Abstract
Nowadays, baked goods are currently manufactured by accelerated processes. Under these circumstances, proteins are subjected to mild or no degradation during manufacturing. An effort was made to study effect of short-time sourdough fermentation on wheat protein. For this purpose, dough was prepared by mixing refined wheat flour with appropriate amount of water along with 20% curd, 2% yeast and 20% curd + 2% yeast followed by fermentation for 90 min at 37°C to study effect of fermentation. The moisture content of dough decreased with increase in fermentation time. The wet gluten content of dough inoculated with curd, yeast and curd + yeast was 27.01, 28.97 and 28.71%, respectively, and remained unaffected during fermentation. SDS-PAGE revealed that intensities of some of the higher molecular weight protein bands decreased in curd and curd + yeast treated dough. The colour parameters (L, a, b values) remain almost unaltered with fermentation.
Public Interest Statement
Sourdough fermentation is widely used to enhance flavour, texture, shelf life and digestibility of baked goods. Hydrolysis of proteins particularly gliadin occurs during sourdough fementation and this property of sourdough products has been widely studied. Mixture of probiotics has been found more effective for protein degradation, particularly gliadin, than the individual strains, due to which curd, which itself is a mixture of different probiotics, was used to study the effect on protein degradation which can be further enhanced to find a cure for coeliac disease from food technology perspective..
Competing interests
The authors declare no competing interest.
1. Introduction
Sourdough, made from water, flour and microbes such as lactic acid bacteria, is a traditional process used to improve the quality and flavour of baked goods (Flander, Suortti, Katina, & Poutanen, Citation2011; Zotta, Piraino, Ricciardi, McSweeney, & Parente, Citation2006). Sourdough fermentation modulates healthiness of cereals in a number of ways: it improves texture and palatability of whole grain, fibre-rich or gluten-free products, stabilise or increase levels of various bioactive compounds, retard starch bioavailability (low glycaemic index products) and improve mineral bioavailability (Katina et al., Citation2005; Lopez et al., Citation2003; Poutanen, Flander, & Katina, Citation2009; Tucker et al., Citation2010).
Sourdough fermentation increases acid production, which results in increase in activity of enzymes like amalyses and proteases (Flander et al., Citation2011). Significant hydrolysis of gliadin and glutenin proteins occurs during sourdough fermentation which is mainly attributed to the acidity and proteolytic enzymes of wheat rather than the proteolytic activities of sourdough Lactobacilli (Clarke, Schober, Dockery, O’Sullivan, & Arendt, Citation2004; Loponen, Mikola, Katina, Sontag-Strohm, & Salovaara, Citation2004; Thiele, Gänzle, & Vogel, Citation2003; Thiele, Grassl, & Gänzle, Citation2004). Proteolytic activities of lactic acid bacteria play only a minor role in overall proteolytic events during fermentation (Loponen et al., Citation2004; Thiele, Gänzle, & Vogel, Citation2002; Thiele et al., Citation2003).
Fermentation of dough by selected lactic acid bacteria can be used as potential tool to decrease the risk of rye contamination of gluten-free products for celiac patients (De Angelis et al., Citation2006; Rizzello et al., Citation2007). Probiotic mixture can be more effective in hydrolysis of gliadin than the individual strains (Angelis et al., Citation2006).
Baked goods are currently manufactured by accelerated processes where long-time fermentation by sourdough, a cocktail of acidifying and proteolytic lactic acid bacteria with yeasts were replaced by the indiscriminate use of chemicals (Angelis et al., Citation2006). Under these circumstances, proteins are subjected to mild or no degradation during manufacturing, probably resulting in lower digestible foods compared to traditional and ancient sourdough baked goods (Gobbetti, Citation1998).
The aim of the present study was to investigate the effect of short-time sourdough fermentation on wheat protein using curd as a source of lactic acid bacteria.
