?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Starches from three organically produced cultivars of potato tuber (Lady Rosetta, Spunta and Voyager) have been studied in relation to (i) acrylamide production (ii) macromolecular integrity after frying with extra virgin olive oil, soybean oil and corn oil. During cultivation, a treatment involving the combination of nitrogen, phosphorus and potassium fertilization under organic farming was applied (N1, P2, K1 where Ν1 = 1.3 g Ν per plant, P2 = 5.2 g P2O5 per plant, Κ1 = 4.0 g K2O per plant).
Potatoes fried in olive oil retained the highest glucose concentrations for all cultivars 0.85 ± 0.2 mmol/kg, followed by 0.48 ± 0.2 for those fried in corn oil and 0.40 ± 0.1 mmol/kg for those fried in soybean oil. The highest average fructose concentration was recorded for the samples fried in corn oil as 0.81 ± 0.2, followed by 0.80 ± 0.2 and 0.68 ± 0.3 mmol/kg for the samples fried in olive and soybean oils, respectively. Asparagine was the most abundant free amino acid in the three varieties tested, followed by glutamine and aspartic acid. The mean initial concentration of asparagine in raw potatoes tubers was 42.8 ± 1.6 mmoles kg−1 for Lady Rosetta, 34.6 ± 1.2 mmoles kg−1 (dry weight) for Spunta and 36.2 ± 2.0 mmoles kg−1 for Voyager. Lady Rosetta contained a significantly higher concentration of asparagine compared to the other two varieties (p < 0.05). The greatest quantity of acrylamide was observed in French fries derived from the potato variety Lady Rosetta when fried in soybean oil and it was 2,600 ± 440 μg/kg, followed by Spunta which was 2,280 ± 340 μg/kg and Voyager 1,120 ± 220 μg/kg. There is a significant reduction in the formation of acrylamide in the variety Voyager compared to the others (p = 0.05).
Public Interest Statement
This paper addresses the effect of thermal processing in oil on the macromolecular integrity and acrylamide formation from starch of three potato cultivars organically fertilized.
This paper is quite significant since it is a farm to fork approach addressing the effect of organic fertilization and different cultivars used by the Greek food industry on the acrylamide production and macromolecular integrity, sugars and amino acid contents as well as oil profile (extra virgin olive oil, corn oil and soybean oil).
It brings new knowledge to the field since it will help the industry to adopt practical, effective and innovative ways to further reduce the levels of acrylamide to help satisfy market and consumer pressures.
The predominant effect of reducing sugar concentration on the acrylamide formation in potato products was confirmed here. Finally, it was found that there was no significant effect of the type of frying oil on the levels of acrylamide.
Competing interests
The authors declare no competing interests.
1. Introduction
The formation of acrylamide, a neurotoxic compound and possible carcinogen, in heated foodstuffs has been a global health concern to markets and consumers, as well as a matter of investigation by research groups and food safety committees (Halford et al., Citation2012). Acrylamide is formed naturally in plant-derived and starch-rich foodstuffs such as potato- and grain-based foods that are cooked at high temperatures (Pedreschi, Mariotti, & Granby, Citation2013).
Acrylamide is formed in heated foods, generally starchy, through the process of the Maillard reaction, in which sugars react with the amino acid asparagine, the role of which has been well established over recent years (Koutsidis, De la Fuente, Dimitriou, Kakoulli, & Wedzicha, Citation2008). However, the relative importance of different sugars and/or carbonyls as reactive species, as well as the conditions employed, may play a crucial role in its formation (Koutsidis et al., Citation2009). It is suggested that both molecular mobility and sugar reactivity would determine the relative effect of sugars on the acrylamide formation, whereas temperature may also play an important role in determining the relative reactants (Wedzicha, Mottram, Elmore, Koutsidis, & Dodson, Citation2005).
There are uncertainties regarding the link between acrylamide and cancer risk in humans: some results suggest a link between human exposure and cancer, whilst others do not support such a conclusion (Hogervorst, Schouten, Konings, Goldbolun, & Vad den Brandt, Citation2007). Exposure data, which are required to evaluate the link between acrylamide and cancer, are limited. In 2002, the European Commission began collecting occurrence data on the levels of acrylamide in foods. In February 2005, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) carried out a safety evaluation on acrylamide in food concluding that the issue poses a human health concern (Food and Agricultural Organisation/World Health Organisation, JECFA, Citation2005). This conclusion was consistent with an opinion published by the Scientific Committee on Food (SCF) in 2002. In co-operation with Member States, the European Food Safety Authority (EFSA) took over responsibility for this task in 2006.
National food safety authorities in the EU Member States, academia and food manufacturers have sought to better understand acrylamide and to reduce its levels in foods. Many countries continue to contribute to the growing body of research and data. Workshops on this issue have been organized by EFSA in 2003 and the European Commission jointly with the European food and drink industry association (CIAA) in 2006 (European Food & Drink Industry Association (CIAA)-Food Drink Europe, Citation2011). CIAA has published an “Acrylamide Toolbox” based on existing knowledge in the food industry, which is regularly updated.
Efforts have been made by food manufacturers to modify recipes and processes to reduce acrylamide occurrence in foods such as French fries, snacks and crisps. Acrylamide formation is also interrelated with quality attributes of heated foodstuffs that arise due to the involvement of common Maillard reaction intermediates. The addition of amino acids has been proposed as a mitigation strategy to reduce the levels of acrylamide in crisps, flat breads, and bread crust, while glycine has received particular attention as an additive that could potentially reduce the acrylamide formation by either competing for available Maillard reaction intermediates or reacting with acrylamide itself through Michael addition type reactions (Low et al., Citation2006). Inevitably, the application of any reduction technique must be a cost-effective solution since it is likely any additional expense will be transferred to the consumers.
