Abstract
Cotton (Gossypium hirsutum L.) being one of the top most cash crop is reckoned as main pillar of textile industry. Cotton cultivation has experienced an outstanding escalation story over the years. The per unit yield and area under cultivation have all incremented to record towering levels. But question is how Bt cotton has contributed and whether it is satisfactory or not. At present for finding a conclusion, we need unfathomable analyses and investigations related to multiple aspects of global cotton cultivation. Genetic engineering is considered as an imperative tool in cotton breeding with a role in empowerment of traditional strategies for improvement in net yield and related factors. Among multitude of reasons for massive shifting to Bt cotton cultivation in the world include inadequate germplasm, climatic conditions, irrigated area, usage of fertilizers and pesticides. We should consider Bt cotton a miracle solution .Therefore, it is probable that Bt cotton along with newly developed strategies, improved irrigation systems and superior chemical application may enhance the production quality and quantity as well. Our review brings into light the success of cotton genetic engineering over the last two decades and probable future prospects.
Public Interest Statement
Importance of cotton can never be over looked in the economy of cotton producing countries because cotton and its subsequent products add significantly in earning foreign exchange. Since the ancient past, breeders have strenuously attempted to perk up cotton quality through conventional breeding means. Spectacular Agricultural and technological advancements during the last few decades have done more than expectation for growing world population. Analysis of last two decades performance offers a good picture of output and present status of Bt cotton in comparison with expectation. The presented information gained from transgenic cotton plants is an important focus for unifying crop research in disciplines like breeding, pathology, biochemistry, and physiology with molecular biology. In spite of criticism many scientists are agreed upon the best contribution of Bt cotton in world economy. This review is helpful in evaluating the criticism and related answers upon Bt cotton and its role in agriculture.
Competing Interests
The authors declare no competing interest.
1. Introduction
Cotton is one of the most valuable multipurpose crop e.g. food and fiber. The highly significant product i.e. lint is a source of better quality natural fiber for textile sector in addition to the use of cotton seed for oil extraction, seed meal and cotton cake (Keshamma, Rohini, Rao, Madhusudhan, & Kumar, Citation2008; Rathore, Sunilkumar, Cantrell, Reding, & Hague, Citation2009; Kouser & Qaim, Citation2012). For the last many years, breeders have attempted to improve cotton by adopting classical plant breeding ways. Although breeding techniques had already being used to solve the problems related to cotton, but disease and insect attack perpetuates that ultimately cause reduction in growth and yield (Chen et al., Citation2008).
Since the commercialization of GM crops in late 1990s, it has won the trust of farmers and other workers. According to an estimate, area under cultivation of genetically modified (GM) cotton and international adaptation rates of biotech cotton are far higher than conventional cotton (James, Citation2013, Citation2014; Marvier, McCreedy, Regetz, & Kareiva, Citation2007). Irrespective of some reported hazards e.g. excessive weedicide usage, Advancement in cotton biotechnology (Wu, Guo, & Chen, Citation2005) is an incredible accomplishment as it has made weed management very easy and somehow Eco-friendly. Fungal attacks on cotton plants, for instance, Verticillium and Alternaria causes serious damages with ultimate loss of gross yield. Viral infections cause matchless destruction of cotton. According to some previous studies, viral attacks resulted in lowering the number and weight of bolls by 87.4 and 38.8% respectively along with 92.2% reduction in the yield of cotton seeds in Nigeria, Sudan and Tanzania (Rahman, Rao, Batool, Shahid, & Husnain, Citation2012).
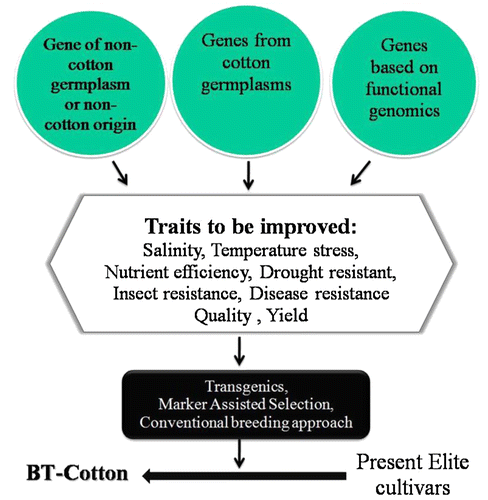
The abiotic stress tolerant cotton development is a challenge for the biotechnologists and genetic engineers. Drought is regarded as a critical factor in cotton growing parts of the world because it adversely influences yield of fiber and lint quality (Maqbool, Citation2009). Both land and water resources are not likely to increase in future. Salinity also devastate cotton yield as being growth impediment causing osmotic stress and leaf senescence (Gorai, Ennajeh, Khemira, & Neffati, Citation2010; Grewal, Citation2010; Munns, Citation2002; Nawaz et al., Citation2010). Consequently, other than raising cotton yield, focus of cotton breeders mainly rely on incrementing plant tolerance to abiotic stresses. Despite multiple objections and oppositions, GM crops are of enormous benefits including sustainable and resource efficient crop management, effective insect-pest control, minimum dependence upon conventional pesticides and environmental health etc. In this review, we have tried to sum up the efforts done by the researchers in the field of GM cotton in different aspects along with probable future perspectives for improvement and development in cotton.
2. Is it right to say, eco-friendly insect and weed resistant technology outcome?
Over recent decades, scientists have made numerous attempts for production of insect resistant cotton plants by transplanting genes from different sources (Rathore et al., Citation2009). In the beginning, genes that were resistant to diverse range of insects i.e. lepidopterans, coleopterans were taken from Bacillus thuringiensis and introduced in cotton (Cohen, Gould, & Bentur, Citation2000). In spite of criticism from opponents of biotechnology, reports highlight great increase in use of Bt cotton Later investigations proved that expression of mentioned altered genes in Gossypium hirsutum (cry1Ac) and Solanum tuberosum (cry3Aa) provided these crops with noteworthy protection against pests of lepidopteran and coleopteran clans (Song, Kain, Cassidy, & Wang, Citation2015). Additionally, several other workers have also regarded Bt cotton cultivars with cry1Ab as superior (Khan, Bakhsh, Ghazanffar, Riazuddin, & Husnain, Citation2013; Tohidfar, Ghareyazie, Mosavi, Yazdani, & Golabchian, Citation2008).