2. Materials and methods
2.1. Raw material
Refined wheat flour having a moisture content 11.37 ± 0.39%, fat 1.25 ± 0.09%, ash 0.63 ± 0.01% and protein content 11.48 ± 0.77% was procured from local market. Curd having a moisture content 87.30 ± 0.28%, protein 3.57 ± 1.35%, fat 3.0 ± 0.56%, ash 0.8 ± 0.02%, pH 3.47 ± 0.07 and titratable acidity 0.138 ± 0.01% was procured from Verka Milk Plant, Amritsar, India. Dried instant yeast having a moisture content 5.32 ± 0.26%, protein 44.33 ± 2.02%, fat 6.68 ± 0.37% and ash content 6.47 ± 0.62% was bought from the local market, Amritsar, India.
2.2. Dough preparation
Dough was prepared using different ingredients as follows:
Control Dough (CN): Refined wheat flour mixed with appropriate amount of water to form dough served as a control.
Curd Dough (CD): Refined wheat flour was mixed with 20% of curd and appropriate amount of water to form dough.
Yeast Dough (YD): Refined wheat flour mixed with 2% yeast and required amount of water to form dough.
Curd + Yeast Dough (CYD): Refined wheat flour was mixed with required amount of water, 2% yeast and 20% curd to make dough.
The fermentation was carried for 90 min at 37°C for all the treatments.
2.3. Physicochemical properties
2.3.1. Composition
Moisture (925.10) and protein (920.87) contents were determined according to the methods of Association of Official Analytical Chemists (AOAC, Citation1990).
2.3.2. Gluten content (American Association of Cereal Chemists, Citation2000, pp. 38–10)
Wet and dry gluten contents in different flour samples were determined by hand washing method American Association of Cereal Chemists (Citation2000).
2.4. Protein extraction
Wheat proteins were extracted sequentially from dough using the method of Concon, Newburg, and Eades (Citation1983), with slight modification. Dough (5 g) was extracted with 20 ml of 0.9% NaCl in 0.02 M phosphate buffer (pH 6.81) for 1 h at 4°C with vortexing at 15 min interval and centrifuged at 8,000 g at 15°C for 10 min. The pellet was extracted twice again with 0.9% NaCl in 0.02 M phosphate buffer (pH 6.81). The supernatant of these three extractions was combined, representing the albumin/globulin protein fraction. The pellet was washed with water to remove salt and then extracted thrice with 20 ml of 70% ethanol (v/v) and centrifuged as described above. The supernatant collected contained gliadin. Finally, the pellets were extracted thrice with 0.075 N NaOH solution and centrifuged as above. The supernatant contained glutenin. All the protein fractions were freeze-dried (Heto PowerDry, Allerod, Denmark) and stored in airtight plastic vials at −20°C.
The protein content in each fraction was determined by the Kjeldahl’s method (N × 5.70) following AOAC (Citation1990).
2.5. SDS-page analysis
Freeze-dried gliadin (5 mg) was re-suspended in 1 ml 2X Laemmli sample buffer solution of pH 6.8 containing 62.5 mM Tris–HCl, 2% SDS, 25% glycerol, 0.01% bromophenol blue. Samples were reduced by 5% ß-mercaptoethanol, incubated for 90 min at 45°C. Then the dispersions were heated in boiling water for 5 min, after centrifugation at 11,000 g for 20 min at 4°C, the supernatant (10 μL for each lane) was used for SDS-PAGE analysis (Mini-Protean 3, Bio-Rad Laboratories, Hercules, USA). Proteins were separated using 4% stacking gel and 12% resolving gel. Runs were performed at 25 mA. Gels were stained overnight with Coomassie Brilliant Blue-R250 and destained using 20% methanol and 10% acetic acid. The stained gels were scanned and molecular weights were determined using Bio-Rad EZ imager (Bio-Rad Laboratories, Hercules, USA).
2.6. Colour measurement
The colour of dough samples was determined using Ultra-scan VIS Hunter Colour lab (Hunter Associates, Reston, USA) following the method of Wani, Sogi, Shivhare, and Gill (Citation2015). Calibration was done using black and white tiles before colour measurement. Colour reading were expressed as “L” for lightness–darkness, “a” for redness–greenness, and “b” for yellowness–blueness.