Numerous pathways that may lead to the formation of acrylamide have been depicted in the literature, for instance the formation of acrolein by the degradation of oxidative lipid, leading to ultimately acrylic acid, which in turn reacts with asparagine to form acrylamide (Yasuhara, Tanaka, Hengel, & Shibamoto, Citation2003). However, several research groups have confirmed that the main route for acrylamide in food is via the Maillard reaction, which is a complex series of non-enzymatic reactions between amino groups and reducing sugars that often determine the colour, flavour and texture of cooked foods (Halford et al., Citation2012).
In the present study, the effect of organic fertilization on sugar and amino acid contents has been examined with respect to the acrylamide formation in potato tubers of three chipping varieties of potato Lady Rosetta, Spunta and Voyager after frying in either extra virgin olive oil, soybean or corn oil was studied. A key question was which oil is most appropriate for frying, and which frying conditions assure quality and safety of the fried products, particularly potato chips (Andrikopoulos, Kalogeropoulos, Falirea, & Barbagianni, Citation2002; Lolos, Oreopoulou, & Tzia, Citation1999; Moreira, Castell-Perez, & Barufet, Citation1999; Tareke, Rydberg, Karlsson, Eriksson, & Törnqvist, Citation2002). We consider the effects of different frying vegetable oils on the creation of acrylamide in potato chips and the estimation of reactants and residuals involved in the acrylamide formation as well as oil uptake of potatoes after frying (Willinger, Citation1964). In addition, the evaluation of the macromolecular integrity of potato amylose and amylopectin is also investigated, in an attempt to correlate their content and configuration with acrylamide formation.
The results will help the industry to adopt practical, effective and innovative ways to further reduce the levels of acrylamide to help satisfy market and consumer pressures.
2. Materials and methods
2.1. Sample materials
For the investigation of the influence of oil type on the acrylamide formation, eight samples of potato tuber were prepared and fried in three different types of oils: olive oil, corn oil and soybean oil.
Potato varieties have been chosen according to suggestions of the Ministry of Agriculture, Greece and the conditions favouring their production in the area of Messinia and those cultivated for use by the major food industries in Greece. (http://varieties.potato.org.uk). They were collected from local companies like Compo Hellas.
Treatments with two different concentrations of nitrogen, phosphorus and potassium have been used plus the control. So for each variety, eight different fertilization treatments have been used. For each treatment, three repetitions have been made with 5 plants each. Experiment was three factorial with two levels for each factor and due to the statistically significant interaction between the factors regression was carried out for each factor separately. The t-test tested the significance at a level of p ≤ 005.
For the effects of the influence of potato cultivar and organic fertilization on the acrylamide formation, five samples of potato tubers of three different chipping varieties of potato (Solanum tuberosum L.) were used. These were, Spunta, Lady Rosetta and Voyager. For the investigation of the influence of oil type on moisture content, titratable acidity, reducing sugar concentration and adsorbed oil from French fries, eight samples of potato tubers from each of the three chipping varieties of potato were used. During cultivation of three potato cultivars (Voyager, Spunta and Lady Rosetta), nine treatments (T) involving the combination of nitrogen, phosphorus and potassium fertilization were applied.
T1: control treatment; T2: N1, P1, K1; T3: N1, P1, K2; T4: N1, P2, K1; T5: N1, P2, K2; T6: N2, P1, K1; T7: N2, P1 K2; T8: N2, P2, K1; and T9: N2, P2, K2, where Ν1 = 1.3 g Ν per plant; N2 = 2.0 g Ν per plant; P1 = 3.1 g P2O5 per plant; P2 = 5.2 g P2O5 per plant; Κ1 = 4.0 g K2O per plant; and Κ2 = 6.6 g K2O per plant.
Results presented here refer to treatment 4. T1: control treatment; T4: N1, P2, K1 where Ν1 = 1.3 g Ν per plant; P2 = 5.2 g P2O5 per plant; Κ1 = 4.0 g K2O per plant. Details of fertilizer application are given in the article by Nikolaou, Varzakas, and Kourkoutas (Citationin press).
Each treatment was applied to five replicates of four plants each and the experiments were carried out at the Technological Educational Institute of Peloponnese (Kalamata, Greece) between March and May 2013.
2.2. General procedure
Tubers were washed and cut into pieces (~1 × 1 × 3 cm) with a French fry-shaped cutter (An industrial peeler (model M591E4, IMC, England) was used. A circular cutting mould was used to provide chips with a diameter of 40 mm.
There was no colour change of potato while cutting as confirmed by a Hunter chroma meter. Potatoes were cut and then immediately transferred to the deep frier.
Potato slices were rinsed immediately after cutting for 1 min in distilled water to eliminate some starch material adhering to the surface prior to frying.
Two litres of oil was weighed and placed in a 5-L domestic deep-fryer (electrical fryer, Greek local company) with a thermostat and preheated. The fryer was equipped with a stirring mechanism to ensure a homogeneous temperature in the oil bath. French fries were weighed and 250 g of each fried at 180 ± 1°C for 8 min. After frying, potatoes remained in the fryer basket mesh, for one min, to remove the excess of surface oil. Then they were placed in pre-weighed filter paper to cool. The oil used in the fryer was refilled after 8 consecutive frying times.
2.3. Absorbed oil measurement
After four consecutive frying times, the oil was allowed to cool and then weighed to calculate the amount of oil absorbed by the potatoes during frying. The results were expressed as g absorbed oil per 100 g raw potatoes.
2.4. Starch sample preparation and characterization
Starch was extracted from raw potato and fried chips using a combined protocol first described by Willinger (Citation1964) and Rosenthal and Espindola (Citation1969). Samples were rinsed with distilled water, peeled, diced and disintegrated in a domestic blender in 1% sodium metabisulphite (Sigma Aldrich, UK, analytical grade) solution to prevent oxidation. The resulting slurry was sifted through a 0.25 mm sieve, rinsed and sieved again.