Leelavathi et al. (Citation2004) have introduced a simple but effective protocol. They co-cultivated embryogenic callus with Agrobacterium tumefaciens that harbored cry1Ia5 gene. According to Wu et al. (Citation2005), in insect-resistant transgenic cotton plants expressing cry1Ac and API-B, Heliothis armigera larval death rate was bit high. However, for maximum sustainability of Bt-transgenic technology, it is vital that the toxin expression levels be expressed at ample quantities in proper plant parts at the required time of season to give defense against major target insect/pests which may primarily include the bollworms (Kranthi et al., Citation2005). Trials conducted by using CIM-482, a Pakistani cotton variety having genes cry1Ac and cry2Ac transformed with the aid of Agrobacterium presented jubilant success by contributing resistance against targeted insect pests. Transgenic plants showed death of nearly 75–100% of second instar of Heliothis armigera as compared to control (Rashid, Saleem, Husnain, & Riazuddin, Citation2008). Later on, Baksh et al. observed high efficacy of the same transgenic lines in field against targeted insects and pests in further descendents (Figures and ). According to some workers, leaf biotoxicity and artificial field assays can be used to check resistance of transgenic cotton cultivars against insects or pests (Khan et al., Citation2013). In two different studies by Bakhsh (Citation2010) and Bakhsh, Siddique, and Husnain (Citation2012), it has been proved that these transgenic cultivars regularly express gene cry1Ac controlled by tissue-specific promoter (RbcS) in comparison with CaMV 35S promoter. Obtained from Bacillus sp, Vip1 and Vip2 genes inducted considerable insecticidal activity against western corn rootworm but remained inactive to cope with lepidopteran insects. While on the other hand Vip3 gene products showed excellent insecticidal activity versus insects of lepidopterans in Zea sp. and Gossypium (Fang et al., Citation2007).
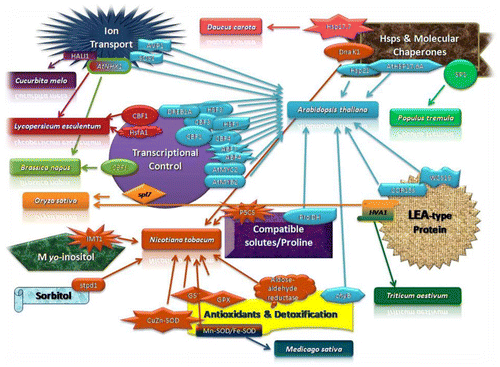
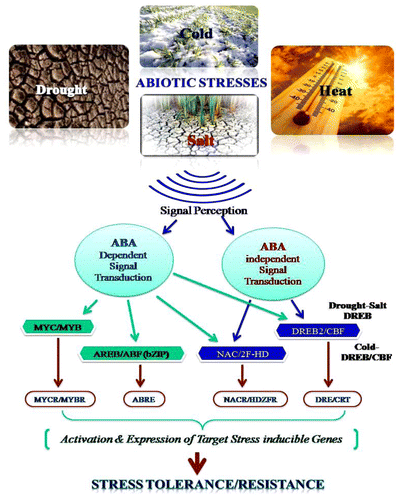
Figure 2. Schematic representation of abiotic stress tolerance/resistance with the help of signal transduction and respective gene response among plants.
Not only the cotton bollworms, but other insect/pests affect cotton yield belongs to order hemiptera mainly e.g. jassids, whiteflies and aphids (Amudha, Balasubramani, Malathi, Monga, & Kranthi, Citation2011). Amazingly, the genes isolated from Bt have not been proved very much effective against this type of pests. Efficacy of Cotton plants with transformed genes for plant lectins is well documented (Chakravarthy, et al., & Vudem, Citation2013; Yarasi, Sadumpati, Immanni, Vudem, & Khareedu, Citation2008). Besides common approaches to achieve targeted insect resistance, an efficient gene silencing strategy, plant-mediated RNAi technology has been given preference due to its better ability in combating insects, and additional action of addressing resistance development among insects or pests (Price & Gatehouse, Citation2008). Therefore, a growing trend is simply a reflection of worldwide trust to cultivate Biotech Cotton.
In the end of 20th century, scientists achieved a milestone by incorporating EPSPS gene of Agrobacterium CP4 strain in cotton. This gene has performed very well in reducing injurious effects of glyphosate herbicide. In 1997, another hall mark in cotton genetic engineering was shown by Monsanto when workers adopted first herbicide-resistant cotton with MON 1445/1698 containing CP4 EPSPS as primary gene trait endowing resistance to glyphosate. While on the other side HT genes have been used as selectable marker like the bar gene, that confer glufosinate-ammonium tolerance (Devine, Citation2005). Blair-Kerth et al. (Citation2001) conducted a series of field experiments by using cultivar Cooker 312 against glufosinate application. Results indicated no injury in glufosinate tolerant plants as compared to those with no expression of gene for tolerance. In China, aroA-M1 gene under 35S promoter was incorporated into cotton cultivar Zhongmian 35 which exhibited high efficacy in T0 and T1 progeny in terms of Glyphosate tolerance.
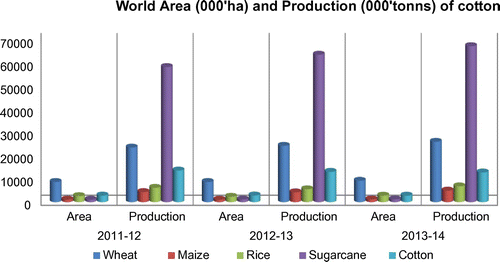
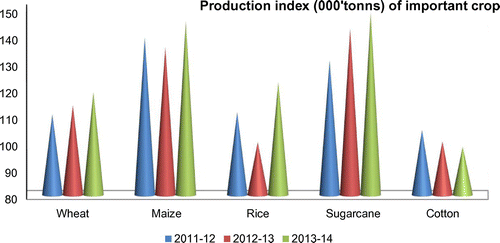
From the discussion, we understand that transgenic plants have exerted in significant influence in reducing environmental issues related to insecticide and herbicide use. Across the globe, genetically engineered cotton plants with insect-pest and herbicide-resistance characters, have earned trust and wide acceptance (Rathore et al., Citation2009). From the biodiversity and environmental point of view, GM crops like cotton with herbicide resistance have got utmost importance in recent years (Ammann, Citation2005). Conclusively, we can hope for a healthier and safe environment not only for humans but for other creatures like natural enemies of insects. Comparative study of economic value unveils the facts and figures that prove success of GM cotton. Higher yields have also been noticed (Figures and ) from small or medium scale cultivators and shows trust worthy benefits by using and adopting such advanced form of crops (Brookes & Barfoot, Citation2008). With full confidence and Contrary to adverse objections by critics over these crops as well as technology, we along with other researchers (Bawa & Anilakumar, Citation2013; Fred, Betz, Hammond, & Roy, Citation2000; Lu et al., Citation2012) declare these transgenic crops quite safe, highly efficient in weed management and environmentally feasible.
