?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pumpkin (Cucurbita moschata) is highly nutritious and antioxidant-rich vegetable widely grown all over the world. Present study reports the effect of storage on physicochemical, microbial, and antioxidant properties of pumpkin candy. Pumpkin and its candy were analyzed for the physicochemical characteristics like moisture content, ash, total soluble solids (TSS), titrable acidity, total sugar, reducing sugar, and color. Beta-carotene and vitamin-C content of pumpkin and its candy were also studied. Antioxidant properties like 1, 1-Diphenyl-2-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), total phenolic content (TPC), reducing power, and lipid peroxidation of methanolic extracts of pumpkin and processed candy were evaluated. During storage, a significant increase in TSS while a non-significant increase in titrable acidity, reducing and total sugars was observed. Beta-carotene, vitamin C, color, and antioxidant properties (DPPH, FRAP, TPC, reducing power, and lipid peroxidation) also showed a non-significant decrease during storage at ambient temperature. Microbial load of pumpkin candy (1.74–3.2 log cfu/g) suggested that candies were safe for human consumption during storage. Hence, candy preparation from pumpkin could be an effective method for preservation of pumpkin and retention of its bioactive components.
Public Interest Statement
Pumpkin (Cucurbita moschata) is highly nutritious and antioxidant-rich vegetable widely grown all over the world. It is a rich source of vitamin A with high amounts of carotenoids, especially β-carotene and lutein. Vitamin C and beta-carotene are present in abundance in pumpkin and impart high antioxidant potential to it. Vitamin C and carotene content of pumpkin can be retained by processing pumpkin into candies. Candy production not only improves the shelf life of vegetable but also maintains its antioxidant potential.
1. Introduction
Pumpkin (Cucurbita moschata) is one of the important cucurbitaceous vegetable widely grown all over Asia. It is a seasonal crop that has been used both as human as well as animal feed. C. moschata is eaten as vegetable and cultivated for its young shoots, flesh, edible flowers, and fruits. The pulp color can vary considerably from brown, completely white, bright orange to greenish light. It can be very sweet, smooth, and usually non-fibrous. Jun, Lee, Song, and Kim (Citation2006) reported high amounts of pectin, mineral salts, carotene, vitamins, and other substances beneficial to human health in C. moschata,. It is a rich source of vitamin A (20 ± 4 mg/g) with high amounts of carotenoids, especially β-carotene and lutein (González, Montenegro, Nazareno, & Lopez de Mishima, Citation2001; Rodriguez-Amaya, Kimura, Godoy, & Amaya-Farfan, Citation2008). Other carotenoids are α-Carotene and minor carotenoides, ζ-carotene (González et al., Citation2001), zeaxanthin, violaxanthin (Rodriguez-Amaya et al., Citation2008), β-carotene 5, 6-epoxide, β-cryptoxanthin, taraxanthin, luteoxanthin, auroxanthin, phytofluene, neurosporene, and neoxanthin (González et al., Citation2001). Epidemiological evidence suggests that a diet rich in carotenoids is associated with enhancement of the immune response and reduction of the risk of degenerative diseases such as cancer, cardiovascular diseases, atherosclerosis, cataracts, and age-related macular degeneration (González et al., Citation2001; Rodriguez-Amaya, Citation2003). The carotenoids protect cells from photoxidation, damage to DNA molecules and lipids, and deactivate singlet oxygen that is mutagenic (González et al., Citation2001). It is a rich source of vitamin C that is associated with the prevention of various degenerative, cardiovascular, and neurological diseases. It has been used traditionally as medicine in many countries such as China, Yugoslavia, Argentina, India, Mexico, Brazil, and America. C. moschata is popularly used in several countries to control diabetes as well as for treating worms and parasites. Consumption of pumpkin is also associated with antihypertension, immunomodulation, antihypercholesterolemia, antitumor, anti-inflammatory, and antalgic responses (Fu, Shi, & Li, Citation2006). González et al. (Citation2001) associated health benefits of C. moschata to antioxidant activity that is often attributed to polyphenolic compounds.
Cooked pumpkin has a limited shelf life in terms of nutritional as well as microbial quality and cooking is usually associated with the loss of vitamins and degradation of polyphenols. Consumption of such kinds of food can result in occurrence of various several food-borne diseases. In wake of consumers towards nutritional and nutraceutical deterioration of food during processing, researchers are focusing on methods that involve minimal processing to retain the bioactive profile of food sources. Thus the proposed investigation was undertaken to minimally process pumpkin into a candy and study its physicochemical, microbial, and antioxidant properties during a storage period of two months.