Samples were centrifuged at 6,000 rpm for 10 min to remove excess solvent and degrease the sample. Peletted material was suspended in 0.1 M sodium hydroxide solution (Fisher Scientific, UK, analytical grade), re-centrifuged and repeated with 0.15 M sodium hydroxide solution. The remaining pellet was rinsed with deionized water until at neutral pH and with 70% ethanol (Fisher Scientific, analytical grade). The sample was dried in an oven at 45°C.
For hydrodynamic characterization, samples were dissolved in 90% DMSO (Fisher Scientific, analytical grade). Intrinsic viscosity [η] was measured using an Ostwald capillary suspended in a water bath temperature controlled to 25.00 ± 0.01°C. Data were analysed using a linear extrapolation of the Huggins (1942) and Kraemer (1938) equations as reported by Harding (Citation1997):(1)
(1)
(2)
(2)
where (ηr) is the relative viscosity (flow time of solution divided by flow time of solvent), (C) is the mass concentration, and (kH) and (kK) are the Huggins and Kraemer constants, respectively.
The hydrodynamic radius (rH) was estimated using Dynamic Light Scattering (DLS) from a ZetaSizer NanoZS (Malvern Instruments, UK) and analysed using Zetasizer Software (v6.20, Malvern Instruments, UK). Intensity fluctuations in the solution were measured at 20.0°C, with backscatter intensities from the solutions from a 632 nm laser registered at an angle of 173°.
3. Sample preparation and analytical procedures
The fried potatoes were retained at room temperature until equilibration. Twenty g was weighed and 150 ml H2O was added. The mixture was homogenized for 2 min and the solution allowed to stand for 10 min before being centrifuged for 15 min at 4,000 rpm. The supernatant solution was filtered on a filter paper with a pore size of 0.45 μm. The collected liquid was stored in glass vials and placed in a −20°C freezer.
The titratable acidity (TA) and the reducing sugar concentration (RS) were measured in the filtered solution. TA was determined according to EC guidelines (Commission Regulation (EC) No 1065/97, Commission Regulation (EC) No 2568/91). Reducing sugars were determined by the DNS method (Miller, Citation1959).
4. Determination of Sugars
4.1. Preparation of the extracts from raw and French fries samples
The samples were prepared using a modification of the method reported by Halford et al. (Citation2012). Four raw potatoes were washed, peeled and cut into small pieces. Thereafter, two g of each was weighted onto a plastic plate and was put into a falcon tube followed by adding 5 ml of an aqueous methanol 50%. The sample was then blended and homogenized using a DI 25 basic dispersing device (KIKA-WERKE GMBH and Co. KG, Staufen, Germany) for 15 min at low speed at room temperature or until uniform, and was then centrifuged by benchtop Jouan C3i centrifuge (Thermo-Fisher Scientific, USA) at 4,000 rpm for 20 min. The aqueous supernatant was transferred to a screw top bottle (being careful to avoid solid particles) and filtered twice through Minisart filter units (0.45 μm), collecting approximately 4 ml of the filtrate, which was stored at −80°C.
4.2. Analysis of the extracted samples using LC-MS
The prepared samples were analysed by LC-MS using an Agilent 1100 high-performance liquid chromatography (HPLC) instrument (Agilent Technologies, Wokingham Berkshire, UK), with a quadrupole mass spectrometer operated in negative electrospray ionization mode (ESI). An isocratic separation was performed at room temperature using Luna 5μ NH2 100A 250 × 2.0 mm (Phenomenex Inc., Macclesfield, UK). The parameters of the instrument were as follows: the column temperature was 40°C. The mobile phase was ACN 80% in purified water, and the flow rate was 0.7 ml/min. The injection volume was 5 μl. Each extract was analysed in triplicate (technical replicate). The run time was 8 min, and the data were collected from 2 to 8 min. Fructose, glucose and sucrose eluted at around 2.07, 2.48 and 3.31 min, respectively. The mode of MS monitoring was selected ion recording (SIR), (where 277.4, 293.5 and 367.4 ions were detected for fructose and glucose) and (341.4, 377.4 and 404.4 ions were detected for sucrose). The dwell time was 0.15 min, and the cone voltage was set at 40 V. Data were reported on a dry weight basis.
MassLynx software was used to obtain data and to transform them into usable results. The chromatogram readings of the gradient mixed standard sugar solutions (10–60 μg/ml) were used to plot a calibration curve for each sugar; glucose, fructose and sucrose, which then were used to determine the unknown concentration corresponding to each of these sugars in a dilution series (50% water/methanol) of the samples under investigation.
5. Determination of amino acids
5.1. Preparation of the extracts from raw and French fried samples
Raw potatoes were peeled after washing, cut, then 1 g of each placed in a falcon tube, to which 4 ml of hydrochloric acid (0.01 mol/L) was added to each tube and the sample was mixed for 15 min at low speed at room temperature until it became homogenized using a DI 25 basic dispersing device (KIKA-WERKE GMBH and Co. KG, Staufen, Germany). The homogenate was then centrifuged by benchtop Jouan C3i centrifuge (Thermo-Electron Corporation, USA) at 4,000 rpm for 20 min. The aqueous supernatant was carefully transferred to a screw top bottle (avoiding the solid partials), followed by filtration using Millipore Millex-HN filter units (0.45 μm) twice, collecting approximately 3 ml of the filtrate and stored at −80°C in a freezer. The amino acids in 25 μl of the filtrate were then derivatized using the EZ-Faast amino acid derivatization kit (Phenomenex, Torrance, CA, USA). Twenty-five microlitre of each filtrate was mixed with 100 μl of norvaline (equivalent to 20 nmol of norvaline) which acted as an internal standard (IS). This mixture was passed through the EZ: fast solid phase extraction sorbent (contained within a pipette tip) which was thereafter washed with 200 μl propanol. A solution of propanol and sodium hydroxide (200 μl) was then used to remove the sorbent (and the amino acids retained on it) from the pipette tip. Fifty microlitre of chloroform was added to the solution followed by the addition of 100 μl of isooctane to derivatize the amino acids. The amino acids were recovered in the upper organic layer, this was dried down using Nitrogen Evaporator and the sample was redissolved in in 100 μl isooctane: chloroform (80:20 v/v). The prepared samples were stored at 4°C in a fridge for 24 h prior to analysing by GC-MS (ThermoQuest, Manchester, UK).