3. Can transgenic cotton thrive in presence of fungal pathogens?
With the help of genetic engineering development of cotton lines with enhanced resistance against fungal pathogens is no more a dream. These fungi e.g. Fusarium and Verticillium hamper cotton growth and development leading to massive production losses. A large number of upland growing cotton varieties appear more prone to Wilt (Klosterman et al., Citation2009).
For so many years, workers have thoroughly sorted out the genetic basis of disease resistance, biochemical pathways, breeding and cloning of resistant genes. By adopting the mentioned ways, in addition to others, they have contributed very significantly in getting resistant cultivars. So far, Tohidfar, Mohammadi, and Ghareyazie (Citation2005) were successful to an extent by developing transgenic cotton which showed better tolerance to Verticillium wilt. It has been described that crude leaf extracts of transgenic cotton expressing chitinase gene have ability to inhibit V. dahliae. Another target achieved in this series of efforts was development of resistant transgenic cotton plants that are well able to be resistant against Fusarium and Alternaria (Ganesan et al., Citation2009). Miao et al. (Citation2010) successfully transformed a susceptible cotton variety with Hpa1Xoo for encoding resistance against V. dahliae. This hairpin protein i.e. Hpa1Xoo taken from Xanthomonas oryzae pv. oryzae is responsible for hypersensitive cell death in plants. Zhang et al. (Citation2012) have reported that the transgenic cotton cultivar having incorporated Gbve1, tomato homologous gene, with enhanced resistance against Verticillium wilt. In addition to other available techniques and strategies, still transgenic approach has authentic role in utilizing cloned genes. Introduction of Xa26 gene in various japonica rice cultivars along with its local promoter provided transgenic plants resistance against bacterial attack (Cao et al., Citation2007). It was then suggested that improved resistance is intimately allied with the lofty gene expression. Contrary to this finding regulation of gene expression by using a pathogen-induced weak promoter decreased the resistance. So from these reports we are in position to infer that optimization of resistant gene expression level by a transgenic technique can be coupled with other strategies i.e. MAS for development of disease resistant cultivars. Given data somehow sums up the success of Bt cotton against fungi in tone and meaning while providing researchers a way out into the world of reality.
4. Rathar than old techniques something new has been introduced for virus defense?
Scientists have used two approaches for producing virus-resistant transgenic cotton (Figure ). One is using a gene from particular virus that encode resistance for the same virus or secondly taken from a different source. The first approach e.g. pathogen-derived resistance uses a portion or whole viral gene for transformation in plant that interrupts viral replication. It was proposed that the small RNAs could be involved in the production of dsRNA for serving as target for PTGS (Hanley-Bowdoin, Bejarano, Robertson and Mansoor, Citation2013). Hammond, Caudy, and Hannon (Citation2001) suggested that small RNAs are ultimate consequence of dsRNA degradation and they would act as silencing signal. The viral CP gene is reckoned as the prime and the most extensively used gene that confers pathogen derived resistance (Prins, Citation2003). Studies unveil the process of Geminivirus replication in the host plant nucleus. Now it has been very clearly described that in addition to transcription resulting in sense mRNA anti-sense transcripts are also generated. So, there emerge more chances of formation of duplex between sense and anti-sense mRNA. Interestingly, dsRNA duplex is considered highly susceptible to decay. For that reason, it is considered that the use of antisense RNA for developing resistance against viruses is a powerful technique (Prins, Citation2003). Transgenic cotton line with resistance against cotton leaf curl disease was developed by using anti-sense coat protein (Amudha et al., Citation2011). In this method, a binary vector that carried ACP gene as well as neomycin phosphotransferase II (nptII) gene with CaMV-35S promoter and NOS (nopaline synthase) terminator was used. Transgenic progeny was tested for resistance by inoculation with viruliferous whiteflies and proved resistant against CLCuV.
According to Sohrab et al. (Citation2014), integration of βC1gene with antisense orientation in CLCuV susceptible plants was highly promising in viral infection in field conditions. It was also observed that virus accumulated in low amounts in transgenic cotton plants because it presented late or reduced symptoms. Finally, they developed cotton plants with single copy integration in genome and their inheritance blueprint exhibited stable integration. Similarly reports show that Begomovirus genes have been used for development of pathogen derived resistance against plant diseases by utilizing viral genomes in different styles like entire, sense or antisense, artificial microRNA (Ali, Amin, Briddon, & Mansoor, Citation2013).
Unequivocally, RNA interference has emerged as a novel technique for its salutary prospective in the control of viral diseases. This evolutionarily conserved phenomenon in plants, animals and fungi is regarded as a mean to protect the cells from viruses and transposons. Chakrabarty et al. (Citation2011) have used this technique successfully for targeting CLCuV. Recently, Cardoso et al. (Citation2014) have described antibody engineering in plants as a novel approach for enabling plants with resistance to pathogens. In this process, plants produce different antibodies or rAb fragments that inactivate pathogens or polypeptides involved in pathogenesis. Data are also available that highlights transgenic plant resistance to viruses caused by defective movement protein (MP) (Hallwass et al., Citation2014). The effect of Bt cotton utilization against Virus is not diffusive. For critics it must be borne in mind that the growing good of the world is partly dependent on unhistorical acts as well. The things are not so ill with us as they might had been.
In a study, Ali et al. (Citation2015) investigated probability of using CRISPR/Cas9 in plants for conferring molecular immunity against viral DNA. During the experiment, they delivered sgRNAs specified for coding as well as non-coding sequences of TYLCV into Nicotiana benthamiana that were stably transformed and express the Cas9 endonuclease. Collected data suggest that CRISPR/Cas9 targeted the said virus and caused mutations on target sequence. Although used sgRNAs presented interference activity and the production of the stem-loop sequence in particular were recognized as the most efficient one. N. benthamiana plants that expressed CRISPR/Cas9 were specifically found with belated or abridged accrual of viral DNA so abolishing or considerably lessening the infection symptoms.
In short, the salient attribute of CRISPR/Cas9 lies in ability to target multiple DNA viruses at once (Jinek et al., Citation2012; Ma, Zhang, & Huang, Citation2014). On the other hand by using this method we can be able to exploit its capacity for expurgation of one or more viruses by using various sgRNAs. Additionally, using this technique offers potential for overcoming resistance after targeting lately evolved viral strains with new-fangled sgRNAs (Fu, Sander, Reyon, Cascio, & Joung, Citation2014; Hsu et al., Citation2013). From the above given facts an opinion can be established that different strategies for producing transgenic cotton can cumulatively work very well in controlling plant viral infections and improving quality and quantity of cotton. Now, we are able to extend the application of latest technology like CRISPR/Cas9 and hope for the opening of a new era in developing viral resistant crops.