2. Materials and methods
2.1. Raw material
Fully ripe pumpkin was purchased from the local farms of Srinagar. Other materials like sugar and polyethylene pouches were also purchased from local market.
2.2. Development of candy
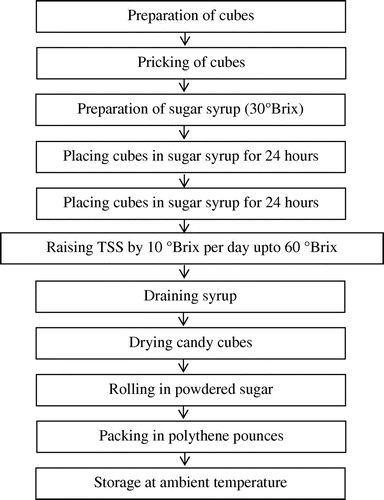
The pumpkin fruit were washed, cut into slices, peeled and after removing seeds, slices were cut into cubes of 1.5 cm. The method for preparation of candy from pumpkin cubes was standardized by following the basic method given by Lal, Sidappa, and Tandon (Citation2010). The unit operation followed is given in Figure .
The fresh pumpkin fruit and pumpkin candy were analyzed for different parameters discussed as under:
2.3. Proximate composition
2.3.1. Moisture content
Moisture was estimated by drying the weighed sample (5 g) to a constant weight in a hot air oven (Association of Official Analytical Chemists [AOAC], Citation2000). The dried samples were then cooled to room temperature in a desiccator prior to weighing. The percent moisture content was calculated as follows:
Where, W1 = Initial weight of empty crucible,
W2 = Weight of crucible + sample before drying and
W3 = Final weight of crucible + sample after
2.3.2. Ash content
Total ash content was determined gravimetrically (Rasool et al., Citation2015) by taking known weight of samples in tarred silica crucible. The dried samples after moisture determination were slowly heated on hot plate until the bulk of organic matter was burnt. The crucibles were then placed in a muffle furnace for ashing at 550°C to obtain a carbon free white ash with a constant weight. Ash content of sample was then calculated and expressed as percent on fresh weight basis:
2.3.3. Pectin content
The method described by Ranganna (Citation1997) was followed for determination of pectin content. Pectin in the samples was saponified with alkali and precipitated as calcium pectate from an acid solution by the addition of calcium chloride. Calcium pectate precipitated was filtered and washed until made free from chlorides, dried and weighed. The pectin content in the given sample was calculated and expressed as percent calcium pectate as given below:
2.3.4. Fiber content
Distilled water (200) was added to a sample weighing 100 g and the contents were brought nearly to a boil. After adding 25 ml of sodium hydroxide solution the contents were boiled for five minutes (Gould, Citation1978). The material was transferred to previously weighed screen and washed thoroughly with water until whole of sodium hydroxide had been removed. The presence of sodium hydroxide was checked by phenolphthalein indicator. The contents were dried for 2 h at 100°C in a hot air oven and fiber content was expressed in percentage.
2.4. Physicochemical characteristics
2.4.1. Color
Color was measured by a colorimeter (12MM Aperture U 59730 Inc., Pittsford, New York, USA) and recorded in the L*, a*, b* color system. This color system consists of a luminance or lightness compound (L*) and two chromatic compounds: a* component for green (-a) to red (+a) and the b* component from blue (-b) to yellow (+b) colors.
2.4.2. TSS (Total soluble sugar), TA (Titrable acidity), and pH
The TSS was determined with the help of hand refractometer and expressed as ∘Brix at 20°C using reference table for temperature correction (Ranganna, Citation1997). Titratable acidity was estimated by titrating a known volume of the sample against 0.1 N NaOH solution using phenolphthalein as an indicator (AOAC, Citation2000). pH was determined using ELTOP 3030 digital pH meter after calibrating with a buffer solution of pH 4.0 and 9.2, following the operational instructions of the instrument.
2.4.3. Sugars
Sugars were estimated by Lane and Eynon’s (1923) volumetric method and sample was prepared for different products as per the method given by Ranganna (Citation1997). The sample was neutralized with 1 N NaOH, using phenolphthalein as an indicator. To this, 2 ml of neutralized lead acetate was added and the solution was kept for 10 min. After that, necessary amount of potassium oxalate solution was added to precipitate excess of lead, made up to desired volume and filtered. The filtered was used for the estimation of reducing and total sugar.