With the exception of the washing and peeling steps, the same steps of the preparation of extracts from raw potato samples have been followed for preparing extracts of French fries.
5.2. Preparation of a linear gradient of standards solutions
A linear gradient of standards solutions was prepared by mixing 10 μl of amino acid calibration standards solution with 100 μl of the internal standard IS, and then the EZ-Faast amino acid derivatization technique was used to derivatize the amino acids. The same steps were followed to prepare 25 and 50 μl standards.
5.3. Analysis of extracted samples using GC-MS
The GC-MS (ThermoQuest, Manchester, UK) was used to analyse the prepared samples. One microlitre of the sample was injected in the splitless mode (split closed for 10 s) using an AS3000 autosampler (ThermoQuest, Manchester, UK), where three technical replicates were performed for each sample. The injector of the Trace GC Ultra gas chromatograph (ThermoQuest, Manchester, UK) was maintained at 250°C, with an initial oven temperature of 90°C which was increased to 320°C at 20°C/min (transfer line from the oven to mass spectrometer, 300°C). Helium (8psi) was used as the carrier gas to elute the amino acids from the ZB-AAA amino acid analysis column (10 m × 0.25 mm ID).
The DSQ II mass spectrometer (ThermoQuest, Manchester, UK) was run in selected ion mode recording ions 84, 101, 114, 116, 130, 144, 146, 155, 156, 158, 172, 180, 184, 243 and 244 with a dwell time of 0.03 s and the running time was 11.50 min. Calibration was achieved by comparison of peak areas for the amino acids in standard and sample runs after adjustment for variation in the peak area of the IS. Data were reported on a dry weight basis. Xcalibur program was used for data acquisition, processing and results delivery, and the data were then exported to excel for the statistical analysis, and the following equation was applied in order to calculate the concentration of amino acids in the sample under investigation:
where PA = Peak area; Conc std = nmols added per unit of IS (one unit = 100 μl).
6. Determination of acrylamide
Acrylamide (0.5–50 μg/ml) in methanol (10%v/v) and formic acid (0.01–1% v/v) solution were prepared as standards. For sample analysis, 2 g of homogenized potato samples (approx. 0.3 mL) were mixed with a 13C-acrylamide internal standard solution to make the isotope ratio match 1:1 and extracted with 40 mL water. The samples were analysed by LC–MS by means of an electrospray ionization interface. Sample extracts and the isotope ratio standard solution were injected (5 μL) on to a Symmetry 300 C4 column (150 mm length, 4.6 mm i.e. 5 μm particle size). The analytical separation was performed using an isocratic mixture of 10% methanol in water containing 0.01% formic acid at a flow rate of 0.2 mLmin−1. The mass spectrometer was operated in selected reaction monitoring (SRM) mode at m/z 72→m/z 55 and m/z 75→m/z 58 for acrylamide and 13C3-acrylamide, respectively.
7. Statistical analysis
T-tests and ANOVA were used to evaluate the significance of the effect of the type of frying oil on the concentration of acrylamide precursors. Means of interest were compared using p-values at level of significance 0.05.
8. Results and discussion
8.1. Moisture content
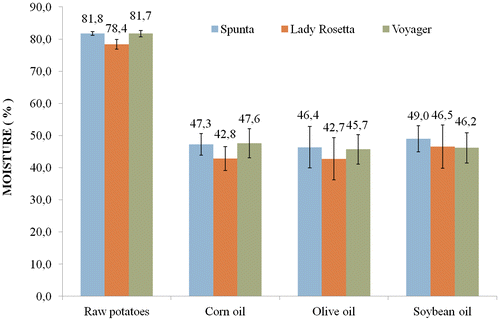
For the three varieties, the mean moisture contents of raw potatoes were 81.8 ± 0.6% for Spunta, 78.4 ± 1.5% for Lady Rosetta and 81.7 ± 0.9% for Voyager (Figure ). During frying, a decrease in the moisture content was observed. The final moisture contents were 45.5 ± 1.3% for Spunta, 44.0 ± 2.1% for Lady Rosetta and 46.5 ± 1.0% for Voyager. The percentage decrease in moisture varied from 30 to 65%. The moisture decrease differed significantly (p < 0.05) between soybean oil and olive oil as a mean of the three varieties, but not in any other comparison between frying oils.
8.2. Absorbed oil in French fries
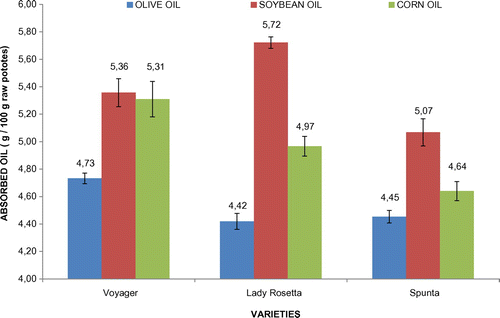
Potatoes fried in olive oil, absorbed significantly (p < 0.05) smaller oil quantities compared to the other two oils, regardless of the potato variety tested. This result agrees with the findings of other studies (Kita & Lisińska, Citation2005) and is most likely due to the fact that the absorption of olive oil in fried potatoes is mainly localized to the surface of potatoes contrary to other vegetable oils where the absorption is usually diffused in the whole mass of the tubers. On the other hand, there was no statistically significant difference between the other two oils (corn oil and soybean oil). The mean value for olive oil absorbed was 4.5 ± 0.1 g/100 g fried potatoes, for corn oil it was 4.9 ± 0.3 g/100 g fried potatoes and for soybean oil it was 5.4 ± 0.3 g/100 g fried potatoes (Figure ).
