Abstract
With increasing consumers demand and tightening of food and environmental regulations, traditional food-processing techniques have lost their optimum performance which gave rise to new and powerful technologies. Ultrasonic is a one of the fast, versatile, emerging, and promising non-destructive green technology used in the food industry from last few years. The ultrasound is being carried out in various areas of food technology namely crystallization, freezing, bleaching, degassing, extraction, drying, filtration, emulsification, sterilization, cutting, etc. Ultrasound is being applied as an effective preservation tool in many food-processing fields viz. vegetables and fruits, cereal products, honey, gels, proteins, enzymes, microbial inactivation, cereal technology, water treatment, diary technology, etc. This review summarizes the latest knowledge on impact and application of ultrasound in food technology.
Public Interest Statement
Ultrasonication is one of the new, fast, and emerging green technology in the field of food science and technology. The review focuses on various applications of ultrasound in food technology and its mode of action and effect on food constituents. The present review work collects informative knowledge that may be of interest to researchers, scientists, and industrialists.
Competing interests
The authors declare no competing interest.
1. Introduction
From the past many years, food industry demand for minimal processed food leads to significant alterations in the processing methods as some processing techniques applied under critical conditions lower their nutrient level and bioavailability by inducing physical and chemical changes, thereby reducing organoleptic acceptability. Thus, in lieu of such techniques, newer mild processing methods in food industry have been devised in order to retain nutrient, non nutrient (bioactive) and sensory characteristics (Czechowska-Biskup, Rokita, Lotfy, Ulanski, & Rosiak, Citation2005). Ultrasonic method is one among those rapidly emerging techniques that were devised to minimize processing, enhance quality, and safeguard the safety of food products (Knorr et al., Citation2011). Ultrasound technology as a key area of research and development in the food industry (Ercan & Soysal, Citation2011) is based on mechanical waves at a frequency above the threshold of human hearing (>16 kHz), and can be categorized into two frequency ranges: low and high energy. Low-energy (low power, low intensity) ultrasound has frequencies higher than 100 kHz at intensities below 1 Wcm−2 while high-energy (high power, high-intensity) ultrasound uses intensities higher than 1 Wcm−2 at frequencies between 20 and 500 kHz (Mason, Chemat, & Vinatoru, Citation2011). The representative range for the frequency that is commonly applied in ultrasonic technology lies between 20 kHz and 500 MHz (Yusaf & Al-Juboori, Citation2014). High-frequency ultrasound as an analytical technique is used to obtain information on the physicochemical properties of food such as acidity, firmness, sugar content, ripeness, etc. While as, low-frequency ultrasound is used to change physical and chemical properties of food (Soria & Villamiel, Citation2010) by inducing pressure, shear, and temperature difference in the medium through which they propagate (Dolatowski, Stadnik, & Stasiak, Citation2007) and is capable of producing cavitations in order to inactivate microorganisms in foods (Piyasena, Mohareb, & McKellar, Citation2003). The typical limit for the frequency that is usually used in ultrasound applications ranges between 20 kHz and 500 MHz (Yusaf & Al-Juboori, Citation2014). Ultrasonication finds its application in quality control of fresh vegetables and fruits in both pre-harvest and post-harvest, cheese processing, commercial cooking oils, bread and cereal products, bulk and emulsified fat-based food products, food gels, aerated, and frozen foods. Other applications include the detection of honey adulteration and assessment of the aggregation state, size, and type of protein. Low power ultrasound (LPU): The frequency range of LPU along with spectroscopy and nuclear magnetic resonance (NMR) are currently the most popular, practical, and widely used nondestructive analytical methods. For many years, LPU has been successfully utilized for studying the physicochemical and structural properties of fluid foods (McClements, Citation1997).
2. Mechanism of action
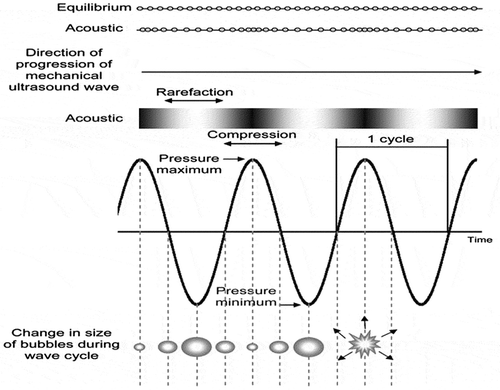
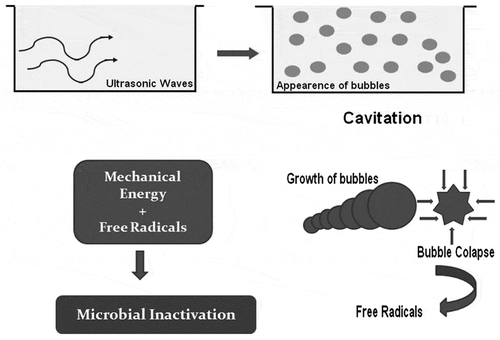
Application of ultrasound to liquid systems causes acoustic cavitation which is the phenomenon of generation, growing and eventual collapse of the bubbles (Figure ). As ultrasound waves propagate, the bubbles oscillate and collapse which causes the thermal, mechanical, and chemical effects. Mechanical effects include collapse pressure, turbulences, and shear stresses (Yusaf & Al-Juboori, Citation2014), while the chemical effects include generation of free radicals (Lateef, Oloke, & Prapulla, Citation2007). The effects in the cavitation zone generate extremely high temperatures (5,000 K) and pressures (1,000 atm) (Soria & Villamiel, Citation2010). Depending on the frequency of the ultrasound, locally produced alternating positive and negative pressures cause expansion or compression of the material, resulting in cell rupture. Ultrasound causes hydrolysis of water inside the oscillating bubbles leading to formation of H+ and OH− free radicals that can be captured in some chemical reactions e.g. free radicals can be scavenged by amino acids of the enzymes involved in structure stability, substrate binding, or catalytic functions. This disruption effect of sonication is significantly resisted by homogenous liquids (Ercan & Soysal, Citation2011). During sonication treatment, bubbles produced are divided into two types on the basis of their structure:
| (1) | Non-linear, forming large bubble clouds with equilibrium size during pressure cycles are known as stable cavitations bubbles. | ||||
| (2) | Non-stable, rapidly collapsing and disintegrating into smaller bubbles are known as internal (transient) cavitations bubbles. | ||||
These small bubbles quickly dissolve, but during bubble stretching, the mass-transfer boundary layer is thinner and the interfacial area is greater than during bubble collapse which implies that more air transfers into the bubble during the stretching phase than leaks out during the collapse phase (Tiwari & Mason, Citation2012).