2.7. Statistical analysis
Analysis of variance (ANOVA) was carried out using Microsoft Excel Software. Fishers least significant difference (LSD) test was used to describe significant difference (p ≤ 0.05).
3. Results and discussion
3.1. Moisture content
Moisture content of the dough samples incubated at 37°C is shown in Table . The initial moisture content of CN, CD, YD and CYD were 43.07, 41.35, 41.15 and 36.97%, respectively. The variation in moisture content in different dough samples might be because of different ingredients added, their water absorption capacity and amount of water added. Ijah, Auta, Aduloju, and Aransiola (Citation2014) also obtained different moisture content in breads when wheat flour was incorporated with varying percentage of potato flour. The moisture content of all dough samples decreased significantly (p ≤ 0.05) with increase in fermentation time. In control dough, it decreased to 39.10%, whereas treated samples, it decreased to 35.88–32.26% after 90 min of fermentation. There was significant difference in moisture content of treated samples (p ≤ 0.05). Loss of moisture is attributed to the evaporation from dough surface during fermentation.
Table 1. Moisture content of curd, yeast and curd + yeast containing dough fermented at 37°C
3.2. Gluten content
Effect of fermentation on wet gluten content of various dough samples is shown in Table . CN contained 27.33% gluten in the beginning and remained unchanged when it was allowed to ferment at 37°C for 90 min. The gluten content, in CD, YD and CYD samples, was 28.97, 27.01 and 28.71%, respectively. After 90 min of fermentation, the wet gluten content for CD, YD, and CYD samples was 27.1, 30.2 and 28.93%, respectively. Statistical analysis showed that wet gluten content did not change significantly (p ≥ 0.05) with time of fermentation; however, it was significantly (p ≤ 0.05) different in various treated samples. Rizzello et al. (Citation2014) observed that degradation of gluten is directly proportional to fementation time. Thiele et al. (Citation2004) observed that proteolytic degradation of glutenin subunits was observed only after 6 h and was more pronounced after 24 h of fermentation. Wheat proteins become more susceptible to proteolysis from proteins to peptides under acidic conditions (Gänzle, Loponen, & Gobbetti, Citation2008; Tuukkanen, Loponen, Mikola, Sontag-Strohm, & Salovaara, Citation2005). Acidic conditions also favour the activation of wheat flour enzymes such as aspartic proteinases and carboxypeptidase (Loponen et al., Citation2004; Tuukkanen et al., Citation2005).
Table 2. Wet gluten content of control, yeast, curd and yeast + curd dough samples fermented at 37°C temperature
Thiele et al. (Citation2004) reported that proteolysis and gluten degradation during sourdough fermentation was due to pH-mediated activation of cereal enzymes. The native proteases of flour degrade cereal prolamins under acid conditions (Loponen et al., Citation2004). Short-time fermentation did not result in pH decrease; therefore, the native proteases of flour did not get activated which can degrade the gluten proteins.
Dry gluten content in CN dough was 9.4% in the beginning (Table ) and after fermentation of 90 min it was 9.02%. The dry gluten content in CD, YD and CYD was 10.21, 9.57 and 10.31%, respectively, in the beginning; however, after 90 min of fermentation it remained unchanged. It indicated that short fermentation time of 90 min was not sufficient to bring degradation in gluten proteins. Statistical analysis followed similar trend as in case of wet gluten. The present findings on wet and dry gluten content are in agreement with previous studies.