9. Hydrodynamic characterization
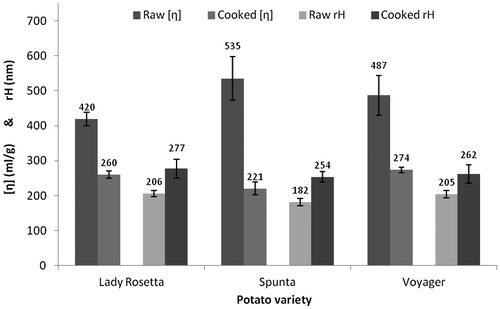
The polymeric integrity of the starches in response to the processing was assessed by viscometry and dynamic light scattering, and the relevant parameters are summarized in Table and Figure . Intrinsic viscosity [η] evaluations were consistent between the Huggins and Kraemer extrapolation methods to zero concentration, and standard errors did not exceed 12%. Raw potato starch samples ranged between 420 and 535 ml/g, a range consistent with previously published intrinsic viscosity values for starch (Millard, Dintzis, Willett, & Klavons, Citation1997), however no significant difference was found in the extrapolation to infinite dilution (p > 0.1) between potato varieties. Upon cooking, starch extracts significantly reduced in intrinsic viscosity (p < 0.05), in all varieties, ranging from 220 to 360 ml/g.
Table 1. Intrinsic viscosity and hydrodynamic radius of starch purified from three types of potato, either raw or deep fried in oil
Distributions of the hydrodynamic radius rH were estimated from measurement of the translational diffusion coefficient from dynamic light scattering, DLS (see Harding, Satelle, & Bloomfield, Citation1992). The polydispersity of all samples was too large for the high-resolution analysis of the distribution, which was expected from an unfractionated preparation of high molecular weight polysaccharide. The z-average values for rH each processed starch are presented in Table . Raw starch samples ranged between 180 and 210 nm, again consistent with other starches (Millard, Wolf, Dintzis, & Willett, Citation1999), with a standard error no more than 6% and with no significant difference between potato varieties (p > 0.14). When fried, the starch significantly increased in radius (p < 0.05) in all varieties. These results are contrary to results found by Roger, Bello-Perez, and Colonna (Citation1999) who found that heating starch using a microwave reduced the hydrodynamic radius of in vitro solutions. The difference observed in these results may be explained by the facts that frying is a fundamentally different heating technique to microwave-heating, and that these starch samples were heated in situ in the cells of potatoes.
Although usually an increase in hydrodynamic radius would accompany an increase in the intrinsic viscosity, the opposite correlation was observed between uncooked and cooked starch samples. This would suggest that there has been a conformational change in the starch. Intrinsic viscosity is more sensitive to shape than hydrodynamic radius, thus the starch granules would have swollen with the uptake of water, and become more spherical as a result.
10. Titratable acidity of oils
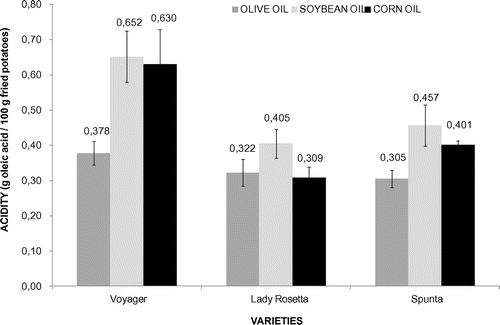
When potatoes were fried in olive oil, the acidity was lower (p < 0.05), compared to corn and soybean oils in spite of the potato variety tested. However, there was no significant difference between the other two oils. The mean value for acidity for olive oil was (0.34 ± 0.10) g/100 g of fried potatoes, (0.45 ± 0.20) g/100 g for corn oil and (0.51 ± 0.10) g/100 g for soybean oil (Figure ). On the other hand, Voyager variety showed higher acidity (p < 0.05), than the other two varieties (Spunta and Lady Rosetta) regardless of the oil being used.
Figure 4. Acidity (g oleic acid/100 g of fried potatoes) of three potato varieties fried in three different oils (p < 0.05).
10.1. Determination of sugar concentration using LC-MS
The means and the standard deviations of the concentrations of the glucose, fructose in the extracts of the French fries samples originating from three potato cultivars and fried in three different types of oils are shown in Figure . The mean of glucose concentrations of all the cultivars was greater when fried in olive oil (0.85 ± 0.2 mmol/kg), compared with 0.48 ± 0.2 mmol/kg for those fried in corn oil and 0.401 ± 0.1 mmol/kg for those fried in soybean oil. The highest average fructose concentration was recorded for the samples fried in corn oil as 0.81 ± 0.2, followed by 0.80 ± 0.2 and 0.68 ± 0.3 mmol/kg for the samples fried in olive and soybean oils, respectively.
Figure 5. The means and the standard deviations of glucose, fructose concentrations in (mmol/kg ± standard deviation) in French fries samples fried in extra virgin olive oil, soybean and corn oil from the three potato varieties.
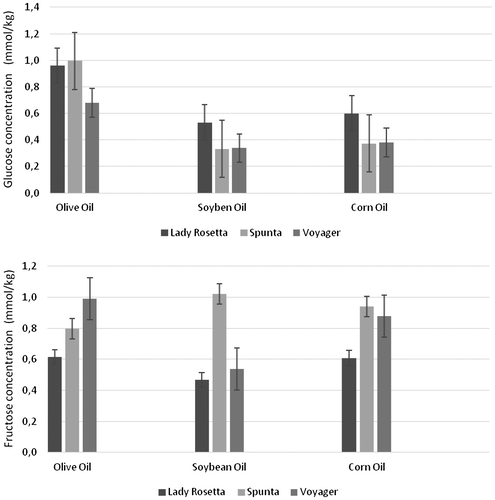
Reducing sugars are consumed in the Maillard reaction between them and the amine groups of the free amino acids Asn and Gln. Of these, the quantities of Asn are largely responsible for the acrylamide formation in French fries (Friedman, Citation2003; Taeymans et al., Citation2004). Frying in oil reduces the concentrations of all sugars.