3. Application
Presently, ultrasound technology has gained wider applications in almost all fields including medical scanning ultrasonic therapy, mineral processing, nanotechnology, food and beverage technology, non-destructive testing, industrial welding, surface cleaning, and environmental decontamination applications (Nithila et al., Citation2014) and in food industry, it has gained enormous attention (Jambrak, Lelas, Mason, Krešić, & Badanjak, Citation2009). Wide spread applicability of ultrasonication as a non-thermal technology in heat-sensitive foods is because it retains sensory, nutritional, and functional characteristics along with enhanced shelf life, microbial safety (Alegria et al., Citation2009), and carrying away of bacterial biofilms (Baumann, Martin, & Hao, Citation2009). Over the past few decades, ultrasonic applications were optimized for processing or testing with the result ultrasonic applications for emulsification, defoaming, decontamination, extraction, wastewater treatment, extrusion, and tenderization of meat existed commercially (Anonymous, Citation2012). In addition, ultrasonic radiation, a type of low-frequency energy (20 kHz–1 MHz), has been enormously utilized for enhancing pretreatment processes like, degassing, crystallization, precipitation, leaching, cleaning, extraction, digestion sample preparation (Jiao & Zuo, Citation2009), changing functional characteristics of food proteins, textural properties of fat products (sonocrystallization), and promoting the extraction of bioactive constituents (Gallego-Juárez, Rodriguez, Acosta, & Riera, Citation2010). Favorable effects of ultrasound in food processing involves enhancement in food preservation, aid in thermal treatments, improved mass transfer, and alteration of food texture and analysis (Knorr et al., Citation2011). Ultrasound technology has achieved significant importance due to advancement of novel ultrasound-based and ultrasound-aided detection systems assisted by modern developments in ultrasound electronic/transducer designs (Jerman Klen & Mozetič Vodopivec, Citation2012).
Ultrasound is applied by three different methods
| • | Applying directly to the product. | ||||
| • | Coupling with the device. | ||||
| • | Submerging in an ultrasonic bath. | ||||
3.1. Effect of ultrasonication on protein
Application of ultrasound in protein modification has received ample attention in recent years either as pretreatment in order to enhance modification or chemical reaction of protein by changing its physical and functional attributes such as, gelation, foamability, emulsification, and solubility. Ultrasonication has proved as an efficient method in producing protein conjugates and to improve the hydrolysis of proteins enzymatically (Chen, Chen, Ren, & Zhao, Citation2011).
3.2. Effect of ultrasonication on microbial inactivation
Combined effect of power ultrasound and heat (thermosonication) has proved to be more efficient method of microbial in cativation than either of the two methods alone (Raviyan, Zhang, & Feng, Citation2005). Microbial inactivation of ultrasound treatment accounts for generation of acoustic cavitations, resulting in increased permeability of membranes, selectivity loss, cell membrane thinning (Sams & Feria, Citation1991), confined heating (Suslick, Citation1998), singlet electron transfer in cooling phase (Lee & Feng, Citation2011), and hydroxyl radical formation (Kadkhodaee & Povey, Citation2008) (Figure ). High-frequency ultrasound method, patented as sonoxide, has more than 600 applications and provided best results in inhibiting bacterial and algal growth in industrial waters (Broekman, Pohlmann, Beardwood, & de Meulenaer, Citation2010). Ultrasonic-treated cells were found to lack internal content when viewed under transmission electron microscopy, but disintegration was not affirmed to be main reason of cell death (Cameron, McMaster, & Britz, Citation2008). Ultrasonication has achieved the FDA requirement of a 5-log reduction in microbial population (Salleh-Mack & Roberts, Citation2007). Earlier, ultrasound as disinfection treatment was used by the electronics industry but now is used as substitute sanitization process in food industry (Sagong et al., Citation2011). Exploitation of ultrasound as means of inhibiting and killing micro-organisms came from the observation that sonar used for anti-submarine warfare resulted in killing of fishes (Scherba, Weigel, & Obrien, Citation1991). Ultrasound frequency of 20 kHz and power of 12.8 W was used on 50 cm3 water contaminated with Streptococcus mutans for a period of 15 min and 97% microbial reduction was achieved (Koda, Miyamoto, Toma, Matsuoka, & Maebayashi, Citation2009). Ultrasonic power of around 100 W was found to be optimal for maximum microbial inactivation (Yusaf & Al-Juboori, Citation2014) and ultrasonication has been found to be effective method for microbial inactivation in Escherichia coli (Furuta et al., Citation2004), Listeria monocytogenes, and other pathogens. Efficiency of ultrasonic treatment as antimicrobial tool depends on the physical (size, hydrophobicity) and biological (gram-status, growth phase) characteristics of the micro-organisms. It has been demonstrated that micro-organisms with “soft” and thicker capsule are extremely resistant to ultrasonic treatment (Gao, Lewis, Ashokkumar, & Hemar, Citation2014).
Figure 2. Cavitation phenomenon and microbial inactivation by ultrasonic waves
3.3. Ultrasonication in meat technology
A large number of applications of ultrasonic treatment are reported in meat technology like, reduction of meat toughness due to large proportion of connective tissue (Jayasooriya, Torley, D’Arcy, & Bhandari, Citation2007), examining the composition of fish, poultry, raw, and fermented meat products by supporting genetic enhancement programs in case of livestock (Gallego-Juárez et al., Citation2010) and in the tenderization of meat products.
