle size (sand: 70.3, silt: 20.5, clay: 9.2), and organic carbon: 1.08%. The pH was measured with the help of pH meter (Mc Lean, Citation1982). The texture of soil in relation to particle size was determined by hydrometer method (Allen, Grimshaw, Parkinson, & Quarmby, Citation1974) and % organic carbon by Walkley (Citation1947). The soil was mixed with river sand and organic manure in the ratio of 3:1, and 15-cm diameter pots were filled with 1 kg of soil (Rizvi et al., Citation2015). The chemical composition of organic manure in the form of Farm Yard Manure (FYM) used in this experiment comprises N (0.50%), P2O5 (0.20%) and K2O (0.28%), bulk density (1.50), water holding capacity (43.62) and porosity (47.32%). About 250 mL of water was poured into each pot to wet the soil before transferring to an autoclave for sterilization at 138 kpa for 20 min. Soil moisture content was maintained in each pot by weighing each vial and adding water (by weight) as required (200 mL). Volumetric moisture content was calculated according to the procedure described by Campbell, Nicholaichuk, Davidson, and Cameron (Citation1977).
2.2. Raising and maintenance of the test plant
The seeds of tomato (Lycopersicon esculentum var. Pusa Ruby) were surface sterilized in 0.01% mercuric chloride (HgCl2) for two minutes and then rinsed three times with sterile water. Seeds were sown in the sterilized soil in 25-cm clay pots. One-week old seedlings were transplanted per pot.
2.3. Preparation of nematode inoculum
Root-knot infected brinjal roots were collected from the infested fields of Aligarh and its adjoining areas. Females of root-knot nematode were collected and identified as M. incognita on the basis of perineal pattern. Pure culture of M. incognita was maintained on Solanum melongena in the greenhouse of the department. Large number of egg masses of root-knot nematode were handpicked, using a sterilized forceps from heavily infected eggplant root. These egg masses were washed in distilled water and then placed in 10 cm diameter 15 mesh sieves containing crossed layers of tissue paper and placed in Petri dishes containing water just deep enough to contact the egg masses. The juveniles hatched were collected after every 24 h and fresh water was added to the Petri dishes. The amount of second-stage juveniles in the water suspension was adjusted so that each mL contained 200 nematodes. Ten milliliters of such suspension containing 2,000 freshly hatched juveniles were inoculated to each pot.
2.4. Procurement and maintenance of R. solani
Pure culture of R. solani was procured from Indian Type Culture Collection of the Plant Pathology Unit, IARI, New Delhi. The culture was then subcultured and maintained on PDA and Richard’s liquid (Riker & Riker, Citation1936) as and when required. The Richard’s liquid medium was prepared in 250-mL Erlenmeyer flasks, each flask containing about 80 mL of Richard’s liquid medium. Small bits of the mycelium of the fungus were transferred to the conical flasks. Inoculated flaks were incubated at 28 ± 2°C for about 15 days to allow growth of the fungus. The pure culture of the fungus was continuously maintained on PDA contained in the test tubes by reinoculation of the fungus after every 15 days. After incubating the conical flasks for about 15 days, the liquid medium was filtered through Whatman filter paper No.1. Thereafter, mycelial mat was washed in distilled water to remove the traces of medium and gently pressed between the folds of blotting paper to remove the excess amount of water. Ten gram fungal mycelium was mixed in 90 mL of sterile water (1:10) and blended for 30 s in a waring blender to prepare the inoculum. Two gram mycelial mat of R. solani was inoculated to each pot.
2.5. Preparation of T. harzianum
Pure culture of T. harzianum was purchased from IARI, New Delhi and further revived on Potato Dextrose Agar (PDA) containing 200-g peeled potato, 20-g dextrose, and 20-g agar agar. The Petri dishes were then placed in an incubator at 24°C for 1–2 weeks. Ten mycelia were carefully scraped from the media and suspended in 90-mL distilled water (1:10). Two gram mycelial mat of T. harzianum was used for each treatment.
2.6. Preparation of N. indicum
Leaves of N. indicum were handpicked and rinsed with distilled water. After washing, the leaves were chopped with sharp sterile knife and incorporated in soil at the rate of 20 g/kg soil/pot. These pots were immediately watered with equal amount to prepare the compost of N. indicum leaves which were left for 20 days.
2.7. Estimation of chlorophyll content
Chlorophyll content of leaf was estimated by the method of Hiscox and Israelstam (Citation1979). One hundred milligrams of leaf pieces were placed in a vial containing 7 mL of dimethyl sulfoxide (DMSO) and the chlorophyll was extracted into the fluid by incubating for 60 min. The extracts was transferred to a graduated tube and made up to 10 mL with DMSO and assayed immediately. A sample of 3 mL of chlorophyll extract was transferred to a cuvette and the optical density (OD) values at 645 and 663 nm were read using a spectrophotometer (Spectronic 1001) against a DMSO blank.