Notably the importance of reducing sugar concentration as the primary determinant of the acrylamide formation is well established, with free asparagine concentration contributing to the variance in some varieties/conditions.
10.2. Determination of free amino acid asparagine concentration using GC-MS
Concentrations of all free amino acids that occur naturally in potato cultivars were measured using GC-MS. Free amino acid concentrations were measured in raw and French fry potato chips. As demonstrated previously, asparagine was the most abundant free amino acid in the three varieties tested here, followed by glutamine and aspartic acid (Halford et al., Citation2012).
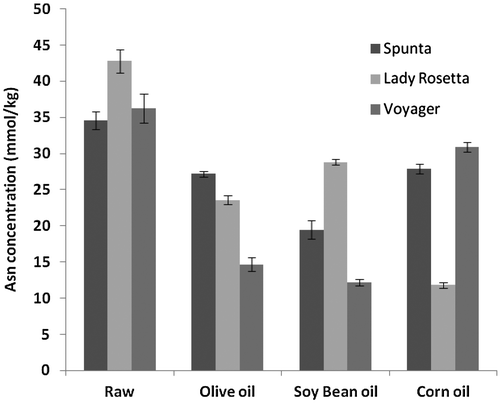
The mean initial concentration of the key amino acid in the acrylamide formation, asparagine, in raw potatoes tubers was 42.8 ± 1.6 mmol kg−1 for Lady Rosetta, 34.6 ± 1.2 mmoles kg−1 for Spunta and 36.2 ± 2.0 mmol kg−1 for Voyager. Lady Rosetta contained a significantly higher concentration of asparagine compared to the other two varieties (p < 0.05).
The residual asparagine contents of French fries were measured after frying in various oils. The asparagine contents of the French fries are not uniformly affected by the choice of oil but notably in these experiments corn oil differentially reduced the asparagine content of Lady Rosetta compared to other varieties in French fries.
The ASN concentrations in the tested samples from the three potato varieties fried in three types of oils are graphically presented in Figure .
10.3. Acrylamide formation
Acrylamide was undetectable in raw potatoes (<20 μg/kg). However, acrylamide formation was detected in all oil fried samples. The greatest quantity of acrylamide was observed in French fries derived from the potato variety Lady Rosetta, followed by Spunta and Voyager. There is a significant reduction in the formation of acrylamide in the variety Voyager compared to the others (p < 0.05). Voyager potatoes contained the least quantity of the reducing sugar glucose and exhibited the smallest reduction upon frying. Glucose would be a substrate for the Maillard reaction leading to the acrylamide formation.
Soybean oil generated the greatest quantity of acrylamide (2,600 ± 440 μg/kg) with Lady Rosetta variety and corn oil the least (1,920 ± 320 μg/kg), but overall the findings suggest that the type of frying oil has no significant impact upon acrylamide formation (Figure ). This observation was found to be consistent with those from the various previous studies.
Figure 7. The effect of potato cultivar and oil type on acrylamide concentration.
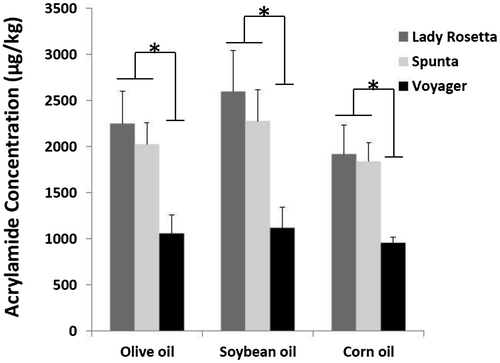
In terms of acrylamide, Lady Rosetta>Spunta>> Voyager. This does not appear to be simply down to the asparagine levels. However, if the secondary impact of sugars is taken into account, the sugars (most notably glucose) are much lowest in voyager, and Lady Rosetta is higher than Spunta. The acrylamide looks like a sugars—asparagine effect.
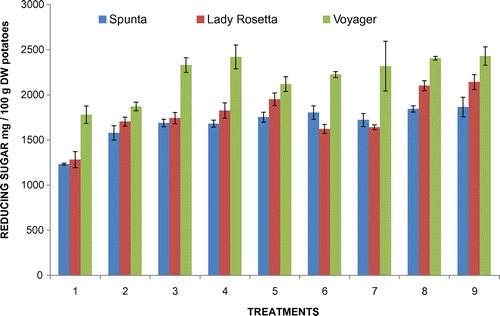
The acrylamide levels are extremely high for the processing method that was used, i.e. the production of French fries, but there is no comparison with other, similar studies or with the European Commission indicative level for French fries of 600 μg/kg. This suggests that reducing sugar concentration in the tubers was very high as shown in Figure .
Figure 8. Initial reducing sugar concentration (mg/100 g DW potatoes) for the three varieties for all 9 treatments.
For example, Mestdagh et al. (Citation2005) reported that the source of frying oils did not appear to influence the formation of acrylamide in fried potatoes and, therefore, the type of frying oil (cotton, olive, peanut, shortening, canola, soybean, seed, and sunflower) is not a significant variable for the acrylamide formation. Mestdagh et al. (Citation2005) investigated the influence of the type of oil on acrylamide formation using a model system based on a closed stainless steel tubular reactor. Their study was also based on the addition of different types of frying oils, including olive oil, corn oil and soybean oil, separately to different artificial potato powder mixtures. These mixtures were heated at 170°C for 5 min. Although the results obtained indicated that the acrylamide levels were not equal for these different oils, no significant differences could be inferred between the tested oils.