3.4. Ultrasonication in fruit and vegetable processing
Ultrasonication is used to maintain both pre- and post-harvest quality attributes in fresh fruits and vegetables(Gallego-Juárez et al., Citation2010) and is considered a substitute for washing of fruit and vegetable in food industry (Alexandre, Brandao, & Silva, Citation2013). In an attempt to meet the consumers needs of not only maintaining but also improving the nutritional value of fruit juices (Bhat, Ameran, Voon, Karim, & Tze, Citation2011; Bhat, Kamaruddin, Min-Tze, & Karim, Citation2011), ultrasonication has proved to be one such technique (Abid et al., Citation2013) and is reported to retain fresh quality, nutritional value, and microbiological safety in guava juice (Cheng, Soh, Liew, & Teh, Citation2007), orange juice (Valero et al., Citation2007), and tomato juice (Wu, Gamage, Vilkhu, Simons, & Mawson, Citation2008). Ultrasound treatment can also be used to recover the nutrient loss occurred during blanching, resulting in achieving the collaborative benefit of both the techniques (Jabbar et al., Citation2014). Ultrasonication cleaners (20–400 kHz) have been efficiently used to produce fruits and vegetables free of contamination (Lin & Erel, Citation1992) and at 40 kHz, it has been applied on strawberry fruits in which decay and infection was considerably reduced along with quality maintenance (Cao et al., Citation2010).
3.5. Ultrasonication in dairy technology
Ultrasound treatment is applied in dairy industry for removal of fat from dairy wastewater using enzyme (Lipase z) as a catalyst (Adulkar & Rathod, Citation2014), improvement in whey ultrafiltration, cutting of cheese blocks, crystallization of ice and lactose, alter the functionality of dairy proteins (Ashokkumar et al., Citation2010), cleaning of equipment, pasteurization, and homogenization which involve minimum loss of flavor, and increased homogeneity and considerable savings in energy (Chouliara, Georgogianni, Kanellopoulou, & Kontominas, Citation2010).
3.6. Ultrasonication in extraction of plant materials or ultrasound-assisted hydrolysis
Extraction of plant components using ultrasound with its lower operating temperatures successfully dodged the limitations of degradation and loss of thermolabile constituents in conventional extraction methods (Jadhav, Rekha, Gogate, & Rathod, Citation2009). Ultrasound extraction involves lower running cost, considerable reduction in time and temperature of extraction with almost same yields (Yang, Zhao, Shi, Yang, & Jiang, Citation2008), and has been employed in extracting various intracellular components such as soybean oil (Hu, Zhao, Liang, Qiu, & Chen, Citation2006; Li, Li, & Guo, Citation2006), isoflavones from oregano (Rostagno, Palma, & Barroso, Citation2007), xyloglucan (Caili, Haijun, Quanhong, Tongyi, & Wengjuan, Citation2005), and cellulose nanofibers from wood. Ultrasonication is reported to induce some secondary plant metabolites such as ginsenoside saponins by 75% in ginseng cell (Lin, Wu, Ho, & Qi, Citation2001), taxol by three times in Taxus baccata cell culture (Rezaei, Ghanati, & Dehaghi, Citation2011), and resveratrol by 8–143 times in whole or sliced peanut kernel (Rudolf & Resurreccion, Citation2005). Ultrasound hydrolysis with higher polyphenol amounts in extracts (Teh & Birch, Citation2014) has gained much popularity in phenolic compound analysis in various plant matrices because of its faster extraction, efficiency, and low consumption of solvent in strawberries, red raspberries, grape seeds, olive fruits, and leaves (Jerman Klen & Mozetič Vodopivec, Citation2012) and it was reported that to extract naringenin, ellagic acid, naringin, rutin, quercetin, and kaempferol in three cycles of 30 s compared to 2–20 h of traditional methods (maceration/stirring) in case of strawberries and conjugated phenolics of cranberry in less than 1.5 h as compared to 16 h by traditional hydrolysis methods. Ultrasonication is used to extract lycopene (Eh & Teoh, Citation2012), to improve the separation of protein-starch in the wet-milling industry (Zhang, Niu, Eckhoff, & Feng, Citation2005), and to reduce particle size of milled corn for sugar release in corn dry-milling (Khanal, Montalbo, van Leeuwen, Srinivasan, & Grewell, Citation2007).
3.7. Ultrasonication in equipment design and analytical operations
Application of ultrasound in food science and technology for improving food quality has widened due to the probable recent advancement in electronics that designed ultrasound instruments and probes with greater convenience and resolution either as sensors (LPU) or as modifiers (high power ultrasound). However, ultrasound equipment are designed for use in a particular application as they cannot be postulated to suit all different applications e.g. in studying functional and physicochemical characteristics of a particular food item selection of suitable processing or sensing system (probe design, frequency, geometry) and operation variables that give optimum outputs in a particular application should be considered (Knorr et al., Citation2011). LPU in conjugation with spectroscopy and NMR are extensively used in non-destructive analytical techniques for studying the characteristics of fluid foods (McClements, Citation1997) and any deviation in ultrasound characteristics helps to evaluate the properties of fluids and to assess foreign gents in foods through container walls thus, allowing measurements using relatively cheap and robust instrument in the lab as well as online (Coupland, Citation2004).
3.8. Ultrasonication in emulsification
Ultrasonication is relatively cheaper technique for emulsion formation with significant effect on emulsion droplet size and structure. In ultrasonic emulsification application of high energy reported viscosity decrease and lesser particle size distribution in sub-micron oil-droplets emulsions. However, change in sonication parameters caused remarkable change in stability and oil droplet size of the emulsion formed (Kaltsa, Michon, Yanniotis, & Mandala, Citation2013). Ultrasonically produced W/O emulsions are used by emulsion liquid membrane for the separation and recapture of cationic dyes, and the stability is governed by operating variables such as emulsification time, carrier, ultrasonic power, surfactant and internal phase concentrations, volume ratios of internal phase to organic phase and of external phase to W/O emulsions, stirring speed, contact time, and diluents (Djenouhat, Hamdaoui, Chiha, & Samar, Citation2008).
3.9. Ultrasonication in oil technology
Ultrasonication stimulates the mixing and required reaction for conversion of soyabean oil to biodiesel, and can achieve optimum yield using 9:1 oil to methanol ratio (Santos, Rodrigues, & Fernandes, Citation2009). Ultrasonic irradiation is also used to increase the rate of transesterification (Deshmane, Gogate, & Pandit, Citation2009).