2.8. Percent pollen fertility
The percent pollen fertility was estimated by the method of Brown (Citation1949) when the plants attained the subsequent stage using stainability of pollen grains in 1% acetocarmine solution.
2.9. Inoculation techniques
Just before inoculations, roots of tomato seedlings were exposed by carefully removing the top layer of soil and the required quantity of nematode suspension and fungus inoculum was poured uniformly around the exposed roots using sterilized pipette. Exposed roots were immediately covered with soil properly. There were five replicates of each treatment, uninoculated plants served as control. Plants were regularly watered in equal amount as and when required with proper thinning in appropriate time.
2.10. Experimental design
Treatments
| • | Control | ||||
| • | NI (Nerium indicum) | ||||
| • | MI (Meloidogyne incognita) | ||||
| • | RS (Rhizoctonia solani) | ||||
| • | TH (Trichoderma harzianum) | ||||
| • | NI + MI | ||||
| • | NI + TH | ||||
| • | NI + RS | ||||
| • | MI + RS | ||||
| • | MI + TH | ||||
| • | RS + TH | ||||
| • | RS + TH + MI | ||||
| • | RS + TH + MI + NI | ||||
| • | RS + MI + NI | ||||
The 14 treatments each with five replicates were arranged in a completely Randomized Block Design (RBD) and maintained in a glasshouse with air temperature. All the plants were watered up to the soil capacity. The tomato plants were uprooted 60 days after nematode inoculation for determining the plant growth and nematode-related parameters.
2.11. Parameters
After termination of the experiment, the following parameters were determined for each treatment;
Plant length (cm)
Plant fresh weight (g)
Plant dry weight (g)
Fruits per plant
Fresh weight of fruits (g)
Percent pollen fertility
Chlorophyll content (mg g−1 fresh leaves)
Nematode population
Number of gall root system−1
Number of egg masses root system−1
Percent root rot
2.12. Nematode related parameters
2.12.1. Nematode population
A 250-g subsample of well-mixed soil from each treatment was processed by Cobb’s sieving and decanting method, followed by Baerman’s funnel extraction to determine the final nematode population in soil (Southey, Citation1986). The mean of five counts was used to calculate the population of nematode per kg soil.
2.13. Galls, egg masses, and root-rot percentage
At termination of the experiment, roots of harvested plants were washed under the tap and examined for the presence of the galls. For the assessment of egg masses, plant roots were immersed for 15 min in 0.015% Phloxine B, which specifically stains the gelatinous matrix of nematode egg masses bright red and the egg masses per root system were counted. Percentage of root-rot was calculated by visual observation on root system/plant.
2.14. Statistical analysis
The entire data collected during the study were statistically analyzed in simple randomized design by the method of Panse and Sukhatme (Citation1985). Critical difference (CD) was calculated at 5% and Duncan’s Multiple Range test (DMRT) was employed to test for significant differences between treatments.
3. Results
Present study revealed that combined application of N. indicum and T. harzianum showed maximum improvement in growth variables as compared to their individual application as well as untreated control. In addition, maximum inhibition in root-rot incidence and nematode multiplication was observed in combined application of both biofertilizers. On the other hand, concomitant application of M. incognita and R. solani showed significant reduction in growth parameters; however, their individual inoculation also reduced the growth parameters but was less than their concomitant inoculation.
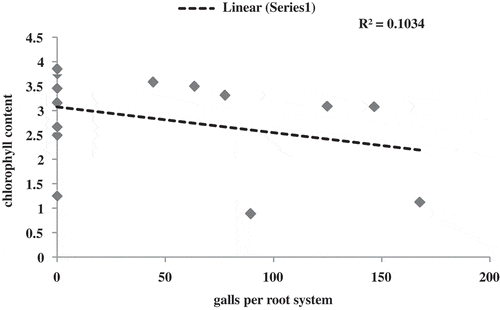
Amendment of organic matter, N. indicum significantly improved the plant growth like plant length, fresh weight, fruits per plant, and dry weight over control (Figure ). However, maximum improvement in plant growth was observed in those plants that received the simultaneous application of T. harzianum and N. indicum. In contrast, minimum plant length was recorded in plants that were given concomitant application of R. solani and M. incognita followed by their individual inoculations. However, individual application of N. indicum was found effective and improved the plant growth significantly followed by T. harzianum. Manifold improvement was recorded in fresh weight of fruits, chlorophyll content, and percent pollen fertility in the experimental set those were given concurrent application of N. indicum and T. harzianum (Table ). However, drastic decreased in plant growth was recorded in concomitantly applied (nematode + fungus) plants (Table ; Figure ). Moreover, maximum chlorophyll contents were recorded in plants that received combined application of N. indicum plus T. harzianum; however, in other treatments, improvement was also recorded but their effectiveness was found to be less than NI + TH. Maximum reductions in chlorophyll content were observed in plants treated with M. incognita + R. solani followed by individual inoculation of M. incognita and R. solani (Table ). To support this hypothesis, a correlation analysis was performed between chlorophyll content and number of galls per root system (Figure ).