Heating fats or vegetable oils at high temperatures produce acrolein, resulting from the thermal degradation of glycerol or lipid. Oxidation of acrolein yields acrylic acid, which then reacts with a nitrogen source to form acrylamide (Skog & Alexander, Citation2006; Umano & Shibamoto, Citation1987; Yasuhara et al., Citation2003). Ehling, Hengel, and Shibamoto (Citation2005) has reported the generation of high levels of acrylamide when fats or oils are heated with amino acid ASN, especially if these oils contain high concentrations of polyunsaturated fatty acid or linoleic acid. In this context they have low frying stability, suggesting that the highest concentration of acrylamide is formed when using soybean oil, followed by corn oil and olive oil. In this pathway, the oxidation of acrolein to acrylic acid seems to be a critical step in the acrylamide formation (Biedermann, Grob, Gutsche, & Weisshaar, Citation2002; Taeymans et al., Citation2004). The inactivity of acrolein in Maillard reaction may be attributed to the fact that this acid immediately reacts with different food components instead of undergoing oxidation to acrylic acid. In addition, the common frying temperatures were found to be above the boiling degree of both acrolein (51°C), and acrylic acid (140°C). Therefore, these compounds that resulted from lipids degradation at elevated temperatures may quickly transform to their gaseous state, and thus do not participate in the formation of acrylamide in fried potatoes (Ehling et al., Citation2005). In general, although lipids and oils may play a minor role in the acrylamide formation and provide a desirable medium for heat transfer, these findings suggest that they do not appear to be significant precursors for the acrylamide formation as compared with the major acrylamide-sugar pathway.
The present study was developed to address the effect of the type of frying oils on the concentrations of acrylamide precursors and the formation of acrylamide in French fries. The influence of the different types of frying oils (olive, corn, and soybean) on the concentrations of these precursors in French fries samples derived from three different potato cultivars (Lady Rosetta, Spunta, and Voyager) was investigated, and taken as an indicator of the acrylamide formation in these samples. As reported previously, differences in the acrylamide precursor concentrations were observed between the different varieties of potato. The variety Lady Rosetta having the highest concentrations of the precursor sugars and free asparagine, which correlated with the highest levels of acrylamide observed in the fried products. The lowest levels of acrylamide were observed in the Voyager variety independent of the oil used to fry the potato.
Finally, it was observed that the acrylamide looks like a sugars—asparagine effect. This means that the predominant effect of reducing sugar concentration on the acrylamide formation in potato products is well established as confirmed by many studies.
Contrary to the hypotheses formulated, it was found that there was no significant effect of the type of frying oil on the levels of acrylamide. The lowest acrylamide concentrations were observed in corn oil.
Additional information
Funding
Notes on contributors
Theo Varzakas
Theo Varzakas is an associate professor in the Department of Food Technology, Technological Educational Institute of Peloponnese (ex Kalamata), Greece specializing in issues of food technology, food processing, food quality and safety. He is the chief editor in Current Research in Nutrition and Food Science since 2015. He is the member of the Editorial board of IJFST since 2007. He has written more than 140 research papers and reviews and has presented more than 140 papers and posters in national and international conferences. He has written and edited two books in Greek, one on genetically modified food and another one on Quality control in food and six in English on sweeteners, biosensors, food engineering, food processing, published by CRC. His main research interests lie on diffusion, solid-state fermentations, bakery and confectionery, emulsions, acrylamide detection, olive oil technology, citrus fruits technology, waste management and production of bioethanol from waste material (potato peel waste, sorghum, maize).
References
- Andrikopoulos, N., Kalogeropoulos, N., Falirea, A., & Barbagianni, M. (2002). Performance of virgin olive oil and vegetable shortening during domestic deep-frying and pan-frying of potatoes. International Journal of Food Science and Technology, 37, 177–190.10.1046/j.1365-2621.2002.00555.x
- Biedermann, M., Grob, K., Gutsche, B., & Weisshaar, R. (2002). Experiments on acrylamide formation and possibilities to decrease the potential of acrylamide formation in potatoes. Mitteilungen aus Lebensmitteluntersuchung und Hygiene, 93, 668–687.
- Ehling, S., Hengel, M., & Shibamoto, T. (2005). Formation of acrylamide from lipids. In M. Friedman & D. Mottram (Eds.), Chemistry and safety of acrylamide in food (pp. 223–233). New York, NY: Springer Science+Business Media.10.1007/b106417
- European Food and Drink Industry Association (CIAA)-Food Drink Europe. (2011). Acrylamide toolbox. Retrieved 13 July, 2014, from http://www.fooddrinkeurope.eu/publication/fooddrinkeurope-updates-industry-wide-toolbox-to-help-manufacturers-further/
- Food and Agricultural Organisation/World Health Organisation. (2005). Summary and conclusions of the sixty-forth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (pp. 1–17). Retrieved from http://www.who.int/ipcs/food/jecfa/summaries/en/summary_report_64_final.pdf
- Friedman, M. (2003). Chemistry, biochemistry, and safety of acrylamide. A review. Journal of Agricultural and Food Chemistry, 51, 4504–4526.10.1021/jf030204+
- Halford, N. G., Muttucumaru, N., Powers, S. J., Gillatt, P. N., Hartley, L., Elmore, J. S., & Mottram, D. S. (2012). Concentrations of free amino acids and sugars in nine potato varieties: Effects of storage and relationship with acrylamide formation. Journal of Agricultural and Food Chemistry, 60, 12044–12055.10.1021/jf3037566
- Harding, S. E. (1997). The intrinsic viscosity of biological macromolecules. Progress in measurement, interpretation and application to structure in dilute solution. Progress in Biophysics and Molecular Biology , 68, 207–262.