3.10. Ultrasonication in water treatment
Ultrasound treatment in combination with other water treatment methods (chlorination, ozonation) is considered efficient and economically feasible technique as in ultrasound equipment, energy requirement is huge (Nithila et al., Citation2014). Ultrasonication is reported to remove all impurities such as worms, sludge, mold, fungi, bacteria, and agrochemicals (Cao et al., Citation2010). Ultrasonication does not use chemicals for mineralization and destruction of recalcitrant organic compounds in water (Gogate, Citation2007). In anaerobic digestion process, ultrasonication is used to increase the process efficiency, leading to more methane production and significant decrease in digestion time. Anaerobic digestion process uses ultrasound treatment either as high or as low strength depending on the irradiation location. High-strength ultrasonication (HS-ultrasonication) is irradiated as a pretreatment to feedstock and low-strength ultrasonication (LS ultrasonication) is irradiated in the aerobic digestion process to the micro-organisms involved (Cho et al., Citation2013).
3.11. Ultrasonication in enzyme technology
Ultrasonication has been used to influence enzyme activity (Fahmi, Khodaiyan, Pourahmad, & Emam-Djomeh, Citation2011) and to obtain intracellular enzymes from microbial cells. Ultrasound treatment helps in the release of glucose-oxidase from Aspergillus niger, galactosidases from Lactobacillus strains and E. coli, and invertase from A. niger. Despite positive implications on enzymatic activity, high-intensity ultrasonication leads to denaturation and hence making ultrasound treatment enzyme-specific and sonication parameter-specific (Lateef et al., Citation2007). Thermosonication a combination treatment of incorporating high static pressure in an ultrasound treatment chamber is used as a means for enzyme inactivation such as lipoxygenase, peroxidase, lipase, and protease, and tomato or orange pectinmethylesterase (Raviyan et al., Citation2005). In cellulose preparation, the cellulolytic activity was found to increase with the ultrasonic intensity because of some minor changes in spatial structure of enzyme molecules that helped in the formation of enzyme–substrate complex and increased the adsorption of cellulase on insoluble cellulose (Nguyen & Le, Citation2013).
3.12. Applications in membrane filtration
Use of ultrasound in conventional membrane filtration has proved to improve process efficiency and utilized in membrane cleaning (Masselin et al., Citation2001). Both cross-flow (Li, Sanderson, & Jacobs, Citation2002) and dead-end filtration (Simon, Gondrexon, Taha, Cabon, & Dorange, Citation2000) uses online ultrasonication. Most commonly ultrasonic water baths are used as ultrasound devices which are associated with high loss of acoustic power of about 90% (Cai, Wang, Zheng, & Liang, Citation2009). In an attempt to improve ultrasound equipment, several workers (Juang & Lin, Citation2004; Mirzaie & Mohammadi, Citation2012; Simon et al., Citation2000) have developed an ultrasonic probe system that in a dead-end filtration process passes ultrasonic waves directly to the feed medium. Also a membrane module fitted with many packed in type ultrasonic transducers are used to apply cross-flow filtration and involve minor loss of ultrasonic energy (Kyllönen, Pirkonen, Nyström, Nuortila-Jokinen, & Grönroos, Citation2006). Filtration performance is measured as the rate of release of permeate flux but is not correlated with processes involved in irreversible fouling and reversible concentration polarization layer in the feed. However, mass-transfer coefficients and concentration of filtrate at the membrane surface have been predicted by modeling and hypothetical methods (Muthukumaran, Kentish, Ashokkumar, & Stevens, Citation2005).
3.13. Ultrasonication in honey
Ultrasound applications in honey include use of velocity of ultrasonic wave propagation as a means to differentiate between different types of honey determination of adulteration in honey and evaluation of the type of protein, aggregation state, and size (Gallego-Juárez et al., Citation2010).
3.14. Other applications
Ultrasonication singly or in combination with other preservation methods have been used to decrease the required processing temperature and time, or both, in pasteurization of liquid foods like milk, wine, and juices. It is used as a substitute or additional process to traditional thermal methods (Valero et al., Citation2007). Numerous other applications of ultrasound are reported in several foods including, cooking oils, bread, cereal products, and emulsified fat-based food products, food gels, aerated foods, and frozen foods (Gallego-Juárez et al., Citation2010). Ultrasound has also been used to determine the interaction of powder with solvent in order to evaluate the reconstitution of powders (Richard et al., Citation2012) and is dependent on product porosity (García-Pérez, Ozuna, Ortuño, Cárcel, & Mulet, Citation2011).
4. Advantages and limitations of ultrasonication
Ultrasound applications offer numerous advantages in the food industry some of which are enlisted as follows:
| • | Ultrasound waves are non-toxic, safe, and environmentally friendly (Kentish & Ashokkumar, Citation2011). | ||||
| • | Ultrasonication in combination with other non-thermal methods is considered an effective means of microbial inactivation (Vercet, Sánchez, Burgos, Montañés, & Lopez Buesa, Citation2002). | ||||
| • | Ultrasonication involves lower running cost, ease of operation, and efficient power output. | ||||
| • | Ultrasonication does not need sophisticated machinery and wide range of technologies (Gallego-Juárez et al., Citation2010). | ||||
| • | Use of ultrasound provides more yield and rate of extraction as compared to other conventional methods of extraction (Balachandran, Kentish, Mawson, & Ashokkumar, Citation2006). | ||||
| • | Ultrasonication involves minimum loss in flavor, superior consistency (viscosity, homogenization), and significant savings in energy expenditure (Chouliara et al., Citation2010). | ||||
| • | Ultrasound has gained huge applications in the food industry such as processing, extraction, emulsification, preservation, homogenization, etc. (Chemat, Zill-e-Huma, & Khan, Citation2011). | ||||
Despite having lot of advantages, use of ultrasonication has also many disadvantages such as:
| • | Ultrasound due to shear stress developed by swirls from the shock waves (mechanical effects) cause inactivation of the released products (Lateef et al., Citation2007). | ||||
| • | Ultrasound application needs more input of energy which makes industrialists to think over while using this technique on commercial scale (Yusaf & Al-Juboori, Citation2014). | ||||
| • | Ultrasound induces physicochemical effects which may be responsible for quality impairment of food products by development of off-flavors, alterations in physical properties, and degradation of components. | ||||
| • | Ultrasonication leads to the formation of radicals as a result of critical temperature and pressure conditions that are responsible for changes in food compounds. The radicals (OH and H) produced in the medium deposit at the surface of cavitation bubble that stimulates the radical chain reactions which involve formation of degradation products and thus lead to considerable quality defects in product (Czechowska-Biskup et al., Citation2005). | ||||
| • | Frequency of ultrasound waves can impose resistance to mass transfer (Esclapez, García-Pérez, Mulet, & Cárcel, Citation2011). | ||||
| • | Ultrasonic power is considered to be responsible for change in materials based on characteristics of medium. So, this power needs to be minimized in food industry in order to achieve maximum results (Feng, Barbosa-Canovas, & Weiss, Citation2011). | ||||
5. Conclusion
Ultrasound being non-toxic and ecofriendly is an emerging technology which is considered as green technology as it saves lot of energy and maximizes production. Ultrasound finds a diverse application in science and food technology which has been employed in studying food composition (fruits, vegetables, and dairy products) and detecting contamination by foreign extraneous materials in canned and dairy foods. A lot of research has been conducted on ultrasound technologies in food technology, but still a great deal of future research is necessary in order to produce industrial-automated ultrasound systems that will help in reduction of labor, cost, energy, and should ensure the maximum production of high value and safe food products.
