Abstract
This study assessed the microbial risk of fresh produce collected from a compost farm, a crop-livestock rotation farm and a traditional farm in Canterbury, New Zealand. A total of 79 vegetable samples were collected from 3 different farming systems. No Escherichia coli O157:H7 was detected, but 95, 92 and 44% of the samples from the organic farm had unsatisfactory aerobic plate count (APC), E. coli, and Staphylococcus aureus levels respectively compared to the traditional farm, with corresponding values of 66, 88 and 27% based on guidelines for ready-to-eat foods. Resistance profiling showed that S. aureus and Salmonella spp. from all farms showed a higher resistance to vancomycin, erythromycin, ampicillin and penicillin antibiotics. The fresh produce collected from the organic farms was more contaminated with a wider range of pathogens than those from the traditional farm. Since the consumer perception of organic produce is because of its ‘chemical free’ status with little regard to the microbial status, it is prudent to conduct further studies to determine the type(s) of microbial hazards and how they behave under different climatic conditions and also address bacterial resistance to antibiotics in farming environments.
Public Interest Statement
Food safety is a vital aspect of vegetable production, especially for trading nations where exports are critical for their economies and well-being. This study provides a detailed description of the discovery of the presence of known human pathogens in a compost farm, crop-livestock rotation farm and a traditional farm in Canterbury, New Zealand. Fresh produce from the organic farms was more contaminated with a wider range of pathogens than those from the traditional farm. Some bacterial isolates in all farms sampled showed a higher resistance to some antibiotics such as vancomycin, erythromycin, ampicillin and penicillin. With the current trend of people favouring more “chemical free” organic (bio) foods, this study will be of great help to the food industry and also the local health authorities.
Competing Interests
The authors declare no competing interest.
1. Introduction
Microbiological food safety is a global concern. As global food chain becomes increasingly complex, additional challenges confront food safety. Nowadays, people eat more fresh produce such as raw vegetables and fruits because these are perceived as healthy foods. In addition, many consumers are concerned about chemicals used in food production such as pesticides and fertilizer. Hence the number of people who purchase organically grown food products are increasing. Organic farming is considered as a sustainable, humane and non-polluting method of producing food products without the use of toxic chemicals and/or genetically modified organisms (Food & Agriculture Organization of the United Nations, Citation1998). In Europe, approximately 5.4% of the agricultural production land is used for organic farming (European Union, Citation2013) compared to 0.6 and 1.6% in the USA and New Zealand, respectively (FiBL and IFOAM, Citation2013).
There are some drawbacks in organic farming. Firstly, production per hectare is less than from traditional farms. Anwar et al. (Citation2005) found that the yield and quality of farm products is higher in traditional farms than from farms that use a combination of organic manure and fertilizer. Secondly, risk of food poisoning from organic fresh produce is probably more than from traditional fresh produce because of the use of manure and other types of soil enrichments. Pell (Citation1997) suggested a higher risk of pathogen contamination of products because manure which is animal faeces can contain in excess of 1010 bacteria/g. Therefore, the concern for food safety in organically grown fresh produce has been growing.
According to Jiang, Morgan, and Doyle (Citation2002), Escherichia coli O157:H7 is able to survive in the manure on soil for 231 days at 21°C. However, Johannessen, Froseth, Solemdal, Jarp, and Wasteson (Citation2004) found no significant difference in the bacteriological quality of organic produce farmed in soils with or without E. coli O157:H7. Most studies did not find E. coli O157:H7 or Salmonella in the fresh organic produce (McMahon & Wilson, Citation2001; Oliveira et al., Citation2010; Sagoo, Little, & Mitchell, Citation2001) and there was no consistent trend in the existence of pathogens (Maffei, Silveira, & Catanozi, Citation2013) or when found were within the acceptable limit compared to the UK guidelines (Public Health Laboratory Service (PHLS), Citation2000) for E. coli and Listeria monocytogenes, and the microbiological guidelines in Norway (Norwegian Food Safety Authority, Citation2000) for E. coli O157:H7and Salmonella spp. (Loncarevic, Johannessen, & Rorvik, Citation2005). This may be because the pathogens are killed by high temperatures generated during the aging of manure. Himathongkham, Bahari, Riemann, and Cliver (Citation1999) reported that destruction speed of pathogens in manure was the highest at 37°C compared to 4 and 20°C. So, appropriate heat treatment of manure can minimise pathogen contamination of food produce.
There have been several outbreaks of E. coli O157:H7 poisonings related to consumption of produce from manure applied farms (Chapman, Siddons, & Manning, Citation1997; Jiang et al., Citation2002). According to Solomon, Yaron, and Matthews (Citation2002), pathogens such as E. coli O157:H7 in the soil-manure mix are “internalized” which means pathogens enter the plant tissues from the roots. In contrast, Gu, Cevallos-Cevallos, Vallad, and van Bruggen (Citation2013) reported that in the tomato plant, “organic soil” prevents internalization of Salmonella. Threshold for the number of Salmonella required to “internalize into produce” is high and therefore the risk of microbial contamination due to internalization from the roots is negligible (Trevor, Citation2011). Also, even when internalized, Salmonella in the upper part of plant is less and also inactive than in the lower part (Ge, Lee, & Lee, Citation2013).