Table 3. Dry gluten content of control, curd, yeast, curd + yeast containing dough fermented at 37°C
3.3. Protein fractionation
The proteins of control and treated samples were fractionated based on their solubility (Table ). Albumin + globulin, gliadin and glutenin fractions of control and treated doughs were observed to be in the range of 1.22–3.51; 3.21–4.26; and 4.68–4.89 g/100 g, respectively. The albumin + globulin content in CD, YD and CYD samples was 3.40 g/100 g, 2.61 g/100 g and 3.51 g/100 g, respectively. The higher content of albumin + globulin in treated samples might be due to different ingredients used which during sequential extraction may have come in supernatant and resulted in higher protein content. The gliadin content in CN, CD, YD and CYD was 3.21 g/100 g, 4.26 g/100 g, 4.31 g/100 g and 3.62 g/100 g, respectively. The glutenin content in CN, CD, YD and CYD was 4.82 g/100 g, 4.89 g/100 g, 4.73 g/100 g and 4.68 g/100 g, respectively. The difference in protein content might be due to use of different ingredients for dough making. The residue content was highest in CN followed by YD, CYD and CD and was 2.20 g/100 g 1.30 g/100 g, 1.28 g/100 g and 0.48 g/100 g, respectively.
Table 4. Protein fractions of control, curd, yeast, curd + yeast containing dough fermented at 37°C
Present study further revealed that albumin + globulin constitute 10.68%, gliadin 28.04%, glutenin 42.04% of the total grain protein in case of control sample. Therefore, the gluten content was 70.08% of the total protein. Previous study showed that gluten proteins constituted about 63–70% of total wheat protein (Emanuelson, Wollenweber, Jorgensen, Andersen, & Jensen, Citation2003). Thus, present findings are in accordance with the previous studies. Soluble protein recovery was 80.76%, while previous reports indicated 88% recovery of soluble proteins using the similar procedure (Concon et al., Citation1983). The lower recovery might be due to difference in cultivars and extraction protocols. The relative proportion of the fraction was remained unaffected by the fermentation by curd, yeast or their combination.
3.4. SDS-PAGE of gliadins
The level of proteolysis was evaluated by SDS-PAGE analysis. gliadin is the major protein which is responsible for immune reaction leading to celiac disease. The peptides were analysed for the effect of fermentation process on 12% resolving gel with Coomassie Brilliant Blue staining technique. CN at 0 min fermentation resolved in 13 subunits with molecular weights of 109.4, 75.9, 48, 44.4, 41.3, 39, 30.1, 20.9, 19.5, 18.4, 17.1, 11.3 and 11 kDa (Figure ). gliadin of CN contained major polypeptides with molecular masses in the range of 44.4–30.1 kDa. The pattern of bands remained same after 60 and 90 min of fermentation (Figure ).
Figure 1. SDS-PAGE analysis of gliadin polypeptides from dough-treated samples incubated for 0, 60 and 90 min at 37°C.
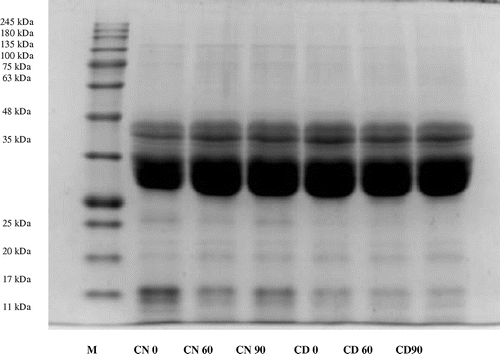
Curd-fermented dough (Figure ) contained polypeptides with molecular weights ranging from 104.6 to 11 kDa in beginning of fermentation process. After 60 min of fermentation, the intensity of bands of 73.5 kDa decreased, however, the effect was more pronounced after 90 min of fermentation with the disappearance of bands in the range of 104.6–73.6 kDa and 20.4 kDa. Angelis et al. (Citation2006) found that gliadin degradation was more pronounced when mixture of probiotic preparations were used rather than individual strains. The results are as per the previous reports.
Yeast-fermented dough (Figure ) had polypeptides in the range of 110.5–11.0 kDa. No change was observed in gliadin profile even after 60 min and 90 min of fermentation. Di Cagno et al. (Citation2004) found that dough fermented with baker’s yeast had the same prolamin profile as that of flour after 2 h of fermentation. Zotta et al. (Citation2006) also reported that proteolysis by yeast was negligible. Thus, the findings of present study are in agreement with the previous reports.