Additional information
Funding
Notes on contributors
Ishrat Majid
Ishrat Majid completed her master’s degree in Food Technology from Jamia Hamdard, New Delhi. Currently, she is a doctoral research fellow at the Department of Food Engineering & Technology, SLIET, Longowal, Punjab, India, having four International publications to her credit.
Gulzar Ahmad Nayik
Gulzar Ahmad Nayik is a doctoral research fellow at the Department of Food Engineering & Technology, SLIET, Longowal, Punjab, India. He has more than 14 International research papers to his credit.
Vikas Nanda
Vikas Nanda received his PhD degree from PAU, Ludhiana in 2006. He is presently working as an associate professor at the Department of Food Engineering & Technology, SLIET, Longowal, Punjab, India. He has already attended various international conferences in Czech Republic, France (Apimondia, 2009), Greece and Argentina (Apimondia 2011), the Netherland (2014), and Thailand (2015). He is also the vice chairman of International Honey Commission. He has more than 25 International research papers to his credit and also published one book.
References
- Abid, M. , Jabbar, S. , Wu, T. , Hashim, M. M. , Hu, B. , Lei, S. , … Zhang, X. (2013). Effect of ultrasound on different quality parameters of apple juice. Ultrasonics Sonochemistry , 20 , 1182–1187.10.1016/j.ultsonch.2013.02.010
- Adulkar, T. V. , & Rathod, V. K. (2014). Ultrasound assisted enzymatic pre-treatment of high fat content dairy wastewater. Ultrasonics Sonochemistry , 21 , 1083–1089.10.1016/j.ultsonch.2013.11.017
- Alegria, C. , Pinheiro, J. , Gonçalves, E. M. , Fernandes, I. , Moldão, M. , & Abreu, M. (2009). Quality attributes of shredded carrot (Daucus carota L. cv. Nantes) as affected by alternative decontamination processes to chlorine. Innovative Food Science and Emerging Technologies , 10 , 61–69.10.1016/j.ifset.2008.08.006
- Alexandre, E. M. C. , Brandao, T. R. S. , & Silva, C. L. M. (2013). Impact of non-thermal technologies and sanitizer solutions on microbial load reduction and quality factor retention of frozen red bell peppers. Innovative Food Science and Emerging Technologies , 17 , 199–205.
- Anonymous . (2012). Extraction of palm oil . Retrieved from http://www.cavitus.com/products/Extraction/palm-oil
- Ashokkumar, M. , Bhaskaracharya, R. , Kentish, S. , Lee, J. , Palmer, M. , & Zisu, B. (2010). The ultrasonic processing of dairy products–An overview. Dairy Science and Technology , 90 , 147–168.10.1051/dst/2009044
- Balachandran, S. , Kentish, S. E. , Mawson, R. , & Ashokkumar, M. (2006). Ultrasonic enhancement of the supercritical extraction from ginger. Ultrasonics Sonochemistry , 13 , 471–479.10.1016/j.ultsonch.2005.11.006
- Baumann, A. R. , Martin, S. E. , & Hao, F. (2009). Removal of Listeria monocytogenes biofilms from stainless steel by use of ultrasound and ozone. Journal of Food Protection , 72 , 1306–1309.
- Bhat, R. , Ameran, S. B. , Voon, H. C. , Karim, A. A. , & Tze, L. M. (2011). Quality attributes of starfruit (Averrhoa carambola L.) juice treated with ultraviolet radiation. Food Chemistry , 127 , 641–644.10.1016/j.foodchem.2011.01.042
- Bhat, R. , Kamaruddin, N. S. B. C. , Min-Tze, L. , & Karim, A. A. (2011). Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrasonics Sonochemistry , 18 , 1295–1300.10.1016/j.ultsonch.2011.04.002
- Broekman, S. , Pohlmann, O. , Beardwood, E. S. , & de Meulenaer, E. C. (2010). Ultrasonic treatment for microbiological control of water systems. Ultrasonics Sonochemistry , 17 , 1041–1048.10.1016/j.ultsonch.2009.11.011
- Cai, M. , Wang, S. , Zheng, Y. , & Liang, H. (2009). Effects of ultrasound on ultrafiltration of Radix astragalus extract and cleaning of fouled membrane. Separation and Purification Technology , 68 , 351–356.10.1016/j.seppur.2009.06.013
- Caili, F. , Haijun, T. , Quanhong, L. , Tongyi, C. , & Wengjuan, D. (2005). Ultrasound assisted extraction of xyloglucan from pomace. Ultrasonics Sonochemistry , 13 , 511–516.