- Harding, S. E., Satelle, D. B., & Bloomfield, V. A. (1992). Laser light scattering in biochemistry. The Royal Society of Chemistry, 33, 341–342.
- Hogervorst, J. G., Schouten, L. J., Konings, E. J., Goldbolun, R. A., & Vad den Brandt, P. A. (2007). A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer, Epidemiology and Prevention, 16, 23–25.
- Kita, A., & Lisińska, G. (2005). The influence of oil type and frying temperatures on the texture and oil content of French fries. Journal of the Science of Food and Agriculture, 85, 2600–2604.10.1002/(ISSN)1097-0010
- Koutsidis, G., De la Fuente, A., Dimitriou, C., Kakoulli, A., & Wedzicha, B. L. (2008). Acrylamide and pyrazine formation in model systems containing asparagine. Journal of Agricultural and Food Chemistry, 56, 6105–6112.10.1021/jf703744k
- Koutsidis, G., Simons, S. P. J., Thong, Y. H., Haldoupis, Y., Mojica-Lazaro, J., & Wedzicha, B. L. (2009). Investigations on the effect of amino acids on acrylamide, pyrazines, and michael addition products in model systems. Journal of Agricultural and Food Chemistry, 57, 9011–9015.10.1021/jf9014763
- Lolos, M., Oreopoulou, V., & Tzia, C. (1999). Oxidative stability of potato chips: Effect of frying oil type, temperature and antioxidants. Journal of the Science of Food and Agriculture, 79, 1524–1528.10.1002/(ISSN)1097-0010
- Low, M. Y., Koutsidis, G., Parker, J. K., Elmore, J. S., Dodson, A. T., & Mottram, D. S. (2006). Effect of citric acid and glycine addition on acrylamide and flavor in a potato model system. Journal of Agricultural and Food Chemistry, 54, 5976–5983.10.1021/jf060328x
- Mestdagh, F., De Meulenaer, B., Van Poucke, C., Detavernier, C., Cromphout, C., & Van Peteghem, C. (2005). Influence of oil type on the amounts of acrylamide generated in a model system and in French fries. Journal of Agricultural and Food Chemistry, 53, 6170–6174.10.1021/jf0506683
- Millard, M. M., Dintzis, F. R., Willett, J. L., & Klavons, J. A. (1997). Light-scattering molecular weights and intrinsic viscosities of processed waxy maize starches in 90% dimethyl sulfoxide and H2O. Cereal Chemistry, 74, 687–691.10.1094/CCHEM.1997.74.5.687
- Millard, M. M., Wolf, W. J., Dintzis, F. R., & Willett, J. L. (1999). The hydrodynamic characterization of waxy maize amylopectin in 90% dimethyl sulfoxide–water by analytical ultracentrifugation, dynamic, and static light scattering. Carbohydrate Polymers, 39, 315–320.10.1016/S0144-8617(99)00021-1
- Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.10.1021/ac60147a030
- Moreira, R. G., Castell-Perez, M. E., & Barufet, M. A. (1999). Fried product processing and characteristics. In Deep-fat frying: Fundamentals and applications (pp. 11–31). Gaithersburg, MD: Chapman & Hall Food Science Book.
- Nikolaou, A., Varzakas, T., & Kourkoutas, Y. (in press). Effect of organic fertilization treatment, frying oil and cultivar variety on the volatile profile of potato tubers. Current Research in Nutrition and Food Science, 4(1).
- Pedreschi, F., Mariotti, M. S., & Granby, K. (2013). Current issues in dietary acrylamide: Formation, mitigation and risk assessment. Journal of the Science of Food and Agriculture, 94, 9–20.
- Roger, P., Bello-Perez, L. A., & Colonna, P. (1999). Contribution of amylose and amylopectin to the light scattering behaviour of starches in aqueous solution. Polymer, 40, 6897–6909.10.1016/S0032-3861(99)00051-8
- Rosenthal, F. R. T., & Espindola, L. (1969). The Mucunã (Dioclea Malacocarpa) the properties of the starch. Starch - Stärke, 21, 262–266.10.1002/(ISSN)1521-379X
- Skog, K., & Alexander, J. (2006). Acrylamide and other hazardous compounds in heat-treated foods. Cambridge: Woodhead Publishing.10.1533/9781845692018
- Taeymans, D., Wood, J., Ashby, P., Blank, I., Studer, A., Stadler, R. H., … Whitmore, T. (2004). A review of acrylamide: An industry perspective on research, analysis, formation, and control. Critical Reviews in Food Science and Nutrition, 44, 323–347.10.1080/10408690490478082
- Tareke, E., Rydberg, P., Karlsson, P., Eriksson, S., & Törnqvist, M. (2002). Analysis of acrylamide, a carcinogen formed in heated foodstuffs. Journal of Agricultural and Food Chemistry, 50, 4998–5006.10.1021/jf020302f
- Umano, K., & Shibamoto, T. (1987). Analysis of acrolein from heated cooking oils and beef fat. Journal of Agricultural and Food Chemistry, 35, 909–912.10.1021/jf00078a014
- Wedzicha, B. L., Mottram, D. S., Elmore, J. S., Koutsidis, G., & Dodson, A. T. (2005). Kinetic models as a route to control acrylamide formation in food. In M. Friedman & D. Mottram (Eds.), Chemistry and safety of acrylamide in food (Vol. 561, pp. 235–253, Advances in experimental medicine and biology). New York, NY: Springer. ISBN 9780387239200.10.1007/b106417
- Willinger A. H. A. (1964). Potato starch. In R. L. Wistler (Ed.), Methods in carbohydrate chemistry (Vol. 4, pp. 9–13). New York, NY: Academic Press.
- Yasuhara, A., Tanaka, Y., Hengel, M., & Shibamoto, T. (2003). Gas chromatographic investigation of acrylamide formation in browning model systems. Journal of Agricultural and Food Chemistry, 51, 3999–4003.10.1021/jf0300947