2.4.4. Total sugars
Clarified filtrate (50 ml) was taken into 250-ml conical flask 5 g citric acid and 50 ml water was added to it. The mixture was boiled gently for 10 minutes and then cooled. The mixture was then neutralized with 1 N NaOH, using phenolphthalein indicator and volume was made up to 250 ml. Total sugars were then determined in a same way as reducing sugars.
2.4.5. Carotenoids
Sample of 1 g was dissolved in the solvent (acetone) and ground till the whole color was extracted, then the liquid was transferred into a separating funnel. Separated colored portion was collected after adding petroleum ether and 3% sodium sulfate solution. The final volume was made to 25 ml. The optical density was taken at 449 nm and the reading was compared with the standard curve (Ranganna, Citation1997). The quantity of carotenoids was calculated using the following formula:
2.5. Antioxidants
2.5.1. Sample extraction
Two grams of sample were weighed accurately and added with 50 ml of aqueous methanol. It was then kept for overnight on magnetic stirrer for 2hrs, then centrifuged on 3,500 rpm for 10 min. The supernatant was recovered and stored for analysis.
2.5.2. Determination of TPC (total phenol content)
The concentration of total phenolics was measured using the method described by Nisar, Baba, and Masoodi (Citation2015), with some modifications. Briefly, an aliquot (300μL) of extract was added to a Folin and Ciocalteu’s phenol reagent and then distilled water and was shaken. After 5 min, Na2CO3 solution was added and the mixture was kept at room temperature for 2 h in the dark. The absorbance versus blank was read at 760 nm. Total phenolic content of extracts were expressed as mg gallic acid equivalents (GAE)/10 g raw pumpkin pulp.
2.5.3. DPPH (1, 1-Diphenyl-2-picrylhydrazyl)
The ability of extracts to scavenge the DPPH radical was measured using the method of Baba et al. (Citation2014) with some modifications. 1,1-Diphenyl-2-picrylhydrazyl radical scavenging activity was determined by preparing 0.08% of DPPH that is 6 mg in 75 ml of methanol. Extract (200μL) was added with 1 ml of 0.08% DPPH, followed by the addition of methanol, respectively. The mixture was vortexed vigorously. The absorbance at 517 nm was measured after incubation in dark for 30 min.
The % inhibition was calculated by the following formula:
where Ac = Absorbance of control at 517 nm; As = Absorbance of sample at 517 nm.
2.5.4. Reducing power
The reducing power of extracts was determined according to the method of Nisar et al. (Citation2015). Extract (200μL) in 1 ml of distilled water was mixed with phosphate buffer (2.5 ml, 0.2 mol/L, pH 6.6) and potassium ferricyanide [K3Fe (CN)6] (2.5 ml, 1%). The mixture was incubated at 50°C for 20 min. A portion (2.5 ml) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3,000 rpm (MSE Mistral 2000, UK) for 10 min. The upper layer of solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1%), and the absorbance was measured at 700 nm.
% reduction was determined by using the following formula:
where: Ac = Absorbance of control at 700 nm; As = Absorbance of sample at 700 nm
2.5.5. Ferric reducing antioxidant power
The total antioxidant potential of extracts was determined using the ferric reducing antioxidant power (FRAP) assay of (Jan et al., Citation2015). A solution of 40 mmol/L HCl and 12 m mol/L ferric chloride was diluted in 300 mmol/L sodium acetate buffer (pH 3.6) at a ratio of 1:1:10. Aliquot (300μL) of extract solutions were added to 3 ml FRAP solution, and allowed to react for 30 min, and the absorbance was taken at 700 nm.
2.5.6. Lipid peroxidation
It was performed according to the method of Wright, Colby, and Miles (Citation1981). The reaction on the total volume of 2 ml, containing 1 ml of linoleic acid, 0.2 ml of ferric nitrate (20 mm), 0.2 ml of ascorbic acid (200 mm), and 0.2 ml of H2O2 (300 mm), 0.2 ml of ddH2O and 200μL of extract. It was followed by incubation at 37°C for 1 h in an incubator. The reaction was stepped by the addition of 1 ml TCA (trichloroacetic acid 10%W/V) followed by 1 ml TBA (1%W/V) and the tubes were placed in water bath preset at 100°C for 10 min. The tubes were then centrifuged at 5000 rpm for 10 min and the absorbance was recorded at 533 nm.
Percent inhibition was taken using the formula:
where Ac = Absorbance of control at 533 nm; As = Absorbance of sample at 533 nm.
2.6. Microbial analysis
Total plate count was determined as per the standard method of (Ranganna, Citation1997) using nutrient agar medium. A primary 10-fold dilution was prepared by homogenizing the sample in sterile saline (0.85w/v) and serially diluted (10−1 to 10−7) in the same diluents. One millimeter (1 mL) of these dilutions was poured plated on the nutrient agar plates. The plates were then incubated at 37°C for 24 h prior to counting. The results of the total plate count were expressed as log of colony forming units per gram (log cfu/g) of sample.
2.7. Sensory analysis
Candy samples were subjected to sensory analysis i.e. Color, odor, texture, and taste using nine-point hedonic scale by nine trained panelists that included staff and faculty of Department of Food Science and Technology, University of Kashmir, India. Before the sensory evaluation was conducted, the panelists were trained using commercial apple pulp to get familiar with the use of rating method, terminology for each attribute and sensory characteristics. The judges rated the quality characteristics of each sample on a nine-point hedonic rating scale where, 9 = like extremely, 8 = like very much, 7 = like moderately, 6 = like slightly, 5 = neither like nor dislike, 4 = dislike slightly, 3 = dislike moderately, 2 = dislike very much, and 1 = dislike extremely. The judges evaluated randomly coded candy samples in terms of color, odor, texture, and taste. Assessors were instructed to cleanse their palate with cold, filtered tap water before tasting each sample. Product characterization was carried out under daylight illumination and in isolated booths within a sensory laboratory.
2.8. Statistical analysis
Results were expressed as mean of triplicate analyses. ANOVA and Duncan’s test were used to establish the significance of differences among the mean values at the 0.05 significance level. The statistical analyses were performed using SPSS software (Systat statistical program version 21 (SPSS Inc., USA).
3. Results and discussions
3.1. Physicochemical characteristics and antioxidant activity of fresh pumpkin fruit (C. moschata)
The data on physicochemical characteristics and anti-oxidant activity of pumpkin are shown in Table . The data indicated that pumpkin fruit recorded the moisture content and ash content of 90.15 and 0.52%, respectively. Total soluble solids of pumpkin was found to be 9.2 ∘B. A low titrable acidity of 0.03% was observed in pumpkin. It is evident from the data that pumpkin fruit is a good source of ascorbic acid (15.65 mg/100 g) and β-carotene (9.685 mg/100 g), which has high nutritional significance. Pumpkin showed a fiber content of 0.66% and pectin content of 1.18%. Reducing and total sugars were found to be 2.65% and 4.80% respectively. The data indicated that the pumpkin pulp recorded the DPPH (% inhibition) of (34.31); FRAP value of (20.57 μM), TPC of (40.16 mg GAE/g), lipid-peroxidation of (40.51) and reducing power of (40.91). L*, a* and b* values of fresh pumpkin was found to be 66.49, 8.31 and 51.96 respectively.
Table 1. Physicochemical characteristics and anti-oxidant activity of fresh pumpkin (Cucurbita moschata)
All the values obtained showed no discrepancy with earlier reports regarding fresh pumpkin values. Fresh pumpkin contains the moisture content of 79–93% and vitamin C of 22.9 mg/100 g. Lee et al. (Citation2002) reported that the ash content of 0.57–0.89% in fresh pumpkin that was similar to our results. β-carotene of pumpkin was found to be 0.006–2340 μg/g (Rodriguez-Amaya et al., Citation2008). Studies conducted by Jacobo-Valenzuela et al. (Citation2008), showed the acidity of (0.06–0.10%), total sugar of (1.90%), TSS of 5.4–11 ∘B, and crude fat of 0.07–0.16% in the fresh pumpkin. Dini, Tenore, and Dini (Citation2013) reported a FRAP value of 41.33 ± 4.0G Trolox Equivalent μM/10 g of raw pumpkin and DPPH Trolox Equivalent μM/10 g of raw pulp. Studies conducted by Tamer, Ncedayi, Yönel, Yonak, and Çopur (Citation2010) showed the total phenolic content of fresh pumpkin pulp as 476.6 (mg GAE/100 g) that was higher than the results obtained in our study. Difference in antioxidant and total phenolic content values can be attributed to varietal and environmental differences. Color values of C. moschata and Waltham butternut species of C. moschata species were found to be L* = 73.7, a* = 14.8, b* = 65.9 & L* = 73.4, a* = 10.4, b*=71.4, respectively (Itle & Kabelka, Citation2009).
3.2. Physicochemical characteristics of pumpkin candy
The moisture content of fresh candy during three months of storage is shown in Table . A non-significant decreasing trend in moisture content of pumpkin candy was seen during storage for one month. With further storage, moisture content decreased significantly. Decrease in moisture may be due to osmosis of the product during storage. Ash content did not vary significantly with storage although a mild decrease was seen. Pectin decreased (0.88–0.65) significantly during three months of storage. This decrease can be attributed to pectin-degrading enzymes.
Table 2. Effect of storage on physicochemical characteristics of the pumpkin candy
3.2.1. Sugars
An increase in sugars of pumpkin candy was observed during storage at ambient temperature (Table ). Data indicated that the total sugar increased significantly from 20.1–27.69% during three months of storage. Reducing sugar also increased significantly from 2.9–3.9% during three months of storage of candy at ambient temperature. Similar trend has been observed in pear candy (Rani & Bhatia, Citation1985) and papaya candy (Sandhu, Citation1994). Sharma, Dhaliwal, and Kalia (Citation1998) revealed that the reducing and total sugars of apple candy under both room and refrigerated temperatures were found to increase significantly with the increase in storage time. Increase in reducing sugar might be due to conversion of sucrose to reducing sugars. However, increase in total sugar content could be due to decrease in moisture content because of osmosis.
3.2.2. Total soluble solids (TSS)
An increasing trend in TSS of pumpkin candy was observed during storage at ambient temperature (Table ). TSS of fresh candy 65.05 ∘B, increased to 68.05 ∘B, 70.05 ∘B, and 74.16 ∘B during one, two, and three months of storage, respectively. During storage at room temperature, an increasing trend in TSS was seen in pear candy (Rani & Bhatia, Citation1985).
Sharma et al. (Citation1998) reported that the increase in TSS of candied apples with respect to method of preparation, packaging materials, storage conditions, and storage intervals varied significantly. The investigation showed that there was significant increase in TSS during storage of pumpkin candy at ambient temperature. The reason for increase in TSS during storage might be due to the decrease in moisture content and inhibition of sugar by the process of osmosis.
3.2.3. Titrable acidity
Titrable acidity of candies showed increase during storage at ambient temperature (Table ). A non-significant increase during first month was followed by a significant increase with further storage at ambient temperature. Increase in acidity can be attributed to the degradation of cell wall components to produce organic acids. Similar results have been observed in pear candy by Rani and Bhatia (Citation1985). Sandhu (Citation1994) also observed an increasing trend in acidity of papaya candy stored at ambient temperature for about five weeks, whereas, samples stored at refrigerated temperature showed significant decrease in acidity during three months of storage. Sharma et al. (Citation1998) reported that the total acidity of apple candy varied non-significantly in response to the methods of candy preparation, storage conditions, and different packaging material. The acid content in pumpkin candy showed a non-significant decreasing trend during storage which could be due to concentrating effect of moisture loss from the product.
3.2.4. Color
Color changes in pumpkin candy during storage with chromatic parameters (L*, a*, b*) are shown in the Table . The redness (a) and the yellowness (b) should be considered as the physical parameters to describe the visual color degradation of the candy during storage. The results showed (L*, a*, b*)) of the fresh candy as (49.43), (7.1), (16.2), respectively. The results indicated the non-significant decrease in chromatic parameters L*, b* during two months of storage followed by a significant decrease when storage period reached three months. a* value of candies showed a non-significant increase up to two months of storage followed by a significant increase with further storage. Decrease in L* value suggests darkening of the product and can be attributed to the occurrence of non-enzymatic browning. A decrease in b* value implies decrease in yellowness of candies. During storage degradation of carotene pigment takes place (Figure ) that is responsible for the decrease in b* value of pumpkin candy. Thus, change in color parameters of pumpkin candy can be due to the occurrence of non-enzymatic browning reactions that also took place together with oxidation and isomerization of beta-carotene (Zhou et al., Citation2013). Gliemmo, Latorre, Gerschenson, and Campos (Citation2009) reported decrease in (a*, b*) values during storage of pumpkin puree. There are many studies on degradation of fresh and processed fruit and vegetables (Chutintrasri & Noomhorm, Citation2007)
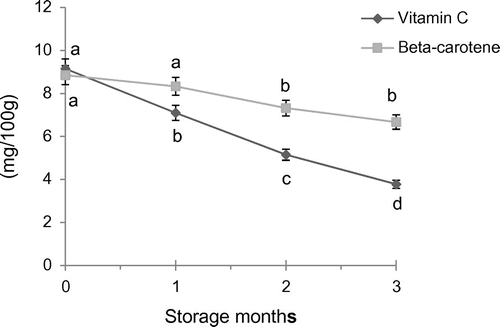
3.2.5. Ascorbic acid
The data (Figure ) indicated that the ascorbic acid (9.15 mg/100 g) of fresh candy that decreased significantly with storage at ambient temperature. Rani and Bhatia (Citation1985) revealed that the ascorbic acid values tend to decrease by 50% with the duration of storage of pear candies. Sandhu (Citation1994) also observed a decreasing trend in ascorbic acid content of papaya candy during storage at both the ambient and refrigerated temperatures. The reduction in ascorbic acid content may be possible due to the oxidation of ascorbic acid as suggested by Rani and Bhatia (Citation1985) in pear candy.
3.2.6. Beta-carotene
Beta-carotene decreases from 8.85–6.67 mg/100 mg during the storage of candy at an ambient temperature. There was a non-significant decrease during first month of storage followed by a significant decrease (Figure ). During storage a decrease in beta-carotene was reported in papaya jam (Saravanan, Godara, Goyal, & Sharma, Citation2004). However Chen, Peng, and Chen (Citation1995) showed higher loss in carotenoids during processing and storage. Decrease in carotene content of pumpkin candy may be attributed to high susceptibility of carotenoids to auto-oxidative degradation during processing and storage of foods as suggested by Sharma and Sethi (Citation2000).
3.3. Antioxidant activity
The investigations showed that there was a non-significant decrease in total phenol content but a significant decrease in DPPH; FRAP value, lipid peroxidation, and reducing power. The decrease in antioxidant activity of pumpkin candy during storage at an ambient temperature is shown in Table . The data indicated that the TPC (mg GAE/300μL) of fresh candy (40.11) decreased to 39.9, 37.6, and 35.94 on one, two, and three months of storage, respectively. FRAP values of fresh candy (20.08 μM/300μL) dropped to 18.96, 16.42, and 15.87 on one month, two months, and three months of storage, respectively. A similar trend was seen in DPPH (34.10–28.27%), reducing power (48.08 to 40.26%), and lipid peroxidation (40.11–33.95%) as reported in Table . The decrease in antioxidant activity was also shown by Zhou et al. (Citation2013).
Table 3. Effect of storage on antioxidant activity of the pumpkin candy
The decrease in total phenolics could mainly be resulted from oxidation, degradation of phenolic compounds, and the polymerization of phenolic compounds with proteins (Varela-Santos et al., Citation2012). Kim and Padilla-Zakour (Citation2004) noted that the decrease in total phenolics could be due to disruption in cell structure during processing. The decrease in antioxidant activity can be due to degradation of total phenolic compounds and vitamin C during storage was also reported by Zhou et al. (Citation2013). Antioxidant activity has been attributed to total phenolic content which have been found to be strongly correlated (Nisar et al., Citation2015). Although TPC content of candy samples showed an initial non-significant followed by significant decrease however, antioxidant activity showed a significant and much greater decrease during storage. However a greater decrease in the antioxidant activity than TPC in pumpkin candy can be due to greater degradation in C content than its carotenoid content.
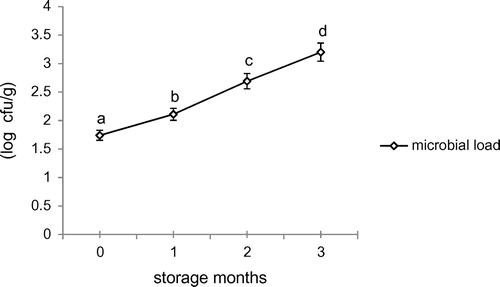
3.4. Microbial analysis
Total plate count (TPC) of pumpkin candy is shown in Figure . A significant increase in TPC (1.74–3.4 log cfu/g) content of pumpkin candy with storage under ambient conditions was seen. Sandhu (Citation1994) reported mold growth within a week when stored in glass jars. On contrary, in our study, pumpkin candy was found to be safe for human consumption during three months of storage. Candy has a high sugar content that limits the growth of microbes by limiting water available for microbial growth.
3.5. Sensory analysis
Sensory analysis of candy during three months of storage is shown in Table . All sensory parameters showed a decrease with storage at ambient temperature. Color of candies showed a non-significant decrease up to first month of storage followed by a significant decrease. Color of pumpkin is primarily due to carotenes and the decrease in color parameter showed a similar trend to carotene degradation of candy. Texture showed prominent changes that might be due to enzymatic breakdown of the middle lamella and cell wall by pectin methylesterase, polygalacturonase, b-galactosidase, and cellulose (Ketsa & Daengkanit, Citation1999). This is in accordance to pectin (%) values seen in candy during storage (table ). Pectin is present in cell wall and contributes to texture of fruits and vegetables. Off-flavor generation is usually attributed to a combined action of compounds generated by microbes and a change in sugar acid ratio in fruits and vegetables. Flavor of candies changed after one month of storage and can be attributed to increased microbial growth that results in production of off flavor compounds. Overall acceptability decreased with storage; however, pumpkin candy was acceptable even after three months of storage.
Table 4. Sensory Analysis of Pumpkin candy during three months of storage
4. Conclusion
Pumpkin is a rich source of nutraceutical compounds such as vitamins and antioxidants Vitamin C and beta-carotene are present in abundance in pumpkin and impart high antioxidant potential to it. Vitamin C and carotene content of pumpkin can be retained by processing pumpkin into candies. Candy production not only improves the shelf life of vegetable but also maintains its antioxidant potential.
Acknowledgement
The authors are thankful to the Department of Biotechnology, Govt. of India.
Additional information
Funding
Notes on contributors
Sabeera Muzzaffar
Sabeera Muzzaffar is an assistant professor in the Department of Food Science and Technology, University of Kashmir.
Waqas N Baba
Waqas N Baba is working as a lab analyst in Department of Food Science and Technology, University of Kashmir.
Nuzhat Nazir
Nuzhat Nazir is a Food Technology student and has completed her master’s in Food Science and Technology from the Department of Food Science and Technology.
F.A. Masoodi
F.A. Masoodi is a professor cum head of the Department of Food Science and Technology, University of Kashmir.
Rafiya Bazaz
Rafiya Bazaz is a doctoral fellow in the Department of Food Science and Technology, University of Kashmir.
References
- Association of Official Analytical Chemists. (2000). Official methods of analysis of AOAC international (17th ed.). Washington, DC: Author.
- Baba, N., Rashid, I., Shah, A., Ahmad, M., Gani, A., & Masoodi, F. A. (2014). Effect of microwave roasting on antioxidant and anti-cancerous activities of barley flour. Journal of Saudi Society of Agricultural Sciences, 27, 143–154.
- Chen, B. H., Peng, H. Y., & Chen, H. E. (1995). Changes of Carotenoids, Color, and Vitamin A Contents during Processing of Carrot Juice. Journal of Agricultural and Food Chemistry, 43, 1912–1918.10.1021/jf00055a029
- Chutintrasri, B., & Noomhorm, A. (2007). Color degradation kinetics of pineapple puree during thermal processing. Journal of Food Science and Technology , 40, 300–306.
- Dini, I., Tenore, G. C., & Dini, A. (2013). Effect of industrial and domestic processing on antioxidant properties of pumpkin pulp. LWT - Food Science and Technology, 53, 382–385.10.1016/j.lwt.2013.01.005
- Fu, C., Shi, H., & Li, Q. (2006). A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum Nutri (formerly qualitas plantarum). Springer, Netherlands, 61, 3–80.
- Gliemmo, M. F., Latorre, M. E., Gerschenson, L. N., & Campos, C. A. (2009). Color stability of pumpkin (Cucurbita moschata, Duchesne ex Poiret) puree during storage at room temperature: Effect of pH, potassium sorbate, ascorbic acid and packaging material. LWT - Food Science and Technology, 42, 196–201.
- González, E., Montenegro, M. A., Nazareno, M. A., & Lopez de Mishima, B. A. (2001). Carotenoid composition and vitamin A value of an Argentinian squash (Cucurbita moschata). Archivos Latinoamericanos de Nutrición, 51, 395–399.
- Gould, W. A. (1978). Food quality assurance (pp. 178–180). Westport Connecticut: AVI Publishing.
- Itle, R. A. & Kabelka, E. A. (2009). Correlation between L*a*b* color space values and carotenoids content in pumpkins and squash (Cucurbita spp.). Hort Science, 44, 633–637.
- Jacobo-Valenzuela, N., Zazueta-Morales, J. J., Pérez-Castañeda, V., Camacho-Hernández, I. L., Gallegos-Infante, J. A., Rocha-Guzmán, N. E., & González-Laredo, R. F. (2008). Rediscovering winter squash (Cucurbita moschata D.) cv. cehualca as a magic food in Sinaloa state. 3rd International Congress of Food Science and Food Biotechnology in Developing Countries. AMECA 14-17 October 475–478.
- Jan, U., Gani, A., Ahmad, M., Shah, U., Baba, W. N., Masoodi, F. A.,... Wani, S. M. (2015). Characterization of cookies made from wheat flour blended with buckwheat flour and effect on oxidant properties. Journal of Food Science and Technology , 1–11.
- Jun, H., Lee, C. H., Song, G. S., & Kim, Y. S. (2006). Characterization of the pectic polysaccharides from pumpkin peel. LWT - Food Science and Technology, 39, 554–561.10.1016/j.lwt.2005.03.004
- Ketsa, S., & Daengkanit, T. (1999). Firmness and activities of polygalacturonase, pectinesterase, β-galactosidase and cellulase in ripening durian harvested at different stages of maturity. Scientia Horticulturae, 80, 181–188.10.1016/S0304-4238(98)00242-8
- Kim, D. O., & Padilla-Zakour, O. I. (2004). Jam processing effect on total phenolics and anti-oxidant activity capacity in anthocyanin rich fruits: cherry, plum, and raspberry, sensory and nutritive qualities of food. Journal of Food Science, 69, 395–400.
- Lal, G., Sidappa, G. S., & Tandon, G. C. (2010). Preservation of fruits and vegetables (pp. 198–213). New Delhi: ICAR.
- Lee, C. H., Cho, J. K., Lee, S. J., Koh, W., Park, W., & Kim, C. H. (2002). Enhancing β-carotene content in Asian noodles by adding pumpkin powder. Cereal Chemistry, 79, 593–595.10.1094/CCHEM.2002.79.4.593
- Nisar, R., Baba, W. N., & Masoodi, F. A. (2015). Effect of chemical and thermal treatments on quality parameters and anti-oxidant activity of apple (pulp) grown in high Himalayan regions. Cogent Food & Agriculture, 1–13.
- Ranganna, S. (1997). Handbook of analysis and quality control for fruit and vegetable products second (pp. 11–12). New Delhi: Tata McGraw Hill Publishing Company Limited.
- Rani, U., & Bhatia, B. S. (1985). Studies on pear candy processing. Indian Food Packer, 39, 40–46.
- Rasool, N., Baba, W. N., Muzzaffar, S., Masoodi, F. A., Ahmad, M., Munaff Bhat, M. M., & Yildiz, F. (2015). A correlation study of proximate composition, physical and cooking properties of new high yielding and disease resistant rice varieties. Cogent Food & Agriculture, 1, 1099175.10.1080/23311932.2015.1099175
- Rodriguez-Amaya, D. B. (2003). Enhancing the carotenoid levels of foods through agriculture and food technology. Food Africa, Internet Forum: Internet Paper for Food Nutrition and Health 31.
- Rodriguez-Amaya, Delia B., Kimura, Mieko, Godoy, Helena T., & Amaya-FarfanJ. (2008). Updated Brazilian database on food carotenoids: Factors affecting carotenoid composition. Journal of Food Composition and Analysis, 21, 445–463.10.1016/j.jfca.2008.04.001
- Sandhu, G. S. (1994). Development of sugar coated candied products (MSc thesis). Punjab Agricultural University, Ludhiana.
- Saravanan, K., Godara, R. K., Goyal, R. K., & Sharma, R. K. (2004). Studies on the storage behavior of papaya jam. Haryana Journal of Horticultural Sciences, 33, 218–220.
- Sharma, K. D., & Sethi, V. (2000). Studies on the storage of apple from three different locations. Beverage Food World, 4, 28–37.
- Sharma, S., Dhaliwal, Y. S., & Kalia, M. (1998). Candied apples: a new perspective. Journal of Food Science and Technology , 35, 79–82.
- Tamer, C. E., Ncedayi, Bİ., Yönel, S. P., Yonak, S., & Çopur, O. U. (2010). Evaluation of several quality criteria of low calorie pumpkin dessert. Notulae Botanicae Horti Agrobotanici Cluj Napoca, 38, 76–80.
- Varela-Santos, E., Ochoa-Martinez, A., Tabilo-Munizaga, G., Reyes, J. E., Pérez-Won, M., Briones Labarca, V., & Morales, J. (2012). Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innovative Food Science & Emerging Technologies, 13, 13–22.10.1016/j.ifset.2011.10.009
- Wright, J. R., Colby, H. D., & Miles, P. R. (1981). Cytosolic factors which affct microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys, 206, 294–304.
- Zhou, C. L., Liu, W., Zhao, J., Yuan, C., Song, Y., Chen, D.,... Li, Q. H. (2013). The effect of high hydrostatic pressure on the microbiological quality and physical-chemical characteristics of pumpkin (Cucurbita maxima Duch.). Innov Food Sci Emerg Technol, 21, 24–34.