- Cameron, M. , McMaster, L. D. , & Britz, T. J. (2008). Electron microscopic analysis of dairy microbes inactivated by ultrasound. Ultrasonics Sonochemistry , 15 , 960–964.10.1016/j.ultsonch.2008.02.012
- Cao, S. , Hu, Z. , Pang, B. , Wang, H. , Xie, H. , & Wu, F. (2010). Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control , 21 , 529–532.10.1016/j.foodcont.2009.08.002
- Chemat, F. , Zill-e-Huma , & Khan, M. K. (2011). Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrasonics Sonochemistry , 18 , 813–835.10.1016/j.ultsonch.2010.11.023
- Chen, L. , Chen, J. , Ren, J. , & Zhao, M. (2011). Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. Journal of Agricultural and Food Chemistry , 59 , 2600–2609.10.1021/jf103771x
- Cheng, L. H. , Soh, C. Y. , Liew, S. C. , & Teh, F. F. (2007). Effects of sonication and carbonation on guava juice quality. Food Chemistry , 104 , 1396–1401.10.1016/j.foodchem.2007.02.001
- Cho, S. K. , Hwang, Y. H. , Kim, D. H. , Jeong, I. S. , Shin, H. S. , & Oh, S. E. (2013). Low strength ultrasonication positively affects the methanogenic granules toward higher AD performance. Part I: Physico-chemical characteristics. Bioresource Technology , 136 , 66–72.10.1016/j.biortech.2013.02.111
- Chouliara, E. , Georgogianni, K. G. , Kanellopoulou, N. , & Kontominas, M. G. (2010). Effect of ultrasonication on microbiological, chemical and sensory properties of raw, thermized and pasteurized milk. International Dairy Journal , 20 , 307–313.10.1016/j.idairyj.2009.12.006
- Coupland, J. N. (2004). Low intensity ultrasound. Food Research International , 37 , 537–543.10.1016/j.foodres.2004.01.011
- Czechowska-Biskup, R. , Rokita, B. , Lotfy, S. , Ulanski, P. , & Rosiak, J. M. (2005). Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydrate Polymers , 60 , 175–184.10.1016/j.carbpol.2004.12.001
- de Sao Jose, J. F. B. , de Andrade, N. J. , Ramos, A. M. , Vanetti, M. C. D. , Stringheta, P. C. , & Chaves, J. B. P. (2014). Decontamination by ultrasound application in fresh fruits and vegetables. Food Control , 45 , 36–50.10.1016/j.foodcont.2014.04.015
- Deshmane, V. G. , Gogate, P. R. , & Pandit, A. B. (2009). Ultrasound-assisted synthesis of biodiesel from palm fatty acid distillate. Industrial and Engineering Chemistry Research , 48 , 7923–7927.10.1021/ie800981v
- Djenouhat, M. , Hamdaoui, O. , Chiha, M. , & Samar, M. H. (2008). Ultrasonication-assisted preparation of water-in-oil emulsions and application to the removal of cationic dyes from water by emulsion liquid membrane. Separation and Purification Technology , 62 , 636–641.10.1016/j.seppur.2008.03.018
- Dolatowski, Z. J. , Stadnik, J. , & Stasiak, D. (2007). Applications of ultrasound in food technology. Acta Scientiarum Polonorum, Technologia Alimentaria , 6 , 89–99.
- Eh, A. , & Teoh, S. G. (2012). Novel modified ultrasonication technique for the extraction of lycopene from tomatoes. Ultrasonics Sonochemistry , 19 , 151–159.10.1016/j.ultsonch.2011.05.019
- Ercan, S. S. , & Soysal, Ç. (2011). Effect of ultrasound and temperature on tomato peroxidase. Ultrasonics Sonochemistry , 18 , 689–695.10.1016/j.ultsonch.2010.09.014
- Esclapez, M. D. , García-Pérez, J. V. , Mulet, A. , & Cárcel, J. A. (2011). Ultrasound-assisted extraction of natural products. Food Engineering Reviews , 3 , 108–120. doi:10.1007/s12393-011-9036-6
- Fahmi, R. , Khodaiyan, F. , Pourahmad, R. , & Emam-Djomeh, Z. (2011). Effect of ultrasound assisted extraction upon the protein content and rheological properties of the resultant soymilk. Advance Journal of Food Science and Technology , 3 , 245–249.
- Feng, H. , Barbosa-Canovas, G. , & Weiss, J. (Eds.). (2011). Ultrasound technologies for food and bioprocessing . New York, NY: Springer.
- Furuta, M. , Yamaguchi, M. , Tsukamoto, T. , Yim, B. , Stavarache, C. E. , Hasiba, K. , & Maeda, Y. (2004). Inactivation of Escherichia coli by ultrasonic irradiation. Ultrasonics Sonochemistry , 11 , 57–60.10.1016/S1350-4177(03)00136-6
- Gallego-Juárez, J. , Rodriguez, G. , Acosta, V. , & Riera, E. (2010). Power ultrasonic transducers with extensive radiators for industrial processing. Ultrasonics Sonochemistry , 17 , 953–964.10.1016/j.ultsonch.2009.11.006
- Gao, S. , Lewis, G. D. , Ashokkumar, M. , & Hemar, Y. (2014). Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria. Ultrasonics Sonochemistry , 21 , 446–453.10.1016/j.ultsonch.2013.06.006
- García-Pérez, J. V. , Ozuna, C. , Ortuño, C. , Cárcel, J. A. , & Mulet, A. (2011). Modeling ultrasonically assisted convective drying of eggplant. Drying Technology , 29 , 1499–1509.10.1080/07373937.2011.576321
- Gogate, P. R. (2007). Application of cavitational reactors for water disinfection: Current status and path forward. Journal of Environmental Management , 85 , 801–815.10.1016/j.jenvman.2007.07.001
- Hu, A. , Zhao, S. , Liang, H. , Qiu, T. , & Chen, G. (2006). Ultrasound assisted supercritical fluid extraction of oil and coixenolide from adley seed. Ultrasonics Sonochemistry , 14 , 219–224.