Figure 1. Soil application of N. indicum and T. harzianum on disease complex involving M. incognita and R. solani on growth parameters (Plant length, fresh weight, fruits/plant, dry weight) of tomato
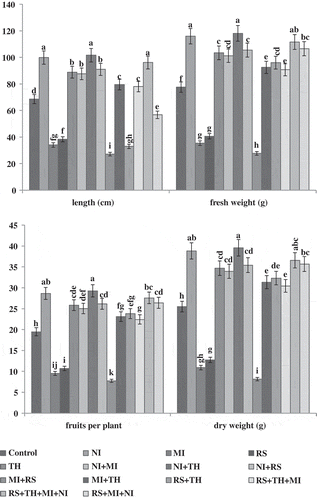
Table 1. Soil application of N. indicum and T. harzianum on disease complex involving M. incognita and R. solani on growth parameters of tomato (five replicates)
Significant reduction in root galls and egg masses/root system was recorded in all the tomato plant treated with various combinations of organic matter and bioinoculant like N. indicum and T. harzianum, respectively (Table ). The multiplication rate of nematode population was also reduced in the tomato plants that were treated with NI + TH. Besides, other treatments were also effective against the disease complex, but their effectiveness was less than the NI + TH (Table ).
Table 2. Soil application of N. indicum and T. harzianum on disease complex involving M. incognita and R. solani on disease incidence and nematode population affecting tomato (five replicates)
Application of N. indicum accounted for a significant reduction in the root-rot percentage (Table ). However, maximum root-rot was recorded in the treatment that received the combine application of M. incognita plus R. solani over control (Table ).
4. Discussion
The results presented in Table emphasized that NI and TH significantly reduced the damaging potential of M. incognita and R. solani and subsequently improved the growth parameters of tomato. This might be partly due to reduction in nematode population and percent disease severity, and partly due to the additive effects of organic matter and TH which also served as manures.
Application of biofertilizer in various combinations constantly improved the plant growth in comparison to untreated control and reduced the damaging potential of M. incognita and R. solani either alone or in combinations. Our results are in conformity with those of (Harish, Saravanakumar, Radjacommare, Ebenezar, & Seetharaman, Citation2008; Mervat, Shawky, & Shaker, Citation2012). Amendments of N. indicum have been found to improve plant growth and yield which has been reported by several other workers (Issac & Abu-Tahan, Citation2014; Moosavi, Citation2012; Radwan, El-Maadawy, & Abu-Elamayem, Citation2007). N indicum has been found to be toxic to various kinds of microbes including phytonematodes (Asif et al., Citation2014; Singh & Singh, Citation1988, Citation1999; Tiwari & Singh, Citation2003). It is known fact that nematotoxic substances present in the organic matter reduce the nematode population upon decomposition. Various theories have been put forwarded by different researchers to explain possible mode of action of decomposed organic additives leading to the control of phytoparasitic nematodes. Management of phytonematodes may be due to nematicidal/nematostatic substances present in such botanicals released after decomposition (Khan, Khan, & Saxena, Citation1974), changes in physical and biological properties of soil (Ramesh, Panwar, Singh, & Ramana, Citation2009), or toxicants released or produced during microbial decomposition. Southey (Citation1978) reported that organic manures may suppress the soil population of nematodes and subsequently enhance crop tolerance and growth variables as well. The pernicious effects of botanicals may be due to the chemicals present in the organics such as alkaloids, flavonoids, saponins, amides including benzamides and ketones that check the rate of nematode reproduction (Adegbite & Adesiyan, Citation2005). Alam (Citation1976) propounded that compounds such as ammonia, H2S, fatty acids, aldehyde, formaldehyde, amino acids, and carbohydrates are released when organic matter partially or fully decomposed. These phytochemicals were found toxic to the plant-parasitic nematodes (Alam, Khan, & Saxena, Citation1977). The biological activity and virulence of N. indicum have been proved to be lethal to M. incognita (Asif et al., Citation2014; Elbadri et al., Citation2008; Xiujuan, Yuxian, Furu, & Tong, Citation2002). Wang et al. (Citation2009) reported that 3β-O-(β-D-diginosyl)-14,15α-di hydroxyl-5α-card-20(22)-enolide, uzarigenin and cardenolide N-1 have the lethal properties to other tylenchids such as Bursaphelenchus xylophilus, Panagrellus redivivus, and Caenorhabditis elegans. Our findings are in agreement with those of Srivastava and Yadav (Citation2008). They reported that leaf extract of neem (Azadirachta indica) inhibited the mycelial growth of Fusarium oxysporum. Singh, Prajapati, Srivastava, Pandey, and Gupta (Citation2007) stated that some botanicals in the form of marigold leaf extract inhibited the growth of Sclerotium rolfsii.
