?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Fresh apricot pulp and its processed products (bar, chutney, and leather) were analyzed for physicochemical (moisture content, titrable acidity (TA), ascorbic acid, and percent reducing sugars) and antioxidant properties (2,2-diphenyl-l-picrylhydrazyl (DPPH), reducing power, total phenolics, lipid peroxidation, ferric reducing antioxidant potential, and hydroxyl radical scavenging activity). Reducing sugars, TA, and ascorbic acid content were found to be higher in the processed products than the fresh pulp. A significant difference in the antioxidant properties between the fresh apricot pulp and its processed products was observed. The difference in antioxidant properties between the fresh and the processed products may be attributed to the partial degradation of the bioactive compounds by the action of heat during processing. Among the processed products, apricot bar showed the highest DPPH radical scavenging activity, lipid peroxidation, and hydroxyl radical scavenging activity. Therefore, production of apricot bar could a suitable option for processing of apricots.
Public Interest Statement
Apricots are widely produced but because of their short shelf life, a lot of the production gets spoiled. Processing of apricots into suitable products not only reduces the postharvest losses, but also increases their market value by making them available in the off season. In this regard, processing products from apricots discussed in this piece of research could be handy.
Competing interests
The authors declare no competing interests.
1. Introduction
Apricots (Prunus armeniaca L.) are grown worldwide. With a worldwide production of 668,231–727,173 tons, the production has increased from 9,900 to 18,000 tons in India during 2000–2013 (FAO, Citation2014). In India apricots are grown commercially in the hills of Himachal Pradesh, Jammu and Kashmir, Uttar Pradesh, and to a limited extent in the north eastern hills. Some apricots are being grown in dry temperate regions of Kinnaur and Lahaul Spiti in Himachal Pradesh and Ladakh in Jammu and Kashmir.
Apricot is a climacteric fruit with a very short storage life due to a high respiration rate and a rapid ripening process (Egea, Martinez-Madrid, Sanchez-Bel, Murcia, & Romojaro, Citation2007). Apricot is a costly fruit and is not available as a raw material in many countries. Due to this, there is scope for fabricating apricot-based products in order to meet the market requirements and earn profit. Apricot-based products are highly appreciated in the market due to their specific taste, aroma, and nutritive value. To extend the shelf life of apricot, different preservation methods have been developed including canning, freezing, drying, packaging in controlled atmospheric packages (Jimenez, Martınez-Tome, Egea, Romojaro, & Murcia, Citation2008), and processing into different products. However, it must be borne in mind that processing can change the concentration of nutrients. The loss of nutrients in fruits and vegetables depends on the type of food, processing time, processing temperature, and storage conditions (Murcia, López-Ayerra, Martínez-Tomé, & García-Carmona, Citation2001; Murcia, Lopez-Ayerra, Martinez-Tome, Vera, & Garcia-Carmona, Citation2000; Murcia, Vera, & Garcia-Carmona, Citation1992). Some preservation methods are also believed to be responsible for depleting the naturally occurring antioxidants in the foods, with a subsequent decrease in their health-protecting capacity (Kalt, Citation2005; Murcia et al., Citation2002; Puupponen-Pimiä et al., Citation2003).
Apricot contains abundant bioactive compounds such as polyphenols, carotenoids, fatty acids, volatiles, polysaccharides, minerals, sugars, and vitamins (Akin, Karabulut, & Topcu, Citation2008; Ali, Masud, & Abbasi, Citation2011), which contribute to its taste, color, and nutritive values. There is a considerable interest in carotenoids and polyphenols of apricot. The principal phenolic compounds in apricot are chlorogenic acid, neochlorogenic acid, (+)-catechin, (−)epicatechin, and rutin, which contribute substantially to its antioxidant potential (Erdogan-Orhan & Kartal, Citation2011). Polyphenols as antioxidants may protect cell constituents against oxidative damage and therefore, limit the risk of various degenerative diseases associated with oxidative stress (Scalbert, Manach, Morand, Rémésy, & Jiménez, Citation2005). Some studies have demonstrated that a diet rich in phenolic compounds correlates with a reduced risk of coronary heart disease (Vallverdú-Queralt, Arranz, Casals-Ribes, & Lamuela-Raventós, Citation2012).
The present piece of work was carried out to assess the physicochemical and antioxidant properties of the fresh apricot pulp, which was further processed into bar, leather, and chutney. The physicochemical and antioxidant properties of the processed products were also assessed to compare them with the fresh apricot pulp.
2. Materials and methods
2.1. Materials
Apricots (P. armeniaca L.) variety Narmo were brought from the Ladakh region of Jammu & Kashmir, India. These were converted into fine pulp and transported to Srinagar under refrigerated conditions. The pulp was stored at −18°C till product development and analysis.
2.2 Preparation of the processed products
2.2.1. Preparation of chutney
Apricot pulp (1.3 kg) was boiled in a large pan along with the spices (2 g each of ginger, cardamom, garlic, onion, and cinnamon) contained in the bag, till much of the water was absorbed. Sugar (1.3 kg), salt (55 g), and vinegar (225 g) were added towards the end of the process and were cooked further until the desired consistency was achieved. The chutney so prepared was packed in aluminum laminates for further analysis.
Preparation of apricot chutney
PULPING OF APRICOT
↓
ADDITION OF WATER
↓
BOILING WITH ADDITION OF SPICES BY BAG METHOD
↓
ADDITION OF SALT, SUGAR AND VINEGAR
↓
PACKAGING
↓
STORAGE
2.2.2. Preparation of apricot leather
Apricot pulp with sugar and citric acid was cooked in a sauce pan until all the sugar was dissolved. It was then laid on a tray lined with a plastic wrap and was spread evenly in a thin layer, using a spatula followed by tunnel drying.
Preparation of apricot leather
PULPLING OF APRICOTS (79 g)
↓
COOKING OF PULP WITH CITRIC ACID (3 g) AND SUGAR (18 g)
↓
POURING INTO TRAYS IN UNIFORM LAYER
↓
DRYING (BY TUNNEL DRY METHOD)
↓
PACKAGING
↓
STORAGE
2.2.3. Preparation of apricot bar
Apricot pulp (100 g) was pasteurized at 91–93°C for 2 min. It was followed by the addition of sugar (70 g) with continuous heating and mixing. Sugar was added followed by the addition of pectin (0.3 g) and the TSS was adjusted to 30°Bx. The mixture was cooled and KMS (0.1%) was added as a preservative. The mixture was poured into trays, which were smeared with butter. It was then dried at 65°C for 36 h followed by cooling. The bars were cut and wrapped in PE pouches; packed in Al pouches, and stored at room temperature.
Preparation of apricot bar
PULPING OF APRICOTS
↓
PASTUERIZATION (91–93°C FOR 2 MINS)
↓
ADDITION OF SUGAR (70 g SUGAR TO 100 g PULP)
↓
HEATING AND MIXING
↓
ADDITION OF PECTIN (0.3 g)
↓
TSS ADJUSTED TO 30 °BRIX
↓
COOLING
↓
ADDITION OF PRESERVATIVE (KMS 0.1%)
↓
POURING INTO TRAYS
↓
DRYING (65 ± 2°C FOR 6 HOURS)
↓
COOLING
↓
PACKAGING
↓
STORAGE
2.3. Quality evaluation of processed products
2.3.1. Titrable acidity
The acid content of the fresh apricot pulp, leather, bar, and chutney was determined by titration. For titrable acidity (TA), 10 g of sample was diluted with 100 ml of distilled water using 3–4 drops of phenolphthalein as indicator. Acids in the fresh apricot pulp and the processed products were titrated with 0.1 N NaOH and the results were expressed as mg of citric acid per 100 ml. The percent acidity was calculated according to the following expression:
2.3.2. Ascorbic acid (vitamin C)
Ascorbic acid content of the fresh apricot pulp, leather, bar, and chutney was estimated by titration method (AOAC, Citation1996), using 2,6-dichlorophenol indophenol dye solution. The method of estimation involves the reduction of 2,6-dichlorophenol indophenol dye to a colorless form by ascorbic acid in an alkaline solution. The reaction is quantitative and particularly specific for ascorbic acid in solution in the pH range of 1–3.5. In the procedure followed, the dye solution was firstly standardized against standard ascorbic acid in order to determine the dye factor. The sample was diluted with 3% metaphosphoric acid and then the phosphoric acid extract of the sample was titrated against the dye solution until a pink color is obtained which persists for 15 s. Dye factor was determined by the following equation:
Ascorbic acid was reported as mg of ascorbic acid/100 g and was determined by the following equation:
2.3.3. Reducing sugars
The quantification of reducing sugars in the samples was carried out using Lane and Eynon method (AOAC, Citation2000). Five grams of each sample were placed in a measuring cylinder, to which 100 ml distilled water was added and stirred thoroughly. The samples were neutralized with 1 N NaOH using phenolphthalein as indicator. It was followed by the addition of 5 ml of 45% lead acetate and then 5 ml of 22% potassium oxalate was added after 10 min. The final volume was made up to 250 ml (using distilled water). The above formed solution was filtered and marked as solution “A.” The Fehling’s solutions were titrated against solution “A” on hot plate till brick red color was developed. After adding 5–7 drops of methylene blue, the same was again titrated till permanent brick red color was obtained.
% reducing sugar was determined using the following equation:
2.4. Antioxidant activity
2.4.1. Sample preparation
The extraction of fresh apricot pulp, leather, bar, and chutney was carried out using methanol as a solvent. Two grams of pulp were mixed with 8 ml methanol, followed by centrifugation at 10000 g for 10 min. The supernatant was collected and was used for the analysis of antioxidant activity. The antioxidant activity of extracts was carried out using the following methods.
2.4.2. DPPH
Radical scavenging activity was measured using the method described by Wani, Wani, Shah, and Masoodi (Citation2013) and Nazir et al. (Citation2013) that involves the use of the free radical 2,2-diphenyl-l-picrylhydrazyl (DPPH). For DPPH, three different concentrations of the extracts (0.1, 0.2, and 0.3 μl) were added to 1.0 ml of a 0.01% methanolic solution of DPPH. Absorbance was then measured after 30 min at 517 nm using the spectrophotometer. The results were expressed as percent inhibition, which was calculated using the formula:
2.4.3. Reducing power
The reducing power of the fresh apricot pulp and its processed extracts was determined according to the method of Oyaizu (Citation1986). About 2.5 ml of the extract in each case was mixed with phosphate buffer (2.5 ml, 2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 1%), the mixture being incubated at 50°C for 20 min. A portion (2.5 ml) of trichloroacetic acid (TCA, 10%) was added to the mixture which was then centrifuged at 1,500 g for 10 min. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1%) and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power.
2.4.4. Total phenolics
The total phenolic content of each extract was determined according to the method of Du, Li, Ma, and Liang (Citation2009). In a 10 ml Eppendorf tube, 7.9 ml of distilled water, 0.1 ml of extract and 0.5 ml of Folin–Ciocalteu reagent (1:1 with water) were mixed. After 1 min, 1.5 ml of sodium carbonate (20%) was added and the mixture was mixed well by shaking. The reaction solution was then incubated at room temperature for 2 h in the dark before absorbance was taken at 765 nm. The total phenolic concentration was calculated from a calibration curve using Gallic acid as the standard and the results were expressed as mg of gallic acid equivalents per 100 gram.
2.4.5. Hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity was assayed using the 2-deoxyribose oxidation method (Chung, Osawa, & Kawakishi, Citation1997) with minor modifications. The reaction mixture contained 0.45 ml of 0.2 M sodium phosphate buffer (pH 7.4), 0.15 ml of 10 mM 2-deoxyribose, about 0.15 ml of 10 mM FeSO4-EDTA, 0.15 ml of 10 mM hydrogen peroxide, 0.525 ml of distilled water, and the varying concentrations of sample extracts were added in a tube. The reaction was started by the addition of hydrogen peroxide. After incubation at 37°C for 1 h, the reaction was stopped by adding 0.75 ml of 2.8% TCA and 0.75 ml of 1.0% thiobarbituric acid (TBA). The mixture was boiled for 10 min and then absorbance was measured at 520 nm. Hydroxyl radical scavenging ability was evaluated as the inhibition rate of 2-deoxyribose oxidation by hydroxyl radicals. The results were expressed as percentage inhibition by applying the following formula:
where absorbance of control (532) was the malondialdehyde (MDA) produced by Fenton reaction treated alone and Absorbance of sample (532) was the MDA produced in presence of the extract.
2.4.6. FRAP
The ferric reducing antioxidant power (FRAP) of the fresh pulp, chutney, leather, and bar extracts was measured according to the modified protocol developed by Benzie and Strain (Citation1999) with minor modifications. FRAP assay measures the ability of the antioxidants contained in the samples to reduce ferric-tripyridyltriazine (Fe3+-TPTZ) to a ferrous form (Fe2+), which absorbs light at 593 nm (Ozgen, Reese, Tulio, Scheerens, & Miller, Citation2006; Szeto, Tomlinson, & Benzie, Citation2002). The assay was carried out with 100, 200, 300, and 400 μl of each extract. To prepare FRAP solution, a mixture of 0.1 M acetate buffer (3.6), 10 Mm TPTZ (2,4,6-tripyridyl-s-triazinesolution in 40 mM HCl) and 20 Mm ferric chloride (10:1:1, V/V/V) was made and 0.1 ml of the sample extract was added to 1.9 ml of the reagent. The absorbance of reaction mixture was measured at 0 min and after 4 min against the blank reagent and the results were expressed as μM FRAP/g of the fresh weight material.
2.4.7. Lipid peroxidation
The antioxidant activity of apricot pulp, chutney, leather, and bar extracts were determined spectrophotometrically according to the method of Wallin, Rosengren, Shertzer, Camejo, and Astra (Citation1993), with minor modifications. Different concentrations (100, 200, 300, and 400 μl) of the extract were mixed with 1 ml of linoleic acid (0.1 g in 100 ml of pure ethanol), 0.2 ml of H2O2 (30 mM), 0.2 ml of ascorbic acid (100 mM), and 0.2 ml of ferric nitrate (20 mM). This was followed by incubation at 37°C in water bath for 1 h. The reaction was stopped by the addition of 1.0 ml TCA (Trichloroacetic acid, 10% w/v), followed by the addition of 1.0 ml of TBA (Thiobarbituric acid, 1% w/v). All the tubes were placed in a boiling water bath for 20 min. The tubes were then centrifuged at 5,000 rpm for 10 min. The amount of malonaldehyde formed in each of the samples was assessed by measuring the absorbance of the supernatant at 535 nm.
2.5. Statistical analysis
One-way analysis of variance (ANOVA) was performed using a commercial statistical package (IBM SPSS Statistics 21. Ink). Means were compared by Duncan’s Multiple Range test at 5% level of significance.
3. Results and discussion
3.1. Physicochemical properties
Table shows the average values of moisture content, TA, reducing sugars, and ascorbic acid for the fresh apricot pulp, leather, bar, and chutney samples. The TA was found to be the highest in leather (1.50%) followed by chutney (0.97%), fresh pulp (0.87%), and the lowest in bar (0.51%). The highest value of acidity in the leather attributes to the addition of lemon juice and the lowest value of acidity in the bar may attribute to the utilization of acids during various biochemical reactions occurring in the product. The moisture content was found to be the highest in apricot pulp (88%) followed by chutney (43.8%), bar (21.9%), and was the lowest in leather (16.45%). The loss in the moisture content is attributed to the loss of water content during cooking. The vitamin C content was found to be 5.0, 6.5, 7.5, and 9.8 mg/100 g in fresh pulp, chutney, bar, and leather, respectively. The increase in ascorbic acid in the products is attributed to concentration of the product. The reducing sugar content was found to be 26.73, 47.61, 59.52, and 66.66% for pulp, bar, leather, and chutney, respectively. The highest value of reducing sugar in chutney may attribute to the inversion of non-reducing sugars into reducing sugars, the conversion of polysaccharides into monosaccharides and due to the addition of table sugar during cooking (Sharma, Chaudhary, Rao, Yadav, & Bisht, Citation2011).
Table 1. Physicochemical properties exhibited by fresh apricot pulp and its processed products
3.2. Antioxidant properties
3.2.1. DPPH
Apricots contain a wide variety of phytochemicals that function as antioxidants. The phytochemicals scavenge free radicals and thus quench a certain amount of DPPH in the experimental essay. Food quality analysis regarding antioxidant components of different products is fast becoming an accepted profile that primarily highlights the antioxidant capacity as a quality index for many fruits and vegetables (Leccese, Bartolini, & Viti, Citation2007).
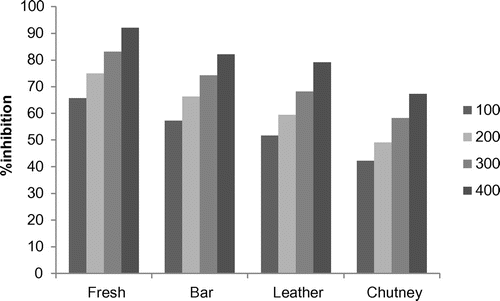
The methanolic extracts of the fresh apricot pulp, bar, leather, and chutney increased in a dose-dependent manner and was found in the range of 65.76–91.98, 57.24–82.19, 51.72–79.07, and 42.2–67.31%, respectively, as shown in Figure . The maximum DPPH radical scavenging activity in fresh pulp can be attributed to the richness of the total phenolic components. There is a rapid degradation of phenolic components in the processed products (bar, leather, and chutney) after being subjected to a higher temperature (Mazza & Minati, Citation1993).
3.2.2. Reducing power
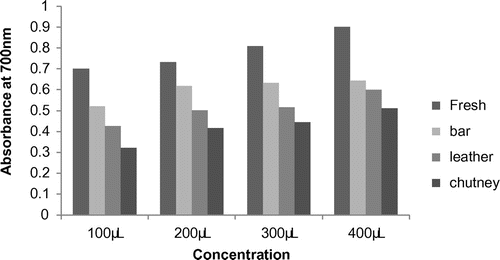
Reducing power is one mechanism of action of antioxidants and may serve as a significant indicator of potential antioxidant activity. Several studies have indicated that the antioxidant activity is related to the development of reductones. Antioxidant activity measured as the reducing activity was found to increase in a dose-dependent manner (Figure ). Fresh pulp (0.70%) showed the highest reducing power followed by leather (0.52%), chutney (0.42%), and bar (0.32%). The decrease in reducing power of leather, chutney and bar could be attributed to decomposition of natural antioxidants at high temperatures encountered during processing (Al-Farsi, Alasalvar, Morris, Baron, & Shahidi, Citation2005).
3.2.3. Lipid peroxidation
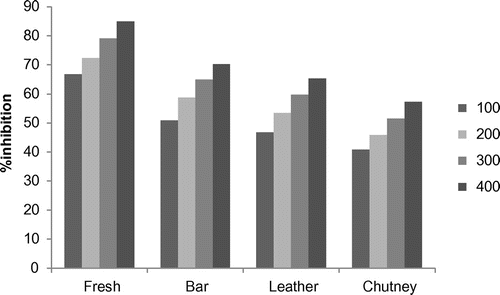
Lipid peroxidation involves the formation and propagation of lipid radicals with numerous deleterious effects, including destruction of membrane lipids, metabolic disorders, and inflammation and production of MDA, is a hallmark of this process. Figure shows the inhibition of lipid peroxidation in the presence of fresh apricot pulp and the processed apricot products (chutney, bar, and leather, respectively). The fresh apricot pulp exhibited the maximum percentage of inhibition (66.70–85.02%) followed by bar (50.90–70.21%), leather (46.80–65.30%) with the minimum percentage of inhibition in chutney (40.80–57.20%). The results are in agreement with those of Jimenez et al. (Citation2008). A much stronger decrease in the percentage of inhibition shown by the three processed products might be due to the enzyme degradation, the combination of leaching, oxidation to biologically inactive forms, and the destruction of ascorbic acid by heat during processing, resulting in the loss of antioxidant activity (Al-Farsi et al., Citation2005).
3.2.4. Total phenols
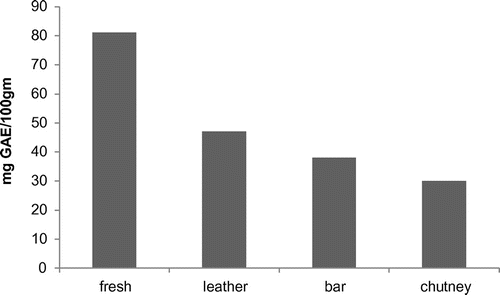
Total phenol content of fresh apricot pulp, leather, bar, and chutney was found to be 81.8, 47, 38, and 30 mg GAE/100 g, respectively (Figure ). The maximum value of total phenolic content attributes to the rich polyphenol content in the fresh pulp, whereas the minimum values for the total polyphenols found in the processed products is due to the degradation of heat labile polyphenols by cooking at a high temperature (Al-Farsi et al., Citation2005) and due to the addition of sugar, which causes dilution.
Polyphenols form a large and diverse class of compounds, many of which occur naturally in a wide range of foods and other plants. The flavonoids are the largest and the best studied group among polyphenols. They are increasingly recognized as playing potentially important roles in health but are not limited to their roles as antioxidants. A range of plant polyphenols is either being actively developed or already sold as dietary supplements and/or herbal-derived medicines in the market. A strong relationship between total phenolic content and antioxidant activity in fruits has well been reported (Battu et al., Citation2011; Chew, Jessica, & Sasidharan, Citation2012; Dorman, Kosar, Kahlos, Holm, & Hiltunen, Citation2003; Duraipandiyan, Baskar, Ignacimuthu, Muthukumar, & Al-Harbi, Citation2012; Kumbhare, Guleha, & Sivakumar, Citation2012).
3.2.5. Hydroxyl radical scavenging activity
Hydroxyl (OH·) radicals are extremely reactive and may be generated in the human body under physiological conditions, where they can react with non-selective compounds, such as proteins, DNA, unsaturated fatty acids, and almost every biological membrane (Murcia, Jimenez, & Martinez-Tome, Citation2001).
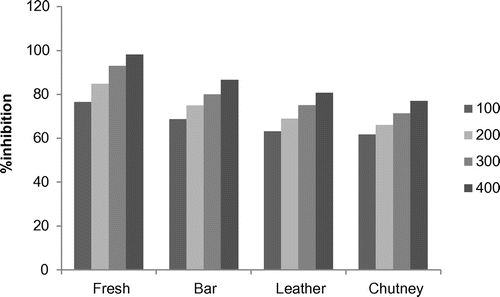
Figure shows the results of the deoxyribose damage caused by OH· in the presence of fresh apricot pulp and its processed products (bar, leather, and chutney). The fresh apricot samples proved to be very good scavengers of OH·, inhibiting deoxyribose damage up to 98.16%. Bar and leather too showed good antioxidant activity of 86.57 and 80.61%, respectively, as compared to apricot chutney (77%). The lowest activity in apricot chutney is due to leaching of anthocyanins and polyphenols during cooking (Chaovanalikit & Wrolstad, Citation2004).
3.2.6. FRAP
The FRAP analysis of fresh apricot pulp, bar, chutney, and leather extracts was found to be 5.2, 4.4, 2.4, and 4.76 μM FRAP/g, respectively. Thus, the highest FRAP values was obtained in the fresh apricot pulp followed by bar, chutney, and leather. The reducing power property indicates that the antioxidant compounds are electron donors and can reduce the oxidized intermediates of the lipid peroxidation process, so that they can act as primary and secondary antioxidants (Yen & Chen, Citation1995). Decrease in FRAP values of the processed products could be attributed to enzyme degradation, leaching, decomposition of polyphenols to inactive forms, and the destruction of ascorbic acid by heat during processing (Al-Farsi et al., Citation2005), resulting in the loss of antioxidant activity.
4. Conclusion
Narmo cultivar of apricots was processed into bar, chutney, and leather that were analyzed for physicochemical and antioxidant properties. A significant decrease in the antioxidant properties of fresh apricot pulp was observed as compared to its processed products. The difference may be attributed to the partial degradation of the phenolic compounds by the action of heat. Processing decreases the total antioxidant properties of the fresh apricots because of heating and addition of sugar. However, considering the least decrease in antioxidant properties during processing, fresh apricots could be suitably processed into apricot bar.
Additional information
Funding
Notes on contributors
S.M. Wani
S.M. Wani is an assistant professor at University of Kashmir, Department of Food Science and Technology. His core areas of research are nutraceuticals and fruit processing. His current research focuses are stability of phytochemicals during processing and storage of perishable temperate fruits.
References
- Akin, E. B., Karabulut, I., & Topcu, A. (2008). Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chemistry, 107, 939–948.10.1016/j.foodchem.2007.08.052
- Al-Farsi, M., Alasalvar, C., Morris, A., Baron, M., & Shahidi, F. (2005). Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. Journal of Agricultural and Food Chemistry, 53, 7592–7599.10.1021/jf050579q
- Ali, S., Masud, T., & Abbasi, K. S. (2011). Physico-chemical characteristics of apricot (Prunus armeniaca L.) grown in Northern Areas of Pakistan. Scientia Horticulturae, 130, 386–392.10.1016/j.scienta.2011.05.040
- AOAC. (1996). Ascorbic acid in vitamin preparations and juices. 2,6-dichloroindophenol titrimetric method. Official methods of Analysis of AOAC International (AOAC Official Method 967.21, pp. 16–17). Arlington, TX: Author.
- AOAC. (2000). Sucrose in fruits and fruit products read with AOAC Official Method 923.09 Lane and Eynon general volumetric method (Official method 925.35 17th ed.) Arlington, TX: Author.
- Battu, G. R., Ethadi, S. R., Priya, G. V., Priya, K. S., Chandrika, K., Rao, A. V., & Reddy, S. O. (2011). Evaluation of antioxidant and anti-inflammatory activity of Euphorbia heyneana Spreng. Asian Pacific Journal of Tropical Biomedicine, 1, S191–S194.10.1016/S2221-1691(11)60154-8
- Benzie, I. F. F., & Strain, J. J. (1999). The ferric reducing ability of plasma as a measure of antioxidant power the FRAP assay. Analytical Biochemistry, 239, 70–76.
- Chaovanalikit, A., & Wrolstad, R. E. (2004). Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. Journal of Food Science, 69, 67–72.
- Chew, A. L., Jessica, J. J. A., & Sasidharan, S. (2012). Antioxidant and antibacterial activity of different parts of Leucas aspera. Asian Pacific Journal of Tropical Biomedicine, 2, 176–180.10.1016/S2221-1691(12)60037-9
- Chung, S. K., Osawa, T., & Kawakishi, S. (1997). Hydroxyl radical scavenging effects of species and scavengers from brown mustard (Brassica nigra). Bioscience, Biotechnology, and Biochemistry, 61, 118–123.10.1271/bbb.61.118
- Dorman, H. J., Kosar, M., Kahlos, K., Holm, Y., & Hiltunen, R. (2003). Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. Journal of Agricultural and Food Chemistry, 51, 4563–4569.10.1021/jf034108k
- Du, G., Li, M., Ma, F., & Liang, D. (2009). Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chemistry, 113, 557–562.10.1016/j.foodchem.2008.08.025
- Duraipandiyan, V., Baskar, A. A., Ignacimuthu, S., Muthukumar, C., & Al-Harbi, N. A., (2012). Anticancer activity of Rhein isolated from Cassia fistula L. flower. Asian Pacific Journal of Tropical Disease, 2, S517–S523.
- Egea, I. M., Martinez-Madrid, M. C., Sanchez-Bel, P., Murcia, M. A., & Romojaro, F. (2007). The influence of electron-beam ionization on ethylene metabolism and quality parameters in apricot (Prunus armeniaca L., cv Búlida). LWT-Food Science and Technology, 40, 1027–1035.10.1016/j.lwt.2006.06.005
- Erdogan-Orhan, I., & Kartal, M. (2011). Insights into research on phytochemistry and biological activities of Prunus armeniaca L. (apricot). Food Research International, 44, 1238–1243.10.1016/j.foodres.2010.11.014
- FAO. (2014). Retrieved from http://faostat.fao.org/faostat
- Jimenez, A. M., Martınez-Tome, M., Egea, I., Romojaro, F., & Murcia, M. A. (2008). Effect of industrial processing and storage on antioxidant activity of apricot (Prunus armeniaca v. bulida). European Food Research and Technology, 227, 125–134.10.1007/s00217-007-0701-1
- Kalt, W. (2005). Effects of production and processing factors on major fruit and vegetable antioxidants. Journal of Food Science, 70, 11–19.10.1111/j.1365-2621.2005.tb09053.x
- Kumbhare, M. R., Guleha, V., & Sivakumar, T. (2012). Estimation of total phenolic content, cytotoxicity and in vitro antioxidant activity of stem bark of Moringa oleifera. Asian Pacific Journal of Tropical Disease, 2, 144–150.10.1016/S2222-1808(12)60033-4
- Leccese, A., Bartolini, S., & Viti, R. (2007). Total antioxidant capacity and phenolics content in apricot fruits. International Journal of Fruit Science, 7, 3–16.10.1300/J492v07n02_02
- Mazza, G., & Minati, E. (1993). Anthocyanins in fruits, vegetables and grains. Boca Raton, FL: CRC Press
- Murcia, M. A., Jimenez, A. M., & Martinez-Tome, M. (2001). Evaluation of the antioxidant properties of Mediterranean and tropical fruits compared with common food additives. Journal of Food Protection, 64, 2037–2046.
- Murcia, M. A., López-Ayerra, B., Martínez-Tomé, M., & García-Carmona, F. (2001). Effect of industrial processing on amino acid content of broccoli. Journal of the Science of Food and Agriculture, 81, 1299–1305.10.1002/jsfa.942
- Murcia, M. A., Lopez-Ayerra, B., Martinez-Tome, M., Vera, A. M., & Garcia-Carmona, F. (2000). Evolution of ascorbic acid and peroxidase during industrial processing of broccoli. Journal of the Science of Food and Agriculture, 80, 1882–1886.10.1002/(ISSN)1097-0010
- Murcia, M. A., Martinez-Tome, M., Jimenez, A. M., Vera, A. M., Honrubia, M., & Parras, P. (2002). Antioxidant activity of edible fungi (truffles and mushrooms): Losses during industrial processing. Journal of Food Protection, 65, 1614–1622.
- Murcia, M. A., Vera, A. M., & Garcia-Carmona, F. (1992). Effect of processing methods on spinach: Proximate composition in fatty acids and soluble protein. Journal of the Science of Food and Agriculture, 59, 473–476.10.1002/(ISSN)1097-0010
- Nazir, A., Wani, S. M., Gani, A., Masoodi, F. A., Haq, E., Mir, S. A., & Riyaz, U. (2013). Nutritional, antioxidant and antiproliferative properties of persimmon (Diospyros kaki)–a minor fruit of J&K India. International Journal of Advanced Research, 1, 545–554.
- Oyaizu, M. (1986). Antiangiogenic properties of natural polyphenols from red wine and green tea. The Japanese Journal of Nutrition and Dietetics, 44, 307–315.10.5264/eiyogakuzashi.44.307
- Ozgen, M., Reese, R. N., Tulio, A. Z., Scheerens, J. C., & Miller, A. R. (2006). Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) methods. Journal of Agricultural and Food Chemistry, 54, 1151–1157.10.1021/jf051960d
- Puupponen-Pimiä, R., Häkkinen, S., Aarni, M., Suortti, T., Lampi, A. M., Eurola, M., & Piironen, V. (2003). Blanching and long-term freezing affect various bioactive compounds of vegetables in different ways. Journal of the Science of Food and Agriculture, 83, 1389–1402.10.1002/jsfa.1589
- Scalbert, A., Manach, C., Morand, C., Rémésy, C., & Jiménez, L. (2005). Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition, 45, 287–306.10.1080/1040869059096
- Sharma, S. K., Chaudhary, S. P., Rao, V. K., Yadav, V. K., & Bisht, T. S. (2011). Standardization of technology for preparation and storage of wild apricot fruit bar. Journal of Food Science and Technology, 48, 675–675.
- Szeto, Y. T., Tomlinson, B., & Benzie, I. F. (2002). Total antioxidant and ascorbic acid content of fresh fruits and vegetables: Implications for dietary planning and food preservation. British Journal of Nutrition, 87, 55–59.10.1079/BJN2001483
- Vallverdú-Queralt, A., Arranz, S., Casals-Ribes, I., & Lamuela-Raventós, R. M. (2012). Stability of the phenolic and carotenoids profile of gazpachos during storage. Journal of Agricultural and Food Chemistry, 60, 1981–1988.10.1021/jf204142j
- Wallin, B., Rosengren, B., Shertzer, H. G., Camejo, G., & Astra, H. A. B. (1993). Lipoprotein oxidation and measurement of thiobarbituric acid reacting substances formation in a single microtiter plate: Its use for evaluation of antioxidants. Analytical Biochemistry, 208, 10–15.10.1006/abio.1993.1002
- Wani, T. A., Wani, S. M., Shah, A. G., & Masoodi, F. A. (2013). Optimizing conditions for antioxidant extraction from Sea Buckthorn leaf (Hippophae rhamnoides L.) as herbal tea using response surface methodology (RSM). International Food Research Journal, 20, 1677–1681.
- Yen, G. C. & Chen, H. Y. (1995). Antioxidant activity of various tea extracts in relation their antimutagenicity. Journal of Agricultural and Food Chemistry, 43, 27–32.10.1021/jf00049a007