- Jabbar, S. , Abid, M. , Hu, B. , Wu, T. , Hashim, M. A. , Lei, S. , … Zeng, X. (2014). Quality of carrot juice as influenced by blanching and sonication treatments. LWT - Food Science and Technology , 55 , 16–21.10.1016/j.lwt.2013.09.007
- Jadhav, D. , Rekha, B. N. , Gogate, P. R. , & Rathod, V. K. (2009). Extraction of vanillin from vanilla pods: A comparison study of conventional soxhlet and ultrasound assisted extraction. Journal of Food Engineering , 93 , 421–426.10.1016/j.jfoodeng.2009.02.007
- Jambrak, A. R. , Lelas, V. , Mason, T. J. , Krešić, G. , & Badanjak, M. (2009). Physical properties of ultrasound treated soy proteins. Journal of Food Engineering , 93 , 386–393.10.1016/j.jfoodeng.2009.02.001
- Jayasooriya, S. D. , Torley, P. J. , D’Arcy, B. R. , & Bhandari, B. R. (2007). Effect of high power ultrasound and ageing on the physical properties of bovine Semitendinosus and Longissimus muscles. Meat Science , 75 , 628–639.10.1016/j.meatsci.2006.09.010
- Jerman Klen, T. , & Mozetič Vodopivec, B. (2012). Optimisation of olive oil phenol extraction conditions using a high-power probe ultrasonication. Food Chemistry , 134 , 2481–2488.10.1016/j.foodchem.2012.04.096
- Jiao, Y. , & Zuo, Y. (2009). Ultrasonic extraction and HPLC determination of anthraquinones, aloe-emodine, emodine, rheine, chrysophanol and physcione, in roots of Polygoni multiflori . Phytochemical Analysis , 20 , 272–278.10.1002/(ISSN)1099-1565
- Juang, R. S. , & Lin, K. H. (2004). Flux recovery in the ultrafiltration of suspended solutions with ultrasound. Journal of Membrane Science , 24 , 3115–3124.
- Kadkhodaee, R. , & Povey, M. J. W. (2008). Ultrasonic inactivation of Bacillus α-amylase I effect of gas content and emitting face of probe. Ultrasonics Sonochemistry , 15 , 133–142.10.1016/j.ultsonch.2007.02.005
- Kaltsa, O. , Michon, C. , Yanniotis, S. , & Mandala, I. (2013). Ultrasonic energy input influence οn the production of sub-micron o/w emulsions containing whey protein and common stabilizers. Ultrasonics Sonochemistry , 20 , 881–891.10.1016/j.ultsonch.2012.11.011
- Kentish, S. , & Ashokkumar, M. (2011). The physical and chemical effects of ultrasound. In H. Feng , G. V. Barbosa-Canovas , & J. Weiss (Eds.), Ultrasound technologies for food and bioprocessing (pp. 1–12). London: Springer.10.1007/978-1-4419-7472-3
- Khanal, S. K. , Montalbo, M. , van Leeuwen, J. , Srinivasan, G. , & Grewell, D. (2007). Ultrasound enhanced glucose release from corn in ethanol plants. Biotechnology and Bioengineering , 98 , 978–985.10.1002/(ISSN)1097-0290
- Knorr, D. , Froehling, A. , Jaeger, H. , Reineke, K. , Schlueter, O. , & Schoessler, K. (2011). Emerging technologies in food processing. Annual Review of Food Science and Technology , 2 , 203–235.10.1146/annurev.food.102308.124129
- Koda, S. , Miyamoto, M. , Toma, M. , Matsuoka, T. , & Maebayashi, M. (2009). Inactivation of Escherichia coli and Streptococcus mutans by ultrasound at 500kHz. Ultrasonics Sonochemistry , 16 , 655–659.10.1016/j.ultsonch.2009.02.003
- Kyllönen, H. , Pirkonen, P. , Nyström, M. , Nuortila-Jokinen, J. , & Grönroos, A. (2006). Experimental aspects of ultrasonically enhanced cross-flow membrane filtration of industrial wastewater. Ultrasonics Sonochemistry , 13 , 295–302.10.1016/j.ultsonch.2005.04.006
- Lateef, A. , Oloke, J. K. , & Prapulla, S. G. (2007). The effect of ultrasonication on the release of fructosyltransferase from Aureobasidium pullulans CFR 77. Enzyme and Microbial Technology , 40 , 1067–1070.10.1016/j.enzmictec.2006.08.008
- Lee, H. , & Feng, H. (2011). Effect of power ultrasound on food quality. In H. Feng , G. V. Barbosa-Canovas , & J. Weiss (Eds.), Ultrasound technologies for food and bioprocessing (pp. 559–582). London: Springer.10.1007/978-1-4419-7472-3
- Li, H. , Li, H. , & Guo, Z. (2006). The application of power ultrasound to reaction crystallization. Ultrasonics Sonochemistry , 13 , 359–363.10.1016/j.ultsonch.2006.01.002
- Li, J. , Sanderson, R. D. , & Jacobs, E. P. (2002). Ultrasonic cleaning of nylon microfiltration membranes fouled by Kraft paper mill effluent. Journal of Membrane Science , 205 , 247–257.10.1016/S0376-7388(02)00121-7
- Lin, I. , & Erel, D. (1992). Dynamic ultrasonic cleaning and disinfecting device and method . US Patent No. 5113881A. Washington, DC: U.S. Patent and Trademark Office.
- Lin, L. , Wu, J. , Ho, K. P. , & Qi, S. (2001). Ultrasound-induced physiological effects and secondary metabolite (saponin) production in Panax ginseng cell cultures. Ultrasound in Medicine and Biology , 27 , 1147–1152.10.1016/S0301-5629(01)00412-4
- Mason, T. J. , Chemat, F. , & Vinatoru, M. (2011). The extraction of natural products using ultrasound or microwaves. Current Organic Chemistry , 15 , 237–247.10.2174/138527211793979871
- Masselin, I. , Chasseray, X. , Durand-Bourlier, L. , Laine, J. M. , Syzaret, P. Y. , & Lemordant, D. (2001). Effect of sonication on polymeric membranes. Journal of Membrane Science , 181 , 213–220.10.1016/S0376-7388(00)00534-2
- McClements, D. J. (1997). Ultrasonic characterization of foods and drinks: Principles, methods, and applications. Critical Reviews in Food Science and Nutrition , 37 (1), 1–46.10.1080/10408399709527766
- Mirzaie, A. , & Mohammadi, T. (2012). Effect of ultrasonic waves on flux enhancement in microfiltration of milk. Journal of Food Engineering , 108 , 77–86.10.1016/j.jfoodeng.2011.07.026
- Muthukumaran, S. , Kentish, S. E. , Ashokkumar, M. , & Stevens, G. W. (2005). Mechanisms for the ultrasonic enhancement of dairy whey ultrafiltration. Journal of Membrane Science , 258 , 106–114.10.1016/j.memsci.2005.03.001
- Nguyen, T. T. T. , & Le, V. V. M. (2013). Effects of ultrasound on cellulolytic activity of cellulase complex. International Food Research Journal , 20 , 557–563.