Figure 2. SDS-PAGE analysis of gliadin polypeptides from dough treated samples incubated for 0, 60 and 90 min at 37°C.
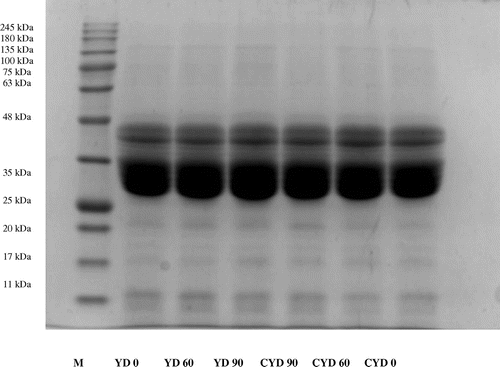
Curd and yeast-fermented dough (Figure ) resolved into subunits with molecular weights range of 110.5–11 kDa. CYD showed also a decrease in intensity in higher molecular weights in the range of 110.5–74.6 kDa in 90 min fermented sample. Gocmen, Gurbuz, Kumral, Dagdelen, and Sahin (Citation2007) observed the protein bands getting fainter in the range of 95–104 kDa while studying the effect of wheat sourdough on glutenin patterns. Kumral (Citation2015) found gliadin band intensities becoming less intense during fermentation.
The differences between SDS-PAGE electropherograms of control and treated dough indicated that lactic acid bacteria have major effect on the banding pattern of the peptides by hydrolysing them depending upon the time. Zotta et al. (Citation2006) also reported that proteolysis by yeast was negligible compared to the activity of LAB and indigenous flour enzymes. Thiele et al. (Citation2004) reported that after 24 h of acid control fermentation, the band intensity of individual high molecular weight glutenins decreased. The results find support from the previous studies that lactic acid bacteria and fermentation time play an important role in hydrolysis of proteins.
3.5. Colour
Colour parameters of control and treated samples are shown in Table . The “L” value for CN at 0 min was 80.53 and it decreased to 78.53 after 90 min of fermentation indicating that dough became a little darker as the fermentation proceeded. The “a” and “b” values of CN sample ranged between 1.51 and 1.28 and 14.62–14.08, respectively. The “a” value for CN at 0 min was 1.51 and after 90 min fermentation it decreased slightly and was 1.28. It indicated decrease in redness. The “b” value for CN at 0 min fermentation was 14.62 and decreased slightly with increase in fermentation time indicating decrease in yellowness.
Table 5. Colour parameters of dough samples fermented at 37°C
The “L” value in CD remained almost constant and ranged from 77.72–78.50. The “a” value CD was 0.93 at 0 min of fermentation and increased with time to 1.38 after 90 min. The “b” value was 13.78 at 0 min fermentation and increased to 15.13 after 90 min fermentation. It indicated increase in both redness and yellowness of the dough on fermentation by lactic acid bacteria. The “L”, “a” and “b” values of YD sample ranged from 73.79–76.59, 1.76–1.92 and 16.06–16.66, respectively. YD had the lowest “L” value when compared with other treated samples. The “L” value in YD decreased to 73.79 after 90 min fermentation indicating decrease in brightness. The “a” value increased with fermentation and was 1.92 after 90 min while “b” remained almost constant indicating increase in redness. YD had the highest “a” and “b” value. CYD had a “L” value of 80.60 at 0 min of fermentation and it decreased to 78.64 after 90 min of fermentation. The “L” value of CYD was highest among all treatments. The “a” value remained constant and ranged from 1.35–1.39. The “b” value also remained constant and ranged from 15.79–15.84. It hinted that the dough did not change its redness or yellowness during fermentation.
Bilgiçli and İbanoğlu (Citation2007) reported that fermentation decreased “L”, “a” and “b” values resulting in darker, more green–less reddish and more blue–less yellow samples in tarhana, a wheat flour–yoghurt mixture. In the present study, the variation in colour parameters was low which might be due to short fermentation time.