Application of N. indicum and T. harzianum decreased the disease intensity in the nematode–fungus infested plants. The detrimental effects of biofertilizers against the disease complex have been observed by earlier workers. (Sahebani & Hadavi, Citation2008; Youssef, Citation2013). In our study, T. harzianum and N. indicum reduced the disease severity of M. incognita–R. solani disease complex. Montealegre et al. (Citation2010) observed the efficacious nature of some mutants of T. harzianum against R. solani. Application of T. harzianum and N. indicum helped to improve the plant growth parameters over control. A similar result against root-knot disease was also observed by Tiyagi, Mahmood, and Rizvi (Citation2009). The plants inoculated with N. indicum and T. harzianum were found to be statistically significant as compared to other treatments which show the antagonistic effect of T. harzianum on nematode parasitism. Rao, Reddy, and Nagesh (Citation2000) reported that Trichoderma sp. individually or in combination with caster or neem cake was able to parasitize the egg masses and ultimately reduced the galling and nematode population of M. incognita. Application of Trichoderma sp. starts penetrating the larval cuticle through hyphae and also the nematodes eggs through some enzymatic activity which dissolves the chitin layer which is supposed to be protective layer of the nematodes against many pathogenic agents inhabiting the soil. Our results are in agreement with Freitas, Pedrosa, Mariano, and Maranhão (Citation2012), who demonstrated that Trichoderma spp. reduced gall index and nematode reproduction which advocates the high potential to control root-knot nematode, M. incognita. T. harzianum can alter the physiology of roots including the root exudates. Soil application of T. harzianum drastically decreased the rotting caused by R. solani on tomato (Strahnoy, Elad, Sivan, Rudich, & Chet, Citation1985). Trichoderma spp. has been reported to be efficient biological agent against various phytopathogenic soil fungi including R. solani and Pythium spp. (Harman, Citation1996; Howell, Citation2006). The efficiency of T. harzianum to inhibit fungal growth may be due to competition for food and space, mycoparasitism and the production of antibiotic compounds. It has been found that the hyphae of the bioagent penetrate the host mycelium through degrading cell wall by secretion of hydrolytic enzymes followed by assimilates of cell contents (Howell, Citation2006; Siameto, Okoth, Amugune, & Cheg, Citation2011). Chlorophyll content was found increased in those plants inoculated with N. indicum and T. harzianum individually and simultaneously as well. The improvement in chlorophyll content in leaves may be due to enhanced nutrient uptake by the addition of organic components which increased photosynthetic efficiency, translocation of nutrients and other metabolites.
5. Conclusion
The present study concludes that disease complex involving M. incognita and R. solani caused significant reduction in various plant growth variables. The effect of biofertilizer N. indicum and T. harzianum either alone or in combination reduced the severity of disease complex. Incorporation of the biofertilizers significantly reduced the nematode multiplication, root-galls, egg mass/ root system, and also reduced percent root-rot of R. solani. Hence, combined application of N. indicum and T. harzianum may be recommended against the disease complex involving R. solani and M. incognita on tomato to the farmers/growers to the developing countries especially in areas where environmental conditions and tomato culture practices may favour the development of these pathogens. Further study may be needed to understand various mechanisms of action of both biofertilizers and their possible synergism with other compounds applied in organic agriculture. In this way, this study may pave the way toward the disease management of sustainable agriculture.
Additional information
Funding
Notes on contributors
Rizwan Ali Ansari
The Aligarh Muslim University, Aligarh has been recognized for its outstanding contributions in the several aspects related to Plant Pathology and Nematology. Corresponding author, Rizwan Ali Ansari has been engaged with the development and formulation of different modules by exploiting the biological organisms and organic matters against the various economically important diseases infesting several agricultural crops. He has recently received a prestigious award by the Nematological Society of India (NSI) for his outstanding contribution in organic farming. In addition, thrust area of Ansari and his groups to promote organic farming across the world by utilizing the organic matters, mycorrhizal fungi, PGPR, biofertilizers, and some other beneficial organisms for sustainable management of plant-parasitic nematodes through biological means as well as enrich the soil with nutrients necessary for plant growth and development.