- Nithila, S. D. , Anandkumar, B. , Vanithakumari, S. C. , George, R. P. , Mudali, U. K. , & Dayal, R. K. (2014). Studies to control biofilm formation by coupling ultrasonication of natural waters and anodization of titanium. Ultrasonics Sonochemistry , 21 , 189–199.10.1016/j.ultsonch.2013.06.010
- Piyasena, P. , Mohareb, E. , & McKellar, R. C. (2003). Inactivation of microbes using ultrasound: A review. International Journal of Food Microbiology , 87 , 207–216.10.1016/S0168-1605(03)00075-8
- Raviyan, P. , Zhang, Z. , & Feng, H. (2005). Ultrasonication for tomato pectinmethylesterase inactivation: Effect of cavitation intensity and temperature on inactivation. Journal of Food Engineering , 70 , 189–196.10.1016/j.jfoodeng.2004.09.028
- Rezaei, A. , Ghanati, F. , & Dehaghi, M. A. (2011). Stimulation of taxol production by combined salicylic acid elicitation and sonication in Taxus baccata cell culture. In International Conference on Life Science and Technology (pp. 193–197). Singapore: IACSIT press.
- Richard, B. , Toubal, M. , Le Page, J. F. , Nassar, G. , Radziszewski, E. , Nongaillard, B. , … Delaplace, G. (2012). Ultrasound tests in a stirred vessel to evaluate the reconstitution ability of dairy powders. Innovative Food Science and Emerging Technologies , 16 , 233–242.10.1016/j.ifset.2012.06.007
- Rostagno, M. A. , Palma, M. , & Barroso, C. G. (2007). Ultrasound-assisted extraction of isoflavones from soy beverages blended with fruit juices. Analytica Chimica Acta , 597 , 265–272.10.1016/j.aca.2007.07.006
- Rudolf, J. R. , & Resurreccion, A. V. A. (2005). Elicitation of resveratrol in peanut kernels by application of abiotic stresses. Journal of Agricultural and Food Chemistry , 53 , 10186–10192.10.1021/jf0506737
- Sagong, H. G. , Lee, S. Y. , Chang, P. S. , Heu, S. , Ryu, S. , Choi, Y. J. , & Kang, D. H. (2011). Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes on organic fresh lettuce. International Journal of Food Microbiology , 145 , 287–292.10.1016/j.ijfoodmicro.2011.01.010
- Salleh-Mack, S. Z. , & Roberts, J. S. (2007). Ultrasound pasteurization: The effects of temperature, soluble solids, organic acids and pH on the inactivation of Escherichia coli ATCC 25922. Ultrasonics Sonochemistry , 14 , 323–329.10.1016/j.ultsonch.2006.07.004
- Sams, A. R. , & Feria, R. (1991). Microbial effects of ultrasonication of broiler drumstick skin. Journal of Food Science , 56 , 247–248.10.1111/jfds.1991.56.issue-1
- Santos, F. F. P. , Rodrigues, S. , & Fernandes, F. A. N. (2009). Optimization of the production of biodiesel from soybean oil by ultrasound assisted methanolysis. Fuel Processing Technology , 90 , 312–316.10.1016/j.fuproc.2008.09.010
- Scherba, G. , Weigel, R. M. , & Obrien, Jr., W. D. (1991). Quantitative assessment of the germicidal efficacy of ultrasonic energy. Applied and Environmental Microbiology , 57 , 2079–2084.
- Simon, A. , Gondrexon, N. , Taha, S. , Cabon, J. , & Dorange, G. (2000). Low-frequency ultrasound to improve dead-end ultrafiltration performance. Separation Science & Technology , 35 , 2619–2637.
- Soria, A. C. , & Villamiel, M. (2010). Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends in Food Science & Technology , 21 , 323–331.
- Suslick, K. S . (1998). Sonochemistry. In Kirk-othmer encyclopedia of chemical technology (4th ed., Vol. 26, pp. 517–541). New York, NY: Wiley.
- Teh, S. S. , & Birch, E. J. (2014). Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrasonics Sonochemistry , 21 , 346–353.10.1016/j.ultsonch.2013.08.002
- Tiwari, B. K. , & Mason, T. J . (2012). Ultrasound processing of fluid foods. In P. J. Cullen , B. K. Tiwari , V. P. Valdramidis (Eds.), Novel thermal and non-thermal technologies for fluid foods (pp. 135–165). London: Academic Press.10.1016/B978-0-12-381470-8.00006-2
- Valero, M. , Recrosio, N. , Saura, D. , Muñoz, N. , Martí, N. , & Lizama, V. (2007). Effects of ultrasonic treatments in orange juice processing. Journal of Food Engineering , 80 , 509–516.10.1016/j.jfoodeng.2006.06.009
- Vercet, A. , Sánchez, C. , Burgos, J. , Montañés, L. , & Lopez Buesa, P. L. (2002). The effects of manothermosonication on tomato pectic enzymes and tomato paste rheological properties. Journal of Food Engineering , 53 , 273–278.10.1016/S0260-8774(01)00165-0
- Wu, J. , Gamage, T. V. , Vilkhu, K. S. , Simons, L. K. , & Mawson, R. (2008). Effect of thermosonication on quality improvement of tomato juice. Innovative Food Science & Emerging Technologies , 9 , 186–195.
- Yang, B. , Zhao, M. , Shi, J. , Yang, N. , & Jiang, Y. (2008). Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chemistry , 106 , 685–690.10.1016/j.foodchem.2007.06.031
- Yusaf, T. , & Al-Juboori, R. A. (2014). Alternative methods of microorganism disruption for agricultural applications. Applied Energy , 114 , 909–923.10.1016/j.apenergy.2013.08.085
- Zhang, Z. , Niu, Y. , Eckhoff, S. , & Feng, H. (2005). Sonication enhanced cornstarch separation. Starch , 57 , 240–245.