5. Has transgenic cotton been successful against fluctuating environment?
According to the expert opinion, it is anticipated that our planet will be facing a pressure by expected addition of 2–4 billion people in coming three or four decades (Cohen, Citation2003). Furthermore, size of arable land increased merely at the rate of 10% in last fifty years. To this worsening situation, reduction in aquifer is adding fuel to fire. As predicted by climate experts, average temperature will increase leading to expansion of dry lands. Saline soil, nutrient acidity, nutrients imbalance, water scarcity are major restraints that severely hamper morpho-anatomical attributes, crop physiology, yield (Arshad et al., Citation2015; Noman, Ali, Hameed, Mehmood, & Iftikhar, Citation2014; Zafar, Ashraf, Anwar, Ali, & Noman, Citation2016). Of these all stresses, drought and soil salinity are more common (Lobell, Ortiz-Monasterio, Gurrola, & Valenzuela, Citation2007; Noman et al., Citation2015).
Different techniques like conventional breeding and genetic engineering of crops are being used to produce Abiotic stress tolerant cultivars (Figures and ). Conventional breeding of plants has driven more for the enhancement of Abiotic stress tolerance, yet development of new cultivars has been slow (Bakhsh, Citation2014). Profiling of gene expression in Gossypium leaf and roots under well-watered and water deficiency presented extensive tissue-specific and stress-responsive alterations in gene expression . A large number of transcripts responding to stress have been identified in root shoot tissues but there have functions are still clandestine. Thus, much more remains to be well-read about abiotic stress responses in cotton.
Many genes in transgenic stress tolerant plants that are engaged in the synthesis of osmoprotectents have been targetted (Vinocur & Altman, Citation2005). Environmentally triggered physiological changes have sound effects fiber development. A strong association has been noted between ABA levels and fiber development in case of stress. These changes were infact ABA induced gene expressional changes (Dasani & Thaker, Citation2006). A number of transcription factors are responsible in plant to respond drought stress like ZIP, MYB, NAC, MYC AP2/ERF and WRKY (Vinocur & Altman, Citation2005). Different genes responsible in the synthesis of ABA have also been incorporated in crop plants (Xiong & Zhu, Citation2003). MYB and ZIP are two major families which are responsible for signaling and activation of ABA. Expression of ABF3 or ABF4 increased drought tolerance in Arabidopsis, with changed expression of ABA/stress responsive genes e.g. rab18, rd29B, ABI1 and ABI2 (Kagaya, Hobo, Murata, Ban, & Hattori, Citation2002). Over expression of LOS5/ABA3 exhibited increased drought tolerance in Chinese cotton cultivars. In transgenic plants due to less transpiration, maximum ABA and proline accumulated and also increased antioxidants activity (Figures and ) that resulted in increased drought tolerance (Yue et al., Citation2012). It is also reported that over expression of LOS5/ABA3 involved in tolerance to drought stress under field condition (Li et al., Citation2013). Recently it is reported that both in green house and field conditions transgenic cotton expressing AtRAV1/2 exhibited resistance to drought stress (Mittal et al., Citation2014). During stress, plants produce heat shock protein. A heat shock protein gene GHSP26 exhibited over expression in a cotton cultivar (Maqbool et al., Citation2010). Under water deficit condition for specific time the transgenic plants accumulated GHSP26 heat shock protein. This protein was highly expressed in transgenic cotton. Universal stress protein has also been introduced in cotton against abiotic stress (Shamim, Rashid, Rahman, & Husnain, Citation2013). According to FAO (2005), almost 800 million hec around the globe are salt affected. Salinity reduces emergence and germination, decrease cotton growth and overall leads to low yield and fiber quality (Khorsandi & Anagholi, Citation2009). A lot of genes are responsible in cotton to regulate response to salinity stress condition. Rodriguez-Uribe et al. (Citation2011) recognized 720 salt responsive genes, in which 695 were down regulated and 25 were up regulated in the salt tolerant mechanism. Therefore, it was believed that transfer of genes like isopentenyl transferase (IPT) gene from Agrobacterium tumifaciens proved efficient in not only enhancing salt stress tolerance but also delaying leaf senescence. Expression of IPT in transgenic cotton of delaying leaf senescence was described by Liu et al. (Citation2012). Similarly it was observed that GhDREB gene was activated in cotton by high salt stress. Gao et al. (Citation2009) reported that transgenic wheat with GhDREB gene had much improved tolerance of salt stress through highly accumulating soluble sugar (Figure ). Targeting the genes encoding enzymes involved in these pathways can yield dual benefits in terms of quality and quantity. These not only will improve growth and tackle stress effects but also increase yield along with maintained fiber size as well as quality. In addition, genes for Lipid biosynthesizing can be bring into focal point. As lipids are chief constituents of membrane and cell wall synthesis, upregulation of these lipid encoding genes can enhance fiber quality.
Production and analysis of transgenic cotton progeny expressing gene cassettes operated by stress induced promoters is now in progress and probably this approach will allow increase in stress tolerant cultivars without pessimistic agronomic consequences. As a result, while we are expected to see firm progress by using conventional and molecular breeding strategies, transgenic alterations will provide greater variety of options for enhancing stress tolerance in Cotton. Although much has to be done yet and critics are considering Bt cotton adaptability in environment as “one soon borne away” by the waves and lost in darkness and distance. But those deficiencies and rigmaroles will be fully answered in the perfect by the wishes, the hopes, the confidence and the predictions of the small band of researchers who witnessed the jubilant success in recent past.
6. Does it focus economically beneficial traits?
Being a source of natural fiber, cotton holds important position in agricultural economy of a number of countries. Fiber characteristics like quality, strength, flexibility, durability make it the most feasible material for textile usage (Chee & Campbell, Citation2009; Haigler, Zhang, & Wilkerson, Citation2005). With advancements in genetic engineering, the workers across the world have focused new ways of exploiting and improving cotton quality with help of alien gene incorporation (Wang et al., Citation2010). Being a polygenic trait, new insights into transcription and expression can be very well understood with the help of molecular genetics for cotton fiber growth and quality (Boopathi & Ravikesavan, Citation2009). Reports show that the cell wall extensibility regulation during cell expansion is partly governed by EXPANSIN genes expression in cotton (Arpat et al., Citation2004). These EXPANSIN genes have reportedly play important role in increasing individual cell and fruit size. Additionally, expression of this gene family has also been noticed during elongation of fiber (Ruan, Llewellyn, & Furbank, Citation2001).
Expression of cotton GhPIP1-2 and GhTIP1 (two aquaporin genes) revealed high and preferential expression at 5 DPA. This evidence further support important role during fiber cell expansion (Liu et al., Citation2008). During fiber elongation, two major respiratory pathways, OPPP (oxidative pentose phosphate Pathway) and glycolysis, supply energy and convert substrates to products. The enzymatic activity in these pathways differ with the requirement for respiratory products e.g. high activity of G6PDH and 6PGDH during the elongation of cell . This raised activity of enzymes reflect the need of NADPH and intermediates during the elongation of fibers.
As a part of defense strategy against insects and pests, cotton plants possess different sesquiterpenes i.e. gossypol in aerial parts and in the root epiblema. But contrary to their defense action, the presence of gossypol above than a certain limit make cotton seed product menial to be used as feed. Data reported in earlier years highlight efforts to tackle gossypol biosynthesis and other terpenoids. d-cadinene is precursor in biosynthesis pathway of sesquiterpenoids. (+)-δ-cadinene is produced from the activity of cadinene synthase. The biochemical pathway involved in d- cadenine synthesis has already been well understood and published (Benedict, Martin, Liu, Puckhaber, & Magill, Citation2004). Due to this crucial role of (+)-δ-cadinene in gossypol synthesis, this gene was targeted using RNAi technique in order to decrease gossypol content in seed but keeping its level same in other parts. In the start, the efforts went ineffective (Martin, Liu, Benedict, Stipanovic, & Magill, Citation2003; Townsend, Poole, Blake, & Llewellyn, Citation2005) but later on marked success was achieved by using a highly specific a-globulin promoter involved in driving a hairpin transcript that targets the above said gene. Prominent reduction in gossypol content has been found in different transgenic cotton cultivars that express hpRNA transcripts (Sunilkumar, Campbell, Puckhaber, Stipanovic, & Rathore, Citation2006). On the basis of extensive field trials by using the transgenic cotton, it was presented by the same researchers that lowered gossypol amount in transgenic lines is main difference between seeds of transgenic and wild cotton cultivars, while the rest of composition remained unchanged (Palle et al., Citation2013).
7. Challenges for tomorrow
We must focus to all that is very well but let us cultivate our garden. Within last twenty years, analysis of these two decades offers a good picture of performance and present status of transgenic crops in comparison with expectation. Now, it is imperative to increase research investment on transgenic crops and raising plants that are well able to thrive in presence of biotic as well as abiotic pressures. Across the world forums should be launched with the responsibility for research innovation in the field of transgenic plants and commercialization of biotechnological outcomes. In presence of question marks upon transgenic crops, it is direly needed to carry on massive public awareness campaign i.e. benefits, biosafety, risk assessment. For welfare of cultivator and consumer, it is suggested that human resource development must be conducted to enhance biotechnology based qualitative and quantitative agricultural productivity.
A daunting challenge ahead is an attempt to merge maximum of the constructive alleles into a single cotton cultivar and they also show expression. It must also be very clear to all of us that latest challenges will appear with the ongoing change in global climate. Presently, a great effort is needed to comprehend the effect of the climatic alterations on genetically altered cotton which will later be used as novel traits for transgenic improvement of cotton.
Another feasible arena is reduction in processing costs by eliminating, anti-nutritive, malnutritive or bad flavor constituents in foods by adopting antisense nucleic acid technology. In addition to this, genes and enzymes taking part in aroma biosynthesis and flavors are significant for this industry and to the public. Gigantic prospect lies in the utilization of crops for commodity like chemical products. Plants have conventionally been a resource of extensive polymers i.e. carbohydrates, rubber, waxes. Nevertheless, related issues of synthetic polymer usage like manufacturing cost, supply and wastage are attributes that are refreshing attention towards using bio-polymers. Evident from the success story, it is now envisioned that Genetic engineering can significantly expand the continuum and sonata of available plant based polymers.
Additional information
Funding
Notes on contributors
Ali Noman
Ali Noman, Madiha Zainab, Waqar Islam and Muhammad Adnan are researchers at Fujian Agriculture and Forestry University, Fuzhou. The authors are working on crop genetic improvement in presence of environmental pressures.
Rohina Bashir
Rohina Bashir and Sara Zafar are Assistant Professors at GC University Faisalabad. These contributors have their specialties in plant Biotechnology and plant abiotic stress tolerance respectively.
Muhammad Aqeel
Muhammad Aqeel and Wasif Iftikhar are MPhil in Botany and working in different organizations. All the authors have published several research papers in journals of international repute.
Sumera Anwer
Sumera Anwer is Post-Doctoral researcher at HZAU, Wuhan. She has experience in studying plant and environmental interactions.
Shahbaz Khan
Shahbaz Khan is a doctorate candidate at HZAU, Wuhan.
References
- Ali, I., Amin, I., Briddon, R. W., & Mansoor, S. (2013). Artificial microRNA-mediated resistance against the monopartite begomovirus Cotton leaf curl Burewala virus. Virology Journal, 10, 231.10.1186/1743-422X-10-231
- Ali, Z., Abulfaraj, A., Idris, A., Ali, S., Tashkandi, M., & Mahfouz, M. M. (2015). CRISPR/Cas9-mediated viral interference in plants. Genome Biology, 16, 267.10.1186/s13059-015-0799-6
- Ammann, K. (2005). Effects of biotechnology on biodiversity, herbicide-tolerant and insect-resistant GM crops. Trends in Biotechnology, 23, 388–394.10.1016/j.tibtech.2005.06.008
- Amudha, J., Balasubramani, G., Malathi, V. G., Monga, D., & Kranthi, K. R. (2011). Cotton leaf curl virus resistance transgenics with antisense coat protein gene (AV1). Current Science, 101, 300–307.
- Arpat, A. B., Waugh, M., Sullivan, J. P., Gonzales, M., Frisch, D., Main, D., … Wilkins, T. A. (2004). Functional genomics of cell elongation in developing cotton fibers. Plant Molecular Biology, 54, 911–929.10.1007/s11103-004-0392-y
- Arshad, M., Ali, S., Noman, A., Ali, Q., Rizwan, M., Farid, M., & Irshad, M. K. (2015). Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Archives of Agronomy and Soil Science, 62, 533–546. doi:10.1080/03650340.2015.1064903
- Bakhsh, A. (2010). Expression of two insecticidal genes in cotton (PhD thesis). The University of Punjab, Lahore, 60.
- Bakhsh, A. (2014). Engineering crop plants against abiotic stress, current achievements and future prospects. Emirates Journal of Food and Agriculture, 27, 24–39.
- Bakhsh, A., Siddique, S., & Husnain, T. (2012). A molecular approach to combat spatio-temporal variation in insecticidal gene (Cry1Ac) expression in cotton. Euphytica, 183, 65–74.10.1007/s10681-011-0497-8
- Bawa, A. S., & Anilakumar, K. R. (2013). Genetically modified foods: Safety, risks and public concerns—A review. Journal of Food Science and Technology, 50, 1035–1046. doi:10.1007/s13197-012-0899-1
- Benedict, C. R., Martin, G. S., Liu, J., Puckhaber, L., & Magill, C. W. (2004). Terpenoid aldehyde formation and lysigenous gland storage sites in cotton, variant with mature glands but suppressed levels of terpenoid aldehydes. Phytochemistry, 65, 1351–1359.10.1016/j.phytochem.2004.03.032
- Blair-Kerth, L., Dotray, P. A., Keeling, J. W., Gannaway, J. R., Oliver, M. J., & Quisenberry, J. E. (2001). Tolerance of transformed cotton to glufosinate. Weed Science, 49, 375–380.10.1614/0043-1745(2001)049[0375:TOTCTG]2.0.CO;2
- Boopathi, M., & Ravikesavan, R. (2009). Emerging trends in enhancement of cotton fiber productivity and quality using functional genomics tools. Biotechnology Molecular Biology, 4, 11–28.
- Brookes, G., & Barfoot, P. (2008). Global impact of biotech crops, socioeconomic and environmental effects. AgBioForum, 11, 21–38.
- Cao, Y., Ding, X., Cai, M., Zhao, J., Lin, Y., Li, X., … Wang, S. (2007). The expression pattern of a rice disease resistance gene Xa3/Xa26 Is differentially regulated by the genetic backgrounds and developmental stages that influence its function. Genetics, 177, 523–533.10.1534/genetics.107.075176
- Cardoso, F. M., Ibanez, L. I., Hoecke, S. V., Baets, S. D., Smet, A., Roose, K., … Saelens, X. (2014). Single domain antibodies targeting Neuraminidasr protect against an H5N1 influenza virus challenge. Journal of Virology, 88, 8278–8296.10.1128/JVI.03178-13
- Chakrabarty, P. K., Kalbande, B., Chavhan, R., Warade, J., Bajaj, D., Sable, S., … Monga, D. (2011). Engineering cotton leaf curl virus resistance cotton through RNA interference approach. Mumbai: Proceedings of World Cotton Research Conference-5.
- Chakravarthy, V. S. K., Sadumpati, V. K., Nunna, H. R., Puligundla, S. K., Vudem, D. R., & Khareedu, V. R. (2013). Development of transgenic cotton lines expressing Allium sativum Agglutinin (ASAL) for enhanced resistance against major sap-sucking pests. PLoS One, 8, e72542.doi:10.1371/journal.pone.0072542
- Chee, P. W., & Campbell, B. T. (2009). Bridging classical and molecular genetics of cotton fiber quality and development. In A. H. Paterson (Ed.), Genome of cotton (pp. 283–311). New York, NY: Springer.
- Chen, M., Zhao, J.-Z., Collins, H. L., Earle, E. D., Cao, J., & Shelton, A. M. (2008). A critical assessment of the effects of Bt transgenic plants on parasitoids. PLoS One, 3(5), e2284. Retrieved from http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0002284. doi:10.1371/journal.pone.0002284
- Cohen, B. M., Gould, F., & Bentur, J. C. (2000). Bt rice, practical steps to sustainable use. International Rice Research Notes, 2, 4–10.
- Cohen, J. E. (2003). Human population: The next half century. Science, 302, 1172–1175.10.1126/science.1088665
- Dasani, S. H., & Thaker, V. S. (2006). Role of abscisic acid in cotton fiber development. Russian Journal of Plant Physiology, 53, 62–67.10.1134/S1021443706010080
- Devine, M. D. (2005). Why are there not more herbicide-tolerant crops? Pest Management Science, 61, 312–317.10.1002/(ISSN)1526-4998
- Fang, J., Xu, X., Wang, P., Zhao, J. Z., Shelton, A. M., Cheng, J., … Shen, Z. (2007). Characterization of chimeric Bacillus thuringiensis Vip3 toxins. Applied Environmental Microbiology, 73, 956–961.10.1128/AEM.02079-06
- Fred, S., Betz, Bruce, Hammond, G., & Roy, L. F. (2000). Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regulatory Toxicology and Pharmacology, 32, 156–173.
- Fu, Y., Sander, J. D., Reyon, D., Cascio, V. M., & Joung, J. K. (2014). Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nature Biotechnology, 32, 279–284.10.1038/nbt.2808
- Ganesan, M., Bhanumathi, P., Ganesh, K. K. L., Prabha, A., Song, P. S., & Jayabalan, N. (2009). Transgenic Indian cotton (Gossypium hirsutum) harboring rice chitinase gene (Chi II) confers resistance to two fungal pathogens. American Journal Plant Biochemistry Biotechnology, 5, 63–74.
- Gao, S., Chen, M., Xia, L., Xiu, H., Xu, Z., Li, L., … Ma, Y. (2009). A cotton (Gossypium hirsutum L.) DRE-binding transcription factor gene, GhDREB, confers enhanced tolerance to drought, high salt, and freezing stresses in transgenic wheat. Plant Cell Reports, 28, 301–311.10.1007/s00299-008-0623-9
- Grewal, H. S. (2010). Water uptake, water use efficiency, plant growth and ionic balance of wheat, barley, canola and chickpea plants on a sodic vertosol with variable subsoil NaCl salinity. Agricultural Water Management, 97, 148–156.10.1016/j.agwat.2009.09.002
- Gorai, M., Ennajeh, M., Khemira, H., & Neffati, M. (2010). Combined effect of NaCl-salinity and hypoxia on growth, photosynthesis, water relations and solute accumulation in Phragmites australis plants. Flora - Morphology, Distribution, Functional Ecology of Plants, 205, 462–470.10.1016/j.flora.2009.12.021
- Haigler, C. H., Zhang, D., & Wilkerson, C. G. (2005). Biotechnological improvement of cotton fibre maturity. Physiologia Plantarum, 124, 285–294.10.1111/ppl.2005.124.issue-3
- Hallwass, M., Oliveira, A., Dianese, E. C., Lohuis, D., Boiteux, L., & Inoue-Nagata, A. K. (2014). The Tomato spotted wilt virus cell-to-cell movement protein (NSM) triggers a hypersensitive response in Sw-5 containing resistant tomato lines and Nicotiana benthamiana transformed with the functional Sw-5b resistance gene copy. Molecular Plant Pathology, 15, 871–880.
- Hammond, S. M., Caudy, A. A., & Hannon, G. J. (2001). Post transcriptional gene silencing by double-stranded RNA. Nature Reviews Genetics, 2, 110–119.10.1038/35052556
- Hanley-Bowdoin, L., Bejarano, E. R., Robertson, D., & Mansoor, S. (2013). Geminiviruses: masters at redirecting and reprogramming plant processes. Nature Reviews in Microbiology, 11, 777–88.
- Hsu, P. D., Scott, D. A., Weinstein, J. A., Ran, F. A., Konermann, S., Agarwala, V., ... Zhang, F. (2013). DNA targeting specificity of RNA-guided Cas9 nucleases. Nature Biotechnology, 31, 827–832.10.1038/nbt.2647
- James, C. (2013). Global status of commercialized biotech/GM crops, ISAAA Brief No. 46. Ithaca, NY: ISAAA.
- James, C. (2014). Global status of commercialized biotech/GM crops, ISAAA Brief No. 49. Ithaca, NY: ISAAA.
- Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337, 816–821.10.1126/science.1225829
- Kagaya, Y., Hobo, T., Murata, M., Ban, A., & Hattori, T. (2002). Abscisic acid-induced transcription is mediated by phosphorylation of an abscisic acid response element binding factor, TRAB1. Plant Cell, 14, 3177–3189.10.1105/tpc.005272
- Keshamma, E., Rohini, S., Rao, K. S., Madhusudhan, B., & Kumar, M. U. (2008). Tissue culture-independent in plant transformation strategy, an Agrobacterium tumefaciens-mediated gene transfer method to overcome recalcitrance in cotton (Gossypium hirsutum L.). Journal Cotton Science, 12, 264–272.
- Khan, G. A., Bakhsh, A., Ghazanffar, M., Riazuddin, S., & Husnain, T. (2013). Development of transgenic cotton pure lines harboring a pesticidal gene (cry1Ab). Emirates Journal Food Agriculture, 25, 434–442.
- Khorsandi, F., & Anagholi, A. (2009). Reproductive compensation of cotton after salt stress relief at different growth stages. Journal of Agronomy and Crop Science, 195, 278–283.10.1111/jac.2009.195.issue-4
- Klosterman, S., Atallah, Z., Vallad, G., & Subbarao, K. (2009). Diversity, pathogenicity, and management of Verticillium species. Annual Review of Phytopathology, 47, 39–62.10.1146/annurev-phyto-080508-081748
- Kouser, S., & Qaim, M. (2012). Valuing financial, health and environmental benefits of Bt cotton in Pakistan. Foz do Iguacu: The International Association of Agricultural Economists (IAAE) Triennial Conference. doi:10.1111/agec.12014
- Kranthi, K. R., Naidu, S., Dhawad, C. S., Tatwawadi, A., Mate, K., Patil, E., … Kranthi, S. (2005). Temporal and intra-plant variability of Cry1Ac expression in Bt-cotton and its influence on the survival of the cotton bollworm, Helicoverpa armigera (Hübner) (Noctuidae, Lepidoptera). Current Science, 89, 291–298.
- Leelavathi, S., Sunnichan, V. G., Kumria, R., Vijaykanth, G. P., Bhatnagar, R. K., & Reddy, V. S. (2004). A simple and rapid Agrobacterium -mediated transformation protocol for cotton (Gossypium hirsutum L.): Embryogenic calli as a source to generate large numbers of transgenic plants. Plant Cell Reports, 22, 465–470.10.1007/s00299-003-0710-x
- Li, Y., Zhang, J., Zhang, J., Hao, L., Hua, J., Duan, L., … Li, Z. (2013). Expression of an Arabidopsis molybdenum cofactor sulphurase gene in soybean enhances drought tolerance and increases yield under field conditions. Plant Biotechnology Journal, 11, 747–758.10.1111/pbi.12066
- Liu, D. Q., Tu, L. L., Wang, L., Li, Y. J., Zhu, L. F., & Zhang, X. L. (2008). Characterization and expression of plasma and tonoplast membrane aquaporins in elongating cotton fibers. Plant Cell Reports, 27, 1385–1394.10.1007/s00299-008-0545-6
- Liu, Y. D., Yin, Z. J., Yu, J. W., Li, J., Wei, H. L., Han, X. L., & Shen, F. F. (2012). Improved salt tolerance and delayed leaf senescence in transgenic cotton expressing the Agrobacterium IPT gene. Biologia Plantarum, 56, 237–246.10.1007/s10535-012-0082-6
- Lobell, D. B., Ortiz-Monasterio, J. I., Gurrola, F. C., & Valenzuela, L. (2007). Identification of saline soils with multiyear remote sensing of crop yields. Soil Science Society of America Journal, 71, 777–783.10.2136/sssaj2006.0306
- Lu, Y., Wu, K., Jiang, Y., Guo, Y., & Desneux, N. (2012). Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature, 487, 362–365.
- Ma, Y., Zhang, L., & Huang, X. (2014). Genome modification by CRISPR/Cas9. Federation of European Biochemical Societies Journal, 281, 5186–5193. doi:10.1111/febs.13110
- Maqbool, A. (2009). Search for drought tolerant genes through differential display (PhD thesis). The University of Punjab, Lahore.
- Maqbool, A., Abbas, W., Rao, A. Q., Irfan, M., Zahur, M., Bakhsh, A., … Husnain, T. (2010). Gossypium arboreum GHSP26 enhances drought tolerance in Gossypium hirsutum L. Biotechnology Progress, 26, 21–25.
- Martin, G. S., Liu, J., Benedict, C. R., Stipanovic, R. D., & Magill, C. W. (2003). Reduced levels of cadinane sesquiterpenoids in cotton plants expressing antisense (+)-δ-cadinene synthase. Phytochemistry, 62, 31–38.10.1016/S0031-9422(02)00432-6
- Marvier, M., McCreedy, C., Regetz, J., & Kareiva, P. (2007). A meta-analysis of effects of Bt cotton and maize on non-target invertebrates. Science, 316, 1475–1477.10.1126/science.1139208
- Miao, W., Wang, X., Li, M., Song, C., Wang, Y., Hu, D., & Wang, J. (2010). Genetic transformation of cotton with a harpin-encoding gene hpaXoo confers an enhanced defense response against different pathogens through a priming mechanism. BMC Plant Biology, 10. doi:10.1186/1471-2229-10-67
- Mittal, A., Gampala, S. S. L., Ritchie, G. L., Payton, P., Burke, J., & Rock, C. D. (2014). Related to ABA-Insensitive3(ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotechnology Journal, 12, 578–589.10.1111/pbi.12162
- Munns, R. (2002). Salinity, growth and phytohormones. In A. Läuchli, U. Lüttge (Eds.), Salinity, environment—plants—molecules (pp. 271–290). Dordrecht: Kluwer Academic.
- Nawaz, K., Hussain, K., Majeed, A., Khan, F., Afghan, S., & Ali, K. (2010). Fatality of salt stress to plants: Morphological, physiological and biochemical aspects. African Journal of Biotechnology, 9, 5475–5480.