4. Conclusion
The moisture content of the dough decreased with fermentation time. Short-time sourdough fermentation proved ineffective in decreasing gluten content which remained almost unchanged after 90 min of fermentation in all the treated samples. SDS-PAGE revealed the intensity of high-molecular weight protein bands in the range of 109–76 kDa decreased with increase in fermentation time in case of curd and curd + yeast; however, no change was observed in YD even after 90 min fermentation.
Additional information
Funding
Notes on contributors
Raushid Ahmad Siddiqi
Raushid Ahmad Siddiqi has done MSc in food technology and in now pursuing PhD in food technology from Guru Nanak Dev University. His area of research is wheat protein.
References
- American Association of Cereal Chemists. (2000). Cereal laboratory methods (10th ed., pp. 38–10). St Paul, MN.
- Angelis, M., Rizzello, C. G., Fasano, A., Clemente, M. G., Simone, C., Silano, M., … Gobbetti, M. (2006). VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for celiac sprue probiotics and gluten intolerance. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1762, 80–93.10.1016/j.bbadis.2005.09.008
- Association of Official Analytical Chemists. (1990). Official methods of analysis (15th ed.). Washington, DC.
- Bilgiçli, N., & İbanoğlu, Ş. (2007). Effect of wheat germ and wheat bran on the fermentation activity, phytic acid content and colour of tarhana, a wheat-flour yoghurt mixture. Journal of Food Engineering, 78, 681–686.10.1016/j.jfoodeng.2005.11.012
- Clarke, C. I., Schober, T. J., Dockery, P., O’Sullivan, K., & Arendt, E. K. (2004). Wheat sourdough fermentation: Effects of time and acidification on fundamental rheological properties. Cereal Chemistry, 81, 409–417.10.1094/CCHEM.2004.81.3.409
- Concon, J. M., Newburg, D. S., & Eades, S. N. (1983). Lectins in wheat gluten proteins. Journal of Agricultural and Food Chemistry, 31, 939–941.10.1021/jf00119a004
- De Angelis, M., Coda, R., Silano, M., Minervini, F., Rizzello, C. G., Di Cagno, R., … Gobbetti, M. (2006). Fermentation by selected sourdough lactic acid bacteria to decrease coeliac intolerance to rye flour. Journal of Cereal Science, 43, 301–314.10.1016/j.jcs.2005.12.008
- Di Cagno, R., De Angelis, M., Auricchio, S., Greco, L., Clarke, C., De Vincenzi, M., … Gobbetti, M. (2004). Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Applied Environmental Microbiology, 70, 1088–1096.10.1128/AEM.70.2.1088-1096.2004
- Emanuelson, J., Wollenweber, B., Jorgensen, J. R., Andersen, S. B. F., & Jensen, C. R. (2003). Wheat grain composition and implications for bread quality ( DIAS Report Plant Production No. 92). Tjele: Danish Institute of Agricultural Sciences.