References
- Abuzar, S. (2013). Antagonistic effects of some fluorescent Pseudomonas strains against root rot fungi (Rhizoctonia solani and Fusarium oxysporum) and root-knot nematodes (Meloidogyne incognita) on chili (Capsicum annum). World Applied Sciences Journal , 27 , 1455–1460.
- Adegbite, A. A. , & Adesiyan, S. O. (2005). Root extracts of plants to control root-knot nematode on edible soybean. World Journal of Agricultural Sciences , 1 , 18–21.
- Affokpon, A. , Coyne, D. L. , Htay, C. C. , Agbèdè R. D. , Lawouin, L. , & Coosemans, J. (2011). Biocontrol potential of native Trichoderma isolates against root-knot nematodes in West African vegetable production systems. Soil Biology and Biochemistry , 43 , 600–608.10.1016/j.soilbio.2010.11.029
- Ahmad, M. U. , Karim, M. R. , & Khan, M. S. A. (1990). Effect of some indigenous plant extracts on juvenile mortality of Meloidogyne javanica . International Journal of Nematology, Network News , 7 , 5–7.
- AL-Shammari, T. A. , Bahkali, A. H. , Elgorban, A. M. , El-Kahky, M. T. , & Al-Sum, B. A. (2013). The use of Trichoderma longibrachiatum and Mortierella alpina against root-knot nematode, Meloidogyne javanica on tomato. Journal of Pure and Applied Microbiology , 7 , 199–207.
- Alam, M. M. (1976). Organic amendments in relation to nematodes ( PhD thesis). Aligarh Muslim University, Aligarh, India.
- Alam, M. M. , Khan, A. M. , & Saxena, S. K. (1977). Persistent action of oil-cakes and nematicides on the population of nematodes in field. Botyu Kagaku , 40 , 159–161.
- Allen, S. E. , Grimshaw, H. M. , Parkinson, J. A. , & Quarmby, C. (1974). Chemical analysis of ecological materials (pp. 1–565). Oxford: Blackwell Scientific. Retrieved from http://www.cabdirect.org/abstracts/19751431633.html;jsessionid=C5F0BF8280CFBE051B96320D269705AF
- Ananhirunsalee, K. , Barker, K. R. , & Beute, M. K. (1995). Infection, reproduction potential and root galling by root-knot nematode species and concomitant populations on peanut and tobacco. Journal of Nematology , 27 , 172–177.
- Anwaar, S. A. , & Mckenry, M. V. (2012). Incidence and population density of plant parasitic nematodes infecting vegetable crops and associated yield losses. Pakistan Journal of Zoology , 44 , 327–333.
- Anwar, A. , & Khan, F. A. (2002). Studies on the interaction between Meloidogyne incognita and Rhizoctonia solani . Annals of Plant Protection Sciences , 10 , 128–130.
- Asif, M. , Parihar, K. , Rehman, B. , Ganaia, M. A. , Usman, A. , & Siddiqui M. A. (2014). Bio-efficacy of some leaf extracts on the inhibition of egg hatching and mortality of Meloidogyne incognita . Archives of Phytopathology and Plant Protection , 47 , 1015–1021. doi:10.1080/03235408.2013.829626
- Bhagawati, B. , Das, B. C. , & Sinha, A. K. (2007). Interaction of Meloidogyne incognita and Rhizoctonia solani on okra. Annals of Plant Protection Sciences , 1 , 533–535.
- Brown, G. T. (1949). Pollen-slide studies . Springfield, IL: Charles C. Thomas.
- Campbell, C. A. , Nicholaichuk, W. , Davidson, H. R. , & Cameron, D. R. (1977). Effects of fertilizer N and soil moisture on growth, N content, and moisture use by spring wheat. Canadian Journal of Soil Science , 57 , 289–310.10.4141/cjss77-035
- Chahal, P. P. K. , & Chhabra, H. K. (1984). Interaction of Meloidogyne incognita with Rhizoctonia solani on tomato. Indian Journal of Nematology , 14 , 56–57.
- Chitwood, D. J. (2002). Phytochemical based strategies for nematode control. Annual Review of Phytopathology , 40 , 221–249.10.1146/annurev.phyto.40.032602.130045
- Duke, S. O. (1990). Natural pesticides from plants. In J. Janick & J. E. Simon (Eds.), Advances in New Crops (pp. 511–517). Portland, ME: Timber Press.
- El-Mohamedy, R. S. R. , Jabnoun-Khiareddine, H. , & Daami-Remadi, M. (2014). Control of root rot diseases of tomato plants caused by Fusarium solani, Rhizoctonia solani and Sclerotium rolfsii using different chemical plant resistance inducers. Tunisian Journal of Plant Protection , 9 , 45–55.