- Noman, A., Ali, Q., Hameed, M., Mehmood, T., & Iftikhar, T. (2014). Comparison of leaf anatomical characteristics of Hibiscus rosa-sinensis grown in Faisalabad region. Pakistan Journal of Botany, 46, 199–206.
- Noman, A., Ali, Q., Naheed, F., Ali, Q., Farid, M., Rizwan, M., & Irshad, M. K. (2015). Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L) cultivars under drought stress. Archives of Agronomy and Soil Science, 61, 1659–1672. doi:10.1080/0365034020151028379
- Palle, S. R., Campbell, L. M., Pandeya, D., Puckhaber, L., Tollack, L. K., Marcel, S., … Rathore, K. S. (2013). RNAi-mediated Ultra-low gossypol cottonseed trait: Performance of transgenic lines under field conditions. Plant Biotechnology Journal, 11, 296–304.10.1111/pbi.12013
- Price, D. R. G., & Gatehouse, J. A. (2008). RNAi-mediated crop protection against insects. Trends in Biotechnology, 26, 393–400.10.1016/j.tibtech.2008.04.004
- Prins, M. (2003). Broad virus resistance in transgenic plants. Trends in Biotechnology, 21, 373–375.10.1016/S0167-7799(03)00183-5
- Rahman, M., Rao, A. Q., Batool, F., Shahid, A. A., & Husnain, T. (2012). Transgene copy number and phenotypic variations in transgenic basmati rice. Journal Animal Plant Science, 22, 1004–1013.
- Rashid, B., Saleem, Z., Husnain, T., & Riazuddin, S. (2008). Transformation and inheritance of Bt genes in Gossypium hirsutum. Journal of Plant Biology, 51, 248–254.10.1007/BF03036123
- Rathore, K. S., Sunilkumar, G., Cantrell, R. G., Reding, H. K., & Hague, S. (2009). Compendium of transgenic crop plants. Transgenic sugar, tuber and fiber crops. Wiley Online Library. doi:10.1002/9781405181099.k0707
- Rodriguez-Uribe, L., Higbie, S. H., Stewart, J., Wilkins, T., Lindemann, W., Sengupta-Gopalan, C., & Zhang, J. (2011). Identification of salt responsive genes using comparative microarray analysis in Upland cotton (Gossypium hirsutum L.). Plant Science, 180, 461–469.10.1016/j.plantsci.2010.10.009
- Ruan, Y. L., Llewellyn, D. J., & Furbank, R. T. (2001). The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin. Plant Cell, 1, 47–60.
- Shamim, Z., Rashid, B., Rahman, S., & Husnain, T. (2013). Expression of drought tolerance in transgenic cotton. ScienceAsia, 39, 1–11.10.2306/scienceasia1513-1874.2013.39.001
- Sohrab, S. S., Kamal, M. A., Ilah, A., Husen, A., Bhattacharya, P. S., & Rana, D. (2014). Development of Cotton leaf curl virus resistant transgenic cotton using antisense ßC1 gene. Saudi Journal of Biological Sciences, 10, 358–362.
- Song, X., Kain, W., Cassidy, D., & Wang, P. (2015). Resistance to Bacillus thuringiensis toxin Cry2Ab in Trichoplusia ni is conferred by a novel genetic mechanism. Applied Environmental Microbiology, 81, 5184–5195.10.1128/AEM.00593-15
- Sunilkumar, G., Campbell, L. M., Puckhaber, L., Stipanovic, R. D., & Rathore, K. S. (2006). Engineering cottonseed for use in human nutrition by tissue-specific reduction of toxic gossypol. Proceedings of the National Academy of Sciences, 103, 18054–18059.10.1073/pnas.0605389103
- Tohidfar, M., Ghareyazie, B., Mosavi, M., Yazdani, S., & Golabchian, R. (2008). Agrobacterium-mediated transformation of cotton (Gossypium hirsutum) using a synthetic cry1Ab gene for enhanced resistance against Heliothis armigera. Iranian Journal Biotechnology, 6, 164–173.
- Tohidfar, M., Mohammadi, M., & Ghareyazie, B. (2005). Agrobacterium mediated transformation of cotton (Gossypium hirsutum) using a heterologous bean chitinase gene. Plant Cell Tissue Organ Culture, 83, 83–96.10.1007/s11240-004-6155-2
- Townsend, B. J., Poole, A., Blake, C. J., & Llewellyn, D. J. (2005). Antisense suppression of a (+)-d-cadinene synthase gene in cotton prevents the induction of this defense response gene during bacterial blight infection but not its constitutive expression. Plant Physiology, 138, 516–528.10.1104/pp.104.056010
- Vinocur, B., & Altman, A. (2005). Recent advances in engineering plant tolerance to abiotic stress, achievements and limitations. Current Opinion in Biotechnology, 16, 123–132.10.1016/j.copbio.2005.02.001
- Wang, Q. Q., Liu, F., Chen, X. S., Ma, X. J., Zeng, H. Q., & Yang, Z. M. (2010). Transcriptome profiling of early developing cotton fiber by deep-sequencing reveals significantly differential expression of genes in a fuzzless/lintless mutant. Genomics, 96, 369–376.10.1016/j.ygeno.2010.08.009
- Wu, K. M., Guo, Y., & Chen, Y. (2005). The evolution of cotton pest management practices in China. Annual Review of Entomology, 50, 31–52.10.1146/annurev.ento.50.071803.130349
- Xiong, L. M., & Zhu, J. K. (2003). Regulation of abscisic acid biosynthesis. Plant Physiology, 133, 29–36.10.1104/pp.103.025395
- Yarasi, B., Sadumpati, V., Immanni, C. P., Vudem, D. R., & Khareedu, V. R. (2008). Transgenic rice expressing Allium sativum leaf agglutinin (ASAL) exhibits high-level resistance against major sap-sucking pests. BMC Plant Biology, 8, 102.10.1186/1471-2229-8-102
- Yue, Y., Zhang, M., Zhang, J., Tian, X., Duan, L., & Li, Z. (2012). Overexpression of the AtLOS5 gene increased abscisic acid level and drought tolerance in transgenic cotton. Journal of Experimental Botany, 63, 3741–3748.10.1093/jxb/ers069
- Zafar, S., Ashraf, M. Y., Anwar, S., Ali, Q., & Noman, A. (2016). Yield enhancement in wheat by soil and foliar fertilization of K and Zn under saline environment. Soil and Environment, 35, 46–55.
- Zhang, B., Yang, Y., Chen, T., Yu, W., Liu, T., Li, H., ... Chang, Y. (2012). Island cotton Gbve1Gene encoding a receptor-like protein confers resistance to both defoliating and non-defoliating isolates of Verticillium dahliae. PLoS One, 7, e51091. doi:10.1371/journal.pone.0051091