- Flander, L., Suortti, T., Katina, K., & Poutanen, K. (2011). Effects of wheat sourdough process on the quality of mixed oat-wheat bread. LWT- Food Science and Technology, 44, 656–664.10.1016/j.lwt.2010.11.007
- Gänzle, M. G., Loponen, J., & Gobbetti, M. (2008). Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends in Food Science and Technology, 19, 513–521.10.1016/j.tifs.2008.04.002
- Gobbetti, M. (1998). The sourdough microflora: Interactions of lactic acid bacteria and yeasts. Trends in Food Science and Technology, 9, 267–274.10.1016/S0924-2244(98)00053-3
- Gocmen, D., Gurbuz, O., Kumral, A. Y., Dagdelen, A. F., & Sahin, I. (2007). The effects of wheat sourdough on glutenin patterns, dough rheology and bread properties. European Food Research and Technology, 225, 821–830.10.1007/s00217-006-0487-6
- Ijah, U. J. J., Auta, H. S., Aduloju, M. O., & Aransiola, S. A. (2014). Microbiological, nutritional and sensory quality of bread produced from wheat and potato flour blends. International Journal Food Science. doi:10.1155/2014/671701
- Katina, K., Arendt, E., & Liukkonen, K., Autio, K., Flander, L., & Poutanen, K. (2005). Potential of sourdough for healthier cereal products. Trends in Food Science and Technology, 16, 104–112.10.1016/j.tifs.2004.03.008
- Kumral, A. (2015). Nutritional, chemical and microbiological changes during fermentation of tarhana formulated with different flours. Chemistry Central Journal, 9, 16. doi:10.1186/s13065-015-0093-4
- Lopez, H. W., Duclos, V., Coudray, C., Krespine, V., Feillet-Coudray, C., Messager, A., … & Rémésy, C. (2003). Making bread with sourdough improves mineral bioavailability from reconstituted whole wheat flour in rats. Nutrition, 19, 524–530.10.1016/S0899-9007(02)01079-1
- Loponen, J., Mikola, M., Katina, K., Sontag-Strohm, T., & Salovaara, H. (2004). Degradation of HMW glutenins during wheat sourdough fermentations. Cereal Chemistry, 81, 87–93.10.1094/CCHEM.2004.81.1.87
- Poutanen, K., Flander, L., & Katina, K. (2009). Sourdough and cereal fermentation in a nutritional perspective. Food Microbiology, 26, 693–699.10.1016/j.fm.2009.07.011
- Rizzello, C. G., Curiel, J. A., Nionelli, L., Vincentini, O., Di Cagno, R. D., Silano, M., … Coda, R. (2014). Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiology, 37, 59–68.10.1016/j.fm.2013.06.017
- Rizzello, C. G., De Angelis, M., Di Cagno, R., Camarca, A., Silano, M., Losito, I., … Gobbetti, M. (2007). Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: New perspectives for celiac disease. Applied and Environmental Microbiology, 73, 4499–4507.10.1128/AEM.00260-07
- Thiele, C., Gänzle, M. G., & Vogel, R. F. (2002). Contribution of sourdough lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavor. Cereal Chemistry, 79, 45–51.10.1094/CCHEM.2002.79.1.45
- Thiele, C., Gänzle, M. G., & Vogel, R. F. (2003). Fluorescence labeling of wheat proteins for determination of gluten hydrolysis and depolymerization during dough processing and sourdough fermentation. Journal of Agricultural and Food Chemistry, 51, 2745–2752.10.1021/jf020897e
- Thiele, C., Grassl, S., & Gänzle, M. (2004). Gluten hydrolysis and depolymerization during sourdough fermentation. Journal of Agricultural and Food Chemistry, 52, 1307–1314.10.1021/jf034470z
- Tucker, A. J., MacKay, K. A., Robinson, L. E., Graham, T. E., Bakovic, M., & Duncan, A. M. (2010). The effect of whole grain wheat sourdough bread consumption on serum lipids in healthy normoglycemic/normoinsulinemic and hyperglycemic/hyperinsulinemic adults depends on presence of the APOE E3/E3 genotype: A randomized controlled trial. Nutrition and Metabolism, 7, 37–49.10.1186/1743-7075-7-37
- Tuukkanen, K., Loponen, J., Mikola, M., Sontag-Strohm, T., & Salovaara, H. (2005). Degradation of secalins during rye sourdough fermentation. Cereal Chemistry, 82, 677–682.10.1094/CC-82-0677
- Wani, I. A., Sogi, D. S., Shivhare, U. S., & Gill, B. S. (2015). Physico-chemical and functional properties of native and hydrolyzed kidney bean (Phaseolus vulgaris L.) protein isolates. Food Research International, 76, 11–18.10.1016/j.foodres.2014.08.027
- Zotta, T., Piraino, P., Ricciardi, A., McSweeney, P. L. H., & Parente, E. (2006). Proteolysis in model sourdough fermentations. Journal of Agricultural and Food Chemistry, 54, 2567–2574.10.1021/jf052504s