In organic farming, compost is widely used instead of fertilizer because of its rich nutritional matrix (Fang, Wong, Li, & Wong, Citation1998). Many types of organic waste such as bark, leaf mould, and treated animal manure, have been used in compost making (Raviv, Chen, & Inbar, Citation1986). Composting of plant wastes effectively eliminates fungi also because of the high temperatures generated during the process (Suarez-Estrella, Vargas-Garcia, Elorrieta, Lopez, & Moreno, Citation2003). E. coli and Salmonella enteritidis in cow manure are destroyed by composting at 45°C (Lung et al., Citation2001). Crop-Livestock rotation farming is a sustainable effective traditional organic farming method with rotational cropping and livestock production on the same land and offers many benefits (Vilrla, Macedo, Júnior, & Kluthcouski, Citation2003). For example, cropping improves pasture growth for livestock and supplies forage even in winter or dry season for livestock and livestock improves soil structure with their organic material and pasture can work as a cover for soil (Vilrla et al., Citation2003). However, Hilimire (Citation2011) reported concerns about food safety in the crop-livestock rotation farming because in one study although E. coli O157:H7 and Campylobacter spp. were not detected while Salmonella spp. were detected in the soils (Hilimire, Citation2011).
To our knowledge, there are no reports of microbiological assessment of fresh produce in the crop-livestock rotation farming system. This preliminary study is on microbiological risk assessment (APC, Coliform, E. coli, E. coli O157:H7, yeast and mould, Salmonella spp. and Staphylococcus aureus) of fresh produce collected from different farming systems (compost farm, crop-livestock rotation farm and a traditional farm) in New Zealand using traditional and advanced microbiological methods. To confirm E. coli O157:H7 identity, a novel molecular detection system (3M, USA) was used. Resistance of the samples to β-lactam, aminoglycoside and macrolide type of antibiotics were also tested.
2. Methods
2.1. Selection, transport and handling of samples
Samples were obtained from three different farming systems, two organic farms and one traditional farm in Lincoln, Canterbury, New Zealand. One of the organic farms used compost (which did not include animal waste), the other used a crop-livestock rotation system (using sheep, rotation every four years). The traditional farm used fertilizer. The collection of vegetable, soil and water samples was conducted from May 2014 to October 2014 which is winter-spring season in New Zealand. The samples collected from each farm type was different because different vegetables were grown in the different farms at the time of this study. The priority was to collect vegetables that are normally consumed without cooking. The collected vegetables were: spinach, coriander, carrot, parsley, cabbage from the compost farm, parsley, cabbage, pak choi, spinach, silver beet and lettuce from the crop-livestock rotation farm, and spring onion, leek, radish, pak choi and silver beet from the traditional farm which were 79 samples in total. In addition, a total of 12 soil samples (approximately 200 g from 1 cm of the soil surface around the vegetable sampled) were collected, 2 samples from the traditional farm and 1 sample each from the 2 organic farms on three different occasions, a month apart. Six water samples (from the tap water hose used to supply water to the vegetables) were collected from each farm on two different occasions, a month apart (at month 1 and month 3 of soil sample collection). Samples were packed in individual plastic bags, transported to the laboratory and placed in a refrigerator (at 4°C). Samples were cut into small pieces (about three square centimetres) and placed in a sterile plastic bag for sample processing. The collection continued once a week for six weeks over 6 months.
2.2. Sample preparation
For APC, coliform, E. coli, E. coli O157:H7, S. aureus and yeast and mould, 25 g of sample was placed in a sterilized stomacher bag with 225 g of 0.1% peptone water and mechanically homogenised for 3 min using a stomacher.
2.3. Enumeration of microorganisms
Plate Count agar plates were used for enumerating APC (ISO 4833:2003). The plates were placed in a 37°C incubator for 24 h. MacConkey agar (ISO 21567) plates were used for enumerating E. coli and coliforms and incubated at 37°C for 24 h. Red to purple pink colonies were counted as E. coli and light pinkish colonies as coliforms. Yeast and mould agar plates were used for enumerating yeast and mould (ISO 7954:1987) and incubated at 30°C for 48 h. For S. aureus, Baird-Parker agar plates were used for enumerating (ISO 6888–2:1999) and incubated at 37°C for 24 h. The results were expressed as colony-forming units per gram (CFU/g).
2.4. Detection of Salmonella spp.
A commercial kit (Reveal® 2.0 for Salmonella, Neogen Corporation, USA) was used for Salmonella spp. detection. 25 g of sample was placed in a sterilized stomacher bag with 200 g of steriled-purified water preheated to 42°C and mixed with supplied reagents (Reveal® 2.0 item 9705, Neogen Corporation, USA). The sample was next mixed by hand (grasping through the stomacher bag) and incubated at 37°C for 4 h. One bottle of reconstitute 2 × RV (Reveal® 2.0 item 9715, Neogen Corporation, USA) was dissolved in 200 g of sterilised, purified water preheated to 37°C and mixed with the prepared samples. The samples were mixed gently and incubated at 42°C for 24 h. Eight drops of samples were transferred into the Reveal sample cup (Reveal® 2.0 item, Neogen Corporation, USA). Results were recorded after 15 min.
2.5. Confirmation of Salmonella spp.
The samples used for the detection of Salmonella spp. were confirmed by using Salmonella test kit based on the latex agglutination test (Oxoid, UK). