?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Fresh strawberries, analyzed for the physicochemical properties, showed average fruit length, width, weight, total soluble solids (TSS), titrable acidity, total sugar, and reducing sugar of 26.27 mm, 24.19 mm 6.33 g, 8.0°Brix, 1.28, 5.25, and 4.26%, respectively. The ascorbic acid and anthocyanin content was found to be 38.64 and 452 mg/100 g FW, respectively. Strawberries were processed into crush and divided into four treatments. T1 was stored at ambient temperature, T2 was pasteurized at 60°C for 20 min before storage at ambient temperature, T3 was stored under refrigeration, and T4 was pasteurized at 60°C for 20 min before storage under refrigeration. Among the treatments, T3 showed the lowest decrease in the acidity (1.43–1.34%), the highest anthocyanin (90.54–45.25 mg/100 g) and ascorbic acid contents (11.00–6.38 mg/100 g). The sensory attributes including color (8.25–7.75), taste (8.50–7.85), flavor (7.50–5.70), and overall acceptability (7.93–7.15) were also superior for T3 over the storage study of 120 days. T2 showed the maximum decrease in the acidity (1.40–1.12%) and corresponding increase in the pH (2.47–2.97), TSS (60.30–60.75°B), and reducing sugar (22.08–26.56%). However, its nutritional and sensory attributes were poor, showing the lowest anthocyanin (36.55–2.68 mg/100 g), ascorbic acid (8.64–0.85 mg/100 g), color (7.50–6.20), consistency (7.75–5.40), and overall acceptability (7.42–6.50) during the storage. Therefore, T3 could be explored for the best preservation and storage of strawberry crush.
Public Interest Statement
Strawberries are good for health as they contain different bioactive components. They are highly perishable and normally last for only a couple of days after harvesting. Processing of strawberry into some suitable products would help preserving them, thereby adding market value to it. This research would provide a know-how of preserving strawberry by developing strawberry crush and the nutritional and nutraceutical changes it undergoes with storage.
Competing interests
The authors declare no competing interests.
1. Introduction
Strawberry (Fragaria x ananassa) is typically a false fruit belonging to family rosaceae, the fleshy red outgrowth being its receptacle (Esau, Citation1977). It is one of the most widely consumed fruits in the world due to its pleasant organoleptic characteristics, nutritive value, and vitamin C (60 mg/100 g FW) content (Proteggente et al., Citation2002). Strawberries are produced in 73 countries worldwide on 256,108 hectares with average yield of 13,000 lbs/acre (F.A.O., Citation2009). The United States are the world’s leading producers of strawberries followed by Turkey and Spain (F.A.O., Citation2013). In India, it is grown in Jammu and Kashmir, Uttaranchal, Himachal Pradesh, and hills of Darjeeling (West Bengal) with a significantly gaining cultivation in the subtropical and tropical areas of other states (Singh & Mittal, Citation1992).
Ripe strawberries consist of approximately 90% water and 10% total soluble solids (TSS). The fresh fruit contains protein, fat, and carbohydrate contents of 0.7 g, 0.4 g, and 8.5 g/100 g, respectively. In addition to being a rich source of vitamin C, it is a good source of vitamin A (60 IU), thiamine (0.03 mg), riboflavin (0.07 mg), and niacin (0.6 mg) per 100 g FW (F.A.O., Citation2009). Strawberries are also an important source of phytochemicals and phenolics, which are responsible for its antioxidant activity (Lester, Lewers, Medina, & Saftner, Citation2012), making it a fruit with the highest antioxidant value among all the fruits (Cordenunsi et al., Citation2005). Phenolic compounds commonly found in strawberries include anthocyanins, hydrolysable tannins (ellagitannins), flavonols (quercetin, kaempferol and myricetin), flavan-3-ols (catequins and epicatechins) anthocyanins (pelargonidin-3-glycoside), β-carotene, and melatonin (Stürtz, Cerezo, Cantos-Villar, & Garcia-Parrilla, Citation2011), including some acids like hydroxybenzoic acids (gallic and ellagic acids) and hydroxycinnamic acids (p-cumaric) (Seeram, Lee, Scheuller, & Heber, Citation2006; Tulipani et al., Citation2008).
Phytochemicals are not only responsible for the antioxidant property, but also influence color and organoleptic attributes of the product (Lester et al., Citation2012; Odriozola-Serrano, Soliva-Fortuny, & Martín-Belloso, Citation2010; Pineli et al., Citation2011). Quality of strawberry fruits depends mainly on their appearance (color and biometrical characteristics), firmness, and chemical composition (Gunness, Kravchuk, Nottingham, D’Arcy, & Gidley, Citation2009). Color is one of the most important quality attributes of strawberries and one of the first parameters evaluated by the consumer, which is directly related to the anthocyanins’ content of the fruit (Crecente-Campo, Nunes-Damaceno, Romero-Rodríguez, & Vázquez-Odériz, Citation2012). In addition to the beneficial effect as antioxidants (Garcia-Alonso et al., Citation2005), the anthocyanins regulate adipocytokine gene expression (Tsuda, Ueno, Yoshikawa, Kojo, & Osawa, Citation2006), which is responsible for various complications like obesity, arterial sclerosis, liver steatosis, insulin resistance, and diabetes.
To extend the shelf life of fruits and increase their market value, they are processed into suitable products like jams, jellies, marmalades, squashes, crushes, and cordials. These products can be processed from almost any fruit and their recipes and specifications have long been standardized (Lal, Siddappaa, & Tandon, Citation1998). According to the Food Safety and Standards Act (FSSAI), 2006 of India, Crush means the product prepared from unfermented but fermentable fruit juice obtained from any suitable fruit by blending it with nutritive sweeteners and water. It should contain 25% fruit juice 55% TSS and maximum of 3.5% acidity. It is more or less similar to squash and is diluted before serving (Srivastava & Kumar, Citation2005). In the present study, strawberry fruits were processed to obtain strawberry crush, which was given a common chemical preservation treatment while varying its storage pretreatments and conditions. To assess the preservative effect of the pretreatments and conditions for the most prolonged shelf life of the product, the crush samples were studied periodically for three months in terms of its chemical and sensory characteristics.
2. Materials and methods
2.1. Raw material
Ripe strawberries (F. x ananassa Duch.) used in this research were of Chandler cultivar, native to Srinagar, Jammu and Kashmir, India. The strawberries were harvested at their physiological maturity in the month of May.
2.2. Preparation of strawberry pulp and development of strawberry crush
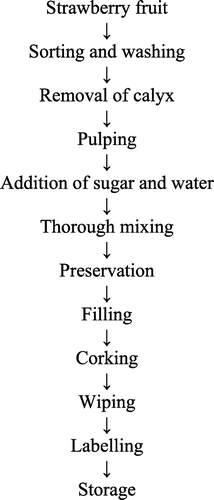
Excluding the defective, injured, and malformed strawberry fruits, the healthy and ripe ones were retained for pulp extraction. The fruit was washed under running tap water to remove any adhered dirt and the calyx was removed manually. To obtain the homogenized pulp, the fruits were crushed in a home scale mixer cum juicer and strained properly to remove the suspended seeds. Crush having 25% pulp and TSS of 60°Brix was prepared as given in Figure .
2.3. Preservation treatments of crush
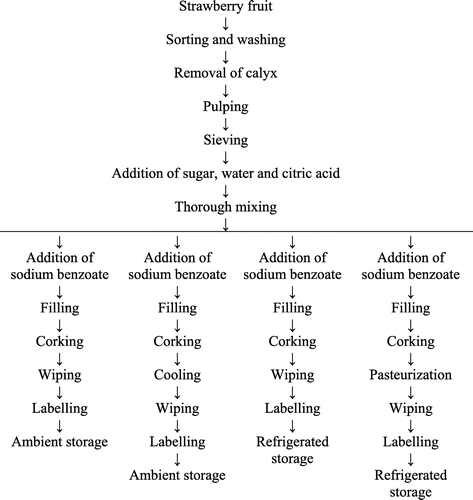
Strawberry crush was divided into four lots viz. T1, T2, T3, and T4 with a common chemical preservation treatment of 600 ppm sodium benzoate. T1 was stored at ambient temperature, T2 was pasteurized at 60°C for 20 min before storage at ambient temperature, T3 was stored under refrigeration, and T4 was pasteurized at 60°C for 20 min before storage under refrigeration. Pre-sterilized glass bottles were used for packaging of all the strawberry crush treatments (Figure ).
2.4. Physicochemical analysis of fresh strawberries and strawberry crush
2.4.1. Fruit weight
Fruit weight (in grams) was determined by taking the mean weight of 10 fruits using a digital electronic balance.
2.4.2. Axial diameter
Axial diameter (fruit length in mm) between the calyx end and the pedicel end of the fruit was measured with the help of a digital vernier caliper.
2.4.3. Radial diameter
The radial diameter (fruit breadth in mm) was measured with the help of a digital vernier caliper.
2.4.4. Total soluble solids
TSS content of the fresh fruit and the processed product was determined using a hand refractometer and the results were expressed as °Brix at 20°C using reference table.
2.4.5. Titrable acidity
Titrable acidity (TA) in fresh fruits was determined by titration of a known quantity of sample (10 mL) against 0.1 N sodium hydroxide using 1% phenolphthalein solution as an indicator to a persisting fade pink end point. The results were expressed as percent anhydrous citric acid (AOAC, Citation1994) using the following equation.
2.4.6. pH
pH of the fresh fruit and the treatments was determined using a digital pH meter (Hanna, USA).
2.4.7. Estimation of reducing sugars
The quantification of reducing sugars in the samples was carried out using Lane & Eynon method (AOAC, Citation2000). Five grams of each sample were placed in a measuring cylinder, to which 100-mL distilled water was added and stirred thoroughly. The samples were neutralized with 1 N NaOH with a phenolphthalein indicator. It was followed by addition of 5 mL of 45% lead acetate and 5 mL of 22% potassium oxalate after 10 min. The final volume of 250 mL (using distilled water) was filtered and marked as solution “A”. It was used to titrate the Fehling’s solution on hot plate until brick red color was observed. After the addition of 5–7 drops of methylene blue, the same was again titrated to permanent brick red color. Reducing sugars were calculated using the formula:
2.4.8. Estimation of total sugars
A measured aliquot (100 mL) of the filtrate used for the estimation of reducing sugars was hydrolyzed by 10 mL of 50% hydrochloric acid for 24 h at room temperature. After neutralization with 40% sodium hydroxide using phenolphthalein indicator, the volume was made up to 250 mL and titrated against Fehling’s solution as above. Total sugars were calculated using the formula:
2.4.9. Estimation of ascorbic acid
Ascorbic acid content of the crush samples was estimated by titration method (AOAC, Citation1996) using 2,6-dichlorophenol indophenol dye solution that gets reduced to a colorless form by ascorbic acid in alkaline solutions. The quantitative reaction is particularly specific for ascorbic acid in solution in the pH range of 1–3.5. It involved the standardization of the dye solution against standard ascorbic acid chiefly in order to determine the dye factor. The sample was diluted with 3% meta-phosphoric acid and then this phosphoric acid extract of the sample was titrated against the dye solution till the end point (pink color persisting for about 15 s). Dye factor was determined by the following equation:
Ascorbic acid was estimated as mg of ascorbic acid/mL and was calculated by the equation:
2.4.10. Estimation of total anthocyanins
Total anthocyanins were estimated according to the method of Fuleki and Francis (Citation1968). Ten milliliters of sample taken with 50 mL of ethanolic HCl were filtered through Whatman No. 4 filter paper. The volume was raised to 250 mL with ethanolic HCl. Two milliliters of this aliquot were again diluted to 100 mL with ethanolic HCl and its absorbance was measured at 535 nm in UV–visible spectrophotometer.
2.5. Sensory evaluation
A nine-point hedonic scale was used for conducting the sensory evaluation of the product. A panel of 15 judges comprising of faculty members and postgraduate students of the Department of Food Science and Technology, University of Kashmir, Hazratbal, Srinagar were selected to evaluate the products for various sensory parameters like color, flavor, consistency, taste, and overall acceptability. The samples were presented to the judges the way they are normally consumed. Coded samples were presented to the judges in separate chambers or places to get unbiased judgments. Plain water was given to the judges to rinse their mouth in between the evaluation of samples. No discussion during sensory evaluation was allowed.
2.6. Statistical analysis
The data recorded were analyzed statistically for interpretations of results using analysis of variance technique for CRD factorial (Gomez & Gomez, Citation1984), while randomized complete block design (RBD) as described by Mahony (Citation1985) was used to analyze the data pertaining to the sensory evaluation of strawberry crush.
3. Results and discussion
3.1. Physicochemical properties of fresh strawberry fruits
Physical properties reflect the maturity of the fruits harvested and in the present study, average length, width, and weight of fresh strawberry fruits were observed to be 26.27 mm, 24.19 mm, and 6.33 g, respectively. The pulp obtained had TSS of 8.0°Brix. TA, total sugar, and reducing sugar contents of 1.28, 5.25, and 4.26%, respectively were recorded. The value of ascorbic acid was observed to be 38.64 mg and that of anthocyanin was observed to be 452 mg per 100 g of sample (Table ). Results of the present analysis are in accordance with the previous literature (Kalt, Forney, Martin, & Prior, Citation1999).
Table 1. Physicochemical characteristics of fresh strawberry fruit (n = 10)
3.2. Effect of preservation methods and storage period of strawberry crush on acidity, pH, and TSS
TA was observed to decrease in all the four treatments with respect to the storage period of 0–120 days (Table ). A corresponding increase in the pH value of the four treatments was observed over the storage period in general (Table ). The pH increased significantly for T2 (2.96 ± 0.10) and T4 (3.06 ± 0.10) for the storage period of 60 and 120 days, respectively, while no significant change in pH was observed for T1 and T3. The increase in pH or decrease in acidity can presumably be in part due to copolymerization of organic acids and formation of brown pigments. The observed results are in conformity with those of Buglione and Lozano (Citation2002).
Table 2. Effect of preservation methods and storage period on pH, acidity, and TSS of strawberry crush (n = 3)
The TSS in general was observed to increase slightly for all the four differently treated crush samples varying from 60.07 to 60.66 °B, 60.30 to 60.75 °B, 60.06 to 60.43 °B, and 60.08 to 60.73 °B, respectively for T1, T2, T3, and T4 over the storage period of 0–120 days (Table ). T1 and T4 showed significant changes in TSS on the 60 days storage period, which further increased in 120 days storage period, while T2 and T3 showed significant increase in TSS only at 120 days storage period. The increase is believed to occur presumably due to conversion of some of the insoluble fraction into soluble fraction during the storage and/or to a lesser extent the moisture loss that is expected before corking of bottles. Further implications reveal that with storage, there was an augmentation in the TSS/TA ratio, which increased the sweetness of the product with storage. A similar kind of effect was observed by Ornelas-Paz et al. (Citation2013) while studying the changes in strawberry fruit upon ripening.
3.3. Effect of preservation methods and storage period on reducing sugars, total sugars, anthocyanins, and ascorbic acid of strawberry crush
The reducing sugars of strawberry crush increased in the entire storage study of the four treatments (Table ). All the treatments showed a significant increase in the reducing sugar content on the 60 days storage period. A further significant rise was seen for T2 (26.56%) and T4 (26.54%) on the 120 days storage period, with T2 displaying the highest value of reducing sugar among the treatments. The increase in reducing sugar content during storage may be due to inversion of sucrose to glucose and fructose or breakdown of polysaccharides into simple sugars. Reducing sugars are chiefly responsible for Maillard reaction (Nayak, Liu, & Tang, Citation2013). A similar trend was observed in case of the total sugars. A significant increase in the total sugar content was observed in the treatments for both the 60 days period and the 120 days period, with T1 showing the highest sugar content values of 58.64 and 60.94%, respectively (Table ). The increase in the total sugar content is expected to be due to the conversion of insoluble polysaccharides to soluble form during storage. Increase in reducing and total sugars upon storage has also been observed by (Mir et al., Citation2015) in quince candy.
Table 3. Effect of preservation methods and storage period on reducing sugars and total sugars of strawberry crush (n = 3)
The anthocyanin content decreased significantly in all the four treatments of strawberry crush from 0 to 120 days of storage (Table ; Figure ). T3 showed the highest anthocyanin content at both 30 days (60.59 mg/100 g) and 120 days (45.25 mg/100 g) of storage, while T2 showed the lowest anthocyanin content for both 30 days (18.54 mg/100 g) and 120 days (2.68 mg/100 g) of storage. The loss of anthocyanin in strawberry crush during storage has been attributed to many factors or combination of factors such as pH and acidity, phenolic compounds, sugar and sugar degradation products, oxygen, and ascorbic acid (Abers & Wrolstad, Citation1979). The anthocyanin content of strawberry crush found in the present study is close to strawberry juice (Lopes-da-Silva, Escribano-Bailón, Perez-Alonso, Rivas-Gonzalo, & Santos-Buelga, Citation2005) with the reported expected variations depending on the strawberry cultivar.
Table 4. Effect of preservation methods and storage period on anthocyanins and ascorbic acid of strawberry crush (n = 3)
The ascorbic acid content decreased significantly in all the four treatments of strawberry crush from 0 to 120 days of storage (Table ). T3 showed the highest ascorbic acid content at both 30 days (9.85 mg/100 g) and 120 days (6.38 mg/100 g) storage period, while T2 showed the lowest ascorbic acid content for both 30 days (4.80 mg/100 g) and 120 days (0.85 mg/100 g) storage period. The decrease in ascorbic acid content during storage might be attributed to its oxidation to dehydro ascorbic acid and further to 2,3 diketo-gluconic acid (Damame, Gaikwad, Patil, & Masalkar, Citation2002).
3.4. Effect of preservation methods and storage period of strawberry crush on sensory parameters (color, taste, flavor, consistency, and overall acceptability)
The color content measured showed a significant decrease in the scores from 0 to 120 days of storage (Table ). T3 showed the highest color score for 60 days (8.10) and 120 days (7.75) storage period, while T2 showed the lowest color score for 120 days (6.20) storage period. The decrease in color score is expected to be due to the browning reaction between reducing sugars and amino acids, accelerated by high temperature and oxidation of phenolic compounds. The taste measured in terms of the scores decreased significantly from 0 to 120 days of storage (Table ). T3 showed the highest taste score for 60 days (8.00) and 120 days (7.85) storage period, while T4 showed the lowest taste score for 60 days (6.75) and 120 days (6.50) storage period. The loss of taste is presumably due to the oxidative and other deteriorative reactions occurring within the product during its storage accompanied with the degradation of ascorbic acid and furfural production (Shimoda & Osajima, Citation1981). A similar decrease was observed in the flavor scores of strawberry crush samples from 0 to 120 days of storage (Table ). T3 showed the highest flavor score of (7.50) for 60 and 120 days storage period, which decreased to the lowest flavor score of (5.70) for 120 days of storage. Golaszewski, Sims, O’keefe, Braddock, and Littell (Citation1998) reported that butyl acetate, ethyl hexanate, and ethyl propionate are the main flavoring volatiles in strawberry in addition to other alcohols. The continuous decline in sensory scores during storage can be attributed to the loss and/or modification of many chemical constituents of the product, especially the flavorants. The slow change in sensory attributers during storage under refrigerated conditions is attributed to the decrease in the rate of these deteriorative reactions at low temperature. The above results are in conformity with the findings of Askar, Ghonaim, Fadeel, and Ali (Citation1996) in peach nectar, Krishnaveni, Manimegalai, and Savavanakumar (Citation2001) in pomegranate squash, and Sogi and Singh (Citation2001) in Kinnow squash.
Table 5. Effect of preservation methods and storage period on sensory parameters (n = 15)
The consistency scores showed a gradual decrease upon storage (Table ). The results obtained were in consonance with that of Saini and Grewal (Citation1995). The overall acceptability also decreased during storage (Table ). T3 showed the significantly highest overall acceptability score for the 0 days (7.93), 60 days (7.62), and 120 (7.15) storage period, while T2 showed the lowest overall acceptability score for 120 days (6.50) storage period. The results obtained are in conformity with the findings of Waskar and Khurdiya (Citation1987) in phalsa squash and nectar, Krishnaveni et al. (Citation2001) in jack fruit RTS beverage, Sogi and Singh (Citation2001) in Kinnow squash, and Kotecha and Kadam (Citation2003) in tamarind RTS beverage.
Table 6. Effect of preservation methods and storage period on sensory parameters continued (n = 15)
4. Conclusion
Based on the study, it is concluded that heat treatment causes a reduction in vitamin content, antioxidant activity, and overall acceptability of strawberry crush and accordingly the best preservation was observed in the treatment with 600 ppm sodium benzoate followed by refrigerated storage. However, it was least preserved when given the same chemical preservation followed by the pasteurization treatment at 60°C for 20 min and stored at ambient temperature. Therefore, sodium benzoate treatment of strawberry crush followed by refrigerated storage could be exploited at the industrial level for its better preservation and marketability.
Acknowledgments
The authors are highly thankful to the Department of Biotechnology, Government of India, for financial support of this project.
Additional information
Funding
Notes on contributors
Sabeera Muzzaffar
Sabeera Muzzaffar is presently working as an assistant professor in the department of Food Science and Technology at University of Kashmir, Hazratbal, Srinagar (India). She did BSc in Agriculture from SKUAST, Kashmir and completed her master’s in post-harvest technology from Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP) in the year 2007. She has 11 research papers in various national and international journals to her credit. Besides, she has more than five years of teaching and research experience. She is presently working on phytochemicals of various plum cultivars for their antioxidant potential.
References
- Abers, J. E., & Wrolstad, R. E. (1979). Causative factors of colour deterioration in strawberry preserves during processing and storage. Journal of Food Science, 44, 75–78.10.1111/jfds.1979.44.issue-1
- AOAC. (1994). Officials methods of analysis. Washington, DC: Author.
- AOAC. (1996). AOAC Official Method 967.21. Ascorbic acid in vitamin preparations and juices. 2,6-dichloroindophenol titrimetric method. Official methods of analysis of AOAC international (pp. 16–17). Arlington, TX: Author.
- AOAC. (2000). Official Method 925.35 Sucrose in fruits and fruit products read with AOAC official method 923.09. Lane and Eynon general volumetric method (17th ed.). Arlington, TX: Author.
- Askar, A., Ghonaim, S. M., Fadeel, A. G. M., & Ali, A. M. (1996). Quality assurance of peach pulp and nectar. Fruit Processing, 6, 151–155.
- Buglione, M., & Lozano, J. (2002). Non-enzymatic and chemical changes during grape juice storage. Journal of Food Science, 67, 1538–1543.10.1111/jfds.2002.67.issue-4
- Cordenunsi, B. R., Genovese, M. I., do Nascimento, J. R. O., Hassimotto, N. M. A., dos Santos, R. J., & Lajolo, F. M. (2005). Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chemistry, 91, 113–121.10.1016/j.foodchem.2004.05.054
- Crecente-Campo, J., Nunes-Damaceno, M., Romero-Rodríguez, M. A., & Vázquez-Odériz, M. L. (2012). Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria X ananassa Duch, cv Selva). Journal of Food Composition and Analysis, 28, 23–30.10.1016/j.jfca.2012.07.004
- Damame, S. V., Gaikwad, R. S., Patil, S. R., & Masalkar, S. D. (2002). Vitamin C content of various aonla products during storage. The Orissa journal of Horticulture, 30, 19–22.
- Esau, K. (1977). Anatomy of seed plants. New York, NY: Wiley.
- F.A.O. (2009). National Agriculture Statistics Service, U.S. Department of Agriculture. Retrieved from http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor
- F.A.O. (2013). FAOSTAT, Food and Agriculture Commodities production. Retrieved from http://www.fao.org/docrep/018/i3107e/i3107e03.pdf
- Fuleki, T., & Francis, F. J. (1968). Quantitative methods for anthocyanins. 1 Extraction and determination of total anthocyanin in cranberries. Journal of Food Science, 33, 72–77.10.1111/jfds.1968.33.issue-1
- Garcia-Alonso, M., Rimbach, G., Sasai, M., Nakahara, M., Matsugo, S., Uchida, Y., … De Pascual-Teresa, S. (2005). Electron spin resonance spectroscopy studies on the free radical scavenging activity of wine anthocyanins and pyranoanthocyanins. Molecular Nutrition and Food Research, 49, 1112–1119.10.1002/(ISSN)1613-4133
- Golaszewski, R., Sims, C. A., O’keefe, S. F., Braddock, R. J., & Littell, R. C. (1998). Sensory attributes and volatile components of stored strawberry juice. Journal of Food Science, 63, 734–738.
- Gomez, K. A., & Gomez, A. A. (1984). Statistical procedures for agricultural research (2nd ed.). New York, NY: Wiley-Interscience Publication, Wiley.
- Gunness, P., Kravchuk, O., Nottingham, S. M., D’Arcy, B. R., & Gidley, M. J. (2009). Sensory analysis of individual strawberry fruit and comparison with instrumental analysis. Postharvest Biology and Technology, 52, 164–172.10.1016/j.postharvbio.2008.11.006
- Kalt, W., Forney, C. F., Martin, A., & Prior, R. L. (1999). Antioxidant capacity, Vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. Journal of Agricultural and Food Chemistry, 47, 4638–4644.10.1021/jf990266t
- Kotecha, P. M., & Kadam, S. S. (2003). Preparation of ready-to-serve beverage, syrup and concentrate from tamarind. Journal of Food Science and Technology, 40, 76–79.
- Krishnaveni, A., Manimegalai, G., & Savavanakumar, R. (2001). Storage stability of jack fruit (Atrocarpus heterophyllus L.) RTS beverage. Journal of Food Science and Technology, 38, 601–602.
- Lal, G., Siddappaa, G. S., & Tandon, G. L. (1998). Preservation of fruits & vegetables. New Delhi: Indian Council of Agricultural Research.
- Lester, G. E., Lewers, K. S., Medina, M. B., & Saftner, R. A. (2012). Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. Journal of Food Composition and Analysis, 27, 102–107.10.1016/j.jfca.2012.05.003
- Lopes-da-Silva, F., Escribano-Bailón, M. T., Perez-Alonso, J. J., Rivas-Gonzalo, J. C., & Santos-Buelga, C. (2005). Anthocyanin pigments in strawberry. Food Science and Technology, 40, 374–382.
- Mahony, M. O. (1985). Sensory evaluation of food: Statistical methods and procedures (pp. 168–169). New York, NY: Marcel Dekker.
- Mir, S. A., Wani, S. M., Ahmad, M., Wani, T. A., Gani, A., Mir, S. A., & Masoodi, F. A. (2015). Effect of packaging and storage on the physicochemical and antioxidant properties of quince candy. Journal of Food Science and Technology, 52, 7313–7320.10.1007/s13197-015-1819-y
- Nayak, B., Liu, R. H., & Tang, J. (2013). Effect of processing on phenolic antioxidants of fruits, vegetables and grains—A review. Critical Reviews in Food Science and Nutrition. doi:10.1080/10408398.2011.654142
- Odriozola-Serrano, I., Soliva-Fortuny, R., & Martín-Belloso, O. (2010). Changes in bioactive composition of fresh-cut strawberries stored under superatmospheric oxygen, low-oxygen or passive atmospheres. Journal of Food Composition and Analysis, 23, 37–43.10.1016/j.jfca.2009.07.007
- Ornelas-Paz, J. J., Yahia, E. M., Ramírez-Bustamante, N., Pérez-Martínez, J. D., Escalante Minakata, M., Ibarra-Junquera, V., … Ochoa-Reyes, E. (2013). Physical attributes and chemical composition of organic strawberry fruit (Fragaria x ananassa Duch, Cv. Albion) at six stages of ripening. Food Chemistry, 138, 372–381.10.1016/j.foodchem.2012.11.006
- Pineli, L. L. O., Moretti, C. L., dos Santos, M. S., Campos, A. B., Brasileiro, A. V., Córdova, A. C., & Chiarello, M. D. (2011). Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. Journal of Food Composition and Analysis, 24, 11–16.10.1016/j.jfca.2010.05.004
- Proteggente, A. R., Pannala, A. S., Paganga, G., Buren, L., Wagner, E., Wiseman, S., & Rice-Evans, C. A. (2002). The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and Vitamin C composition. Free Radical Research, 36, 217–233.10.1080/10715760290006484
- Saini, S. P. S., & Grewal, V. S. (1995). Physico-chemical changes during manufacture and storage of sand Pear (Pyrus pyrifolia) juice into concentrate. Indian Food Packer, 3, 5–13.
- Seeram, N. P., Lee, R., Scheuller, H. S., & Heber, D. (2006). Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chemistry, 97, 1–11.10.1016/j.foodchem.2005.02.047
- Shimoda, M., & Osajima, Y. (1981). Studies on off-flavour formed during storage of Satsuma mandarin juice. Journal of The Agricultural Chemical Society of Japan, 55, 319–324.10.1271/nogeikagaku1924.55.319
- Singh, S., & Mittal, J. P. (1992). Energy in production agriculture. New Delhi: Mittal Pub.
- Sogi, D. S., & Singh, S. (2001). Studies on bitterness development in Kinnow juice, ready-to- serve beverages, squash, jam and candy. Journal of Food Science and Technology, 38, 433–438.
- Srivastava, P. R., & Kumar, S. (2005). Fruit and vegetable preservation, principles and practices (3rd ed., p. 192). International Book Distribution Company.
- Stürtz, M., Cerezo, A. B., Cantos-Villar, E., & Garcia-Parrilla, M. C. (2011). Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chemistry, 127, 1329–1334.10.1016/j.foodchem.2011.01.093
- Tsuda, T., Ueno, Y., Yoshikawa, T., Kojo, H., & Osawa, T. (2006). Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochemical Pharmacology, 71, 1184–1197.10.1016/j.bcp.2005.12.042
- Tulipani, S., Mezzetti, B., Capocasa, F., Bompadre, S., Beekwilder, J., de Vos, C. R., … Battino, M. (2008). Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. Journal of Agricultural and Food Chemistry, 56, 696–704.10.1021/jf0719959
- Waskar, D. P., & Khurdiya, D. S. (1987). Processing and storage of phalsa beverages. Indian Food Packer, 41, 7–16.