- Elbadri, G. A. , Lee, D. W. , Park, J. C. , Yu, H. B. , & Choo, H. Y. (2008). Evaluation of various plant extracts for their nematicidal efficacies against juveniles of Meloidogyne incognita . Journal of Asia-Pacific Entomology , 11 , 99–102.10.1016/j.aspen.2008.04.004
- FAO . (2012). Food and Agriculture Organization of the United Nations . Retrieved 21 November, 2014, from http://en.wikipedia.org/wiki/Tomato
- Freitas, M. A. , Pedrosa, E. M. R. , Mariano, R. L. R. , & Maranhão, S. R. V. L. (2012). Screening Trichoderma spp. as potential agents for biocontrol of Meloidogyne incognita in Sugarcane. Nematropica , 42 , 115–122.
- Giovannucci, E . (1999). Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. JNCI Journal of the National Cancer Institute , 91 , 317–331.10.1093/jnci/91.4.317
- Golden, J. K. , & Van Gundy, S. D. (1974). A disease complex of okra and tomato involving the nematode, Meloidogyne incognita and the soil inhabiting fungus Rhizoctonia solani . Phytopathology , 65 , 265–273.
- Goswami, B. K. , Seth, M. L. , Gupta, J. N. , & Singh, D. V. (1975). Interrelationship of Meloidogyne javanica and Rhizoctonia bataticola in tomato. Indian Phytopathology , 28 , 387–388.
- Hameed, S. F. (1990). Note on the effect of some organic additives on the incidence of root-knot nematodes in tomato (Lycopersicon esculentum Mill.). Indian Journal of Agricultural Science , 40 , 207–210.
- Harish, S. , Saravanakumar, D. , Radjacommare, R. , Ebenezar, E. G. , & Seetharaman, K. (2008). Use of plant extracts and biocontrol agents for the management of brown spot disease in rice. BioControl , 53 , 555–567. doi:10.1007/s10526-007-9098-9
- Harman, G. E. (1996). Trichoderma for biocontrol of plant pathogens: From basic research to commercialized products. In Cornell community, conference on biological control (p. 207). New York, NY: Cornell University.
- Hiscox, J. D. , & Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany , 57 , 1332–1334.10.1139/b79-163
- Howell, C. R. (2006). Understanding the mechanisms employed by Trichoderma virens to effect biological control of cotton diseases. Phytopathology , 96 , 178–180.10.1094/PHYTO-96-0178
- Issac, G. S. , & Abu-Tahan, M. A. (2014). In vitro antifungal activity of medicinal plant extract against Fusarium oxysporum f. sp. Lycopersici race 3 the control agent of tomato. Biology , 65 , 107–118.
- Keshari, A. K. , & Gupta, R. (2015). Management of root-knot nematode, Meloidogyne incognita on tomato using extracts of indigenous plants of Nepal. Annals of Plant Protection Scienes , 23 , 131–134.
- Khan, M. W. , Khan, A. M. , & Saxena, S. K. (1974). Effect of water soluble fractions of oil-cakes and bitter principles of neem on some fungi and nematodes. Acta Botanica Indica , 2 , 120–128.
- Kim, D. I. , Park, J. D. , Kim, S. G. , Kuk, H. , Jang, M. J. , & Kim, S. S. (2005). Screening of some crude plant extracts for their acaricidal and insecticidal efficacies. Journal of Asia-Pacific Entomology , 8 , 93–100.10.1016/S1226-8615(08)60076-X
- Kumar, S. (2013). Trichoderma: A biological weapon for managing plant diseases and promoting sustainability. International Journal of Agricultural Sciences and Veterinary Medicine , 1 , 106–121.
- Kumar, V. , & Haseeb, A. (2009). Interactive effect of Meloidogyne incognita and Rhizoctonia solani on the growth and yield of tomato. Indian Journal of Nematology , 39 , 178–181.
- Mascarin, G. M. , Junior, M. F. B. , Filho, J. V. de A. , & de, A. (2012). Trichoderma harzianum reduces population of Meloidogyne incognita in cucumber plants under greenhouse conditions. Journal of Entomology and Nematology , 4 , 54–57.
- Mc Lean, E. O. (1982). Soil pH and lime requirement. In A. L. Page , R. H. Miller , & D. R. Keeney (Eds.), Methods of soil analyses, Part 2, Chemical and microbiological properties (2nd ed., pp. 199–224). Madison, WI: American Society of Agronomy.
- Mervat, A. A. , Shawky, S. M. , & Shaker, G. S. (2012). Comparative efficacy of some bioagents, plant oil and plant aqueous extracts in controlling Meloidogyne incognita on growth and yield of grapevines. Annals of Agricultural Sciences , 57 , 7–18.10.1016/j.aoas.2012.03.009
- Mokbel, A. A. , Ibrahim, I. K. A. , Shehata, M. R. A. , & El-Saedy, M. A. M. (2007). Interaction between certain root rot disease fungi and rootknot nematode Meloidogyne incognita on sunflower plants. Egyptian Journal of Phytopathology , 35 , 1–11.
- Montealegre, J. , Valderrama, L. , Sánchez, S. , Herrera, R. , Besoain, X. , Chile, Q. , & Pérez, L. M. (2010). Biological control of Rhizoctonia solani in tomatoes with Trichoderma harzianum mutants. Electronic Journal of Biotechnology , 13 (2), 1–11.
- Moosavi, M. R. (2012). Nematicidal effect of some herbal powders and their aqueous extracts against Meloidogyne javanica . Nematropica , 42 , 48–56.
- Naserinasab, F. , Sahebani, N. , & Etebarian, H. R. (2011). Biological control of Meloidogyne javanica by Trichoderma harzianum BI and salicylic acid on tomato. African Journal of Food Science , 5 , 276–280.
- Panse, V. G. , & Sukhatme, P. V. (1985). Statistical methods for agricultural workers . New Delhi: Indian Council of Agricultural Research.
- Radwan, M. A. , El-Maadawy, E. K. , & Abu-Elamayem, M. M. (2007). Comparison of the nematicidal potentials of dried leaves five plant species against Meloidogyne incognita infecting tomato. Nematologia Medittrenea , 35 , 81–84.
- Ramesh, P. , Panwar, N. R. , Singh, A. B. , & Ramana, S. (2009). Effect of organic nutrient management practices on the production potential, nutrient uptake, soil quality, input-use efficiency and economics of mustard (Brassica juncea). Indian Journal of Agricultural Sciences , 79 , 40–44.
- Rao, M. S. , Reddy, P. P. , & Nagesh, M. (1998). Evaluation of plant based formulations of Trichoderma harzianum for the management of Meloidogyne incognita on eggplant. Nematologia Meditterenea , 26 , 59–62.
- Rao, M. S. , Reddy, P. P. , & Nagesh, M. (2000). Management of root-knot nematode (Meloidogyne incognita) on tomato by integrating Glomus mosseae with Pasteuria penetrans under field conditions. Pest Management in Horticultural Ecosystems , 6 , 130–134.
- Riker, A. J. , & Riker, R. S. (1936). Introduction to research on plant diseases (117 p.). St. Louis, MO: John’s Swift.
- Rizvi, R. , Mahmood, I. , Tiyagi, S. A. , & Khan, Z. (2012). Effect of some botanicals for the management of plant-parasitic nematodes and soil-inhabiting fungi infesting chickpea. Turkish Journal of Agriculture and Forestry , 36 , 710–719.
- Rizvi, R. , Singh, G. , Safiuddin , Ansari, R. A. , & Tiyagi, S. A. , & Mahmood, I. (2015). Sustainable management of root-knot disease of tomato by neem cake and Glomus fasciculatum . Cogent Food & Agriculture , 1 , 1008859. doi:10.1080/23311932.2015.1008859
- Safiuddin , Tiyagi, S. A. , Rizvi, R. , & Mahmood, I . (2014). Biological control of diseases complex involving Meloidogyne incognita and Rhizoctonia solani on growth of okra through microbial inoculants. Journal of Microbiology and Biotechnology , 4 , 46–51.
- Sagar, B. V. , Rao, V. K. , & Varaprasad, K. S. (2012). Interaction of Rhizoctonia solani and Meloidogyne incognita on Tomato. Indian Journal of Nematology , 42 , 66–70.
- Sahebani, N. , & Hadavi, N. (2008). Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum . Soil Biology and Biochemistry , 40 , 2016–2020.10.1016/j.soilbio.2008.03.011
- Sharon, E. , Bar-Eyal, M. , Chet, I. , Herrera-Estrella, A. , Kleifeld, O. , & Spiegel, Y. (2001). Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum . Phytopathology , 91 , 687–693.10.1094/PHYTO.2001.91.7.687
- Sharon, E. , Chet, I. , & Spiegel, Y. (2011). Trichoderma as a biological control agent. In K. Davies & Y. Spiegel (Eds.), Biological control of plant-parasitic nematodes: Building coherence between microbial ecology and molecular mechanisms, progress in biological control (pp. 183–201). Dordrecht: Springer. doi:10.1007/978-1-4020-9648-8
- Sharon, E. , Chet, I. , Viterbo, A. , Bar-Eyal, M. , Nagan, H. , Samuels, G. J. , & Spiegel, Y. (2007). Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Euoropean Journal of Plant Pathology , 118 , 247–258.
- Siameto, E. N. , Okoth, S. , Amugune, N. O. , & Cheg, N. C. (2011). Molecular characterization and identification of biocontrol isolates of Trichodema harzianum from Embu District, Kenya. Tropical and Subtropical Agroecosystems , 13 , 81–90.
- Siddiqui, I. A. , & Shahid Shaukat, S. (2003). Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: Importance of bacterial secondary metabolite, 2,4-diacetylpholoroglucinol. Soil Biology and Biochemistry , 35 , 1615–1623.10.1016/j.soilbio.2003.08.006
- Sikora, R. A. , & Fernández, E. (2005). Nematode parasites of vegetables. In M. Luc , R. A. Sikora , & J. Bridge (Eds.), Plant parasitic nematodes in subtropical and tropical agriculture (pp. 319–392). Wallingford, CT: CAB.10.1079/9780851997278.0000
- Singh, S. , & Singh, D. K. (1988). Molluscicidal activity of Nerium indicum bark. Brazilian Journal of Medical and Biological Research , 31 , 951–954.
- Singh, S. , & Singh, D. K. (1999). Effect of molluscicidal components of Abrus precatorius, Argemone mexicana and Nerium indicum on certain biochemical parameters of Lymnaeu acuminate . Phytotherapy Research , 13 , 210–213.
- Singh, S. , Rai, A. B. , Singh, R. , & Singh, A. K. (2011). Population dynamics of phytonematodes in vegetable crops. Annals of Plant Protection Scienes , 19 , 503–504.
- Singh, S. R. , Prajapati, R. K. , Srivastava, S. S. L. , Pandey, R. K. , & Gupta, P. K. (2007). Evaluation of different botanicals and non-target pesticides against Sclerotium rolfsii causing collar rot of lentil. Indian Phytopathology , 60 , 499–501.
- Singh, T. , & Patel, B. A. (2013). Management of root-knot nematode (Meloidogyne incognita) in bottle gourd using different botanicals in pots. Journal of Parasitic Diseases , 2013 . doi:10.1007/s12639-013-0361-y
- Southey, J. F. (1978). Plant nematology . London: Ministry of Agriculture, Fisheries and Food, HMSO.
- Southey, J. F. (1986). Laboratory methods for work with plant and soil nematodes (Ministry of Agriculture, Fisheries and Food Reference Book 402, 202 pp.). London: HMSO.
- Spiegel, Y. , Sharon, E. , & Bar-Eyal, M. (2007). Evaluation and mode of action of Trichoderma isolates as biocontrol agents against plant parasitic nematodes. IOBC WPRS Bull , 30 , 129–133.
- Srivastava, D. K. , & Yadav, H. L. (2008). Antifungal activity of some medicinal plants against Fusarium oxysporum f. sp. lycopersici . Indian Phytopathology , 61 , 99–102.
- Strahnoy, Y. , Elad, Y. , Sivan, A. , Rudich, Y. , & Chet, I. (1985). Control of Rhizoctonia solani fruit rot of tomatoes by Trichoderma harzianum Rifai. Crop Protection , 4 , 359–364.
- Tiwari, S. , & Singh, A. (2003). Control of common freshwater predatory fish, Channa punctatus, through Nerium indicum leaf extracts. Chemosphere , 53 , 865–875.10.1016/S0045-6535(03)00595-2
- Tiyagi, S. A. , Mahmood, I. , & Rizvi, R. (2009). Application of some latex-bearing plants for the management of phytonematodes infecting tomato and eggplant. Thai Journal of Agricultural Science , 42 , 183–189.
- Walkley, A. (1947). A critical examination of a rapid method for determining organic carbon in soils—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Science , 63 , 251–264.10.1097/00010694-194704000-00001
- Wang, X. B. , Li, G. H. , Zheng, L. J. , Ji, K. Y. , Lü, H. , Liu, F. F. , … Zhang, K. Q. (2009). Nematicidal cardenolides from Nerium indicum Mill. Chemistry and Biodiversity , 6 , 431–436.10.1002/cbdv.v6:3
- Xiujuan, Y. , Yuxian, H. , Furu, C. , & Tong, R. (2002). Evaluation of nematicidal activity of different plant extracts. Acta Agriculturae Universitatis Jiangxiensis , 24 , 386–389.
- Youssef, M. M. A. (2013). Efficacy of different medicinal plants as green and dry leaves and extracts of leaves on root knot nematode, Meloidogyne incognita infecting eggplant. Eurasian Journal of Agricultural and Environmental Medicine , 2 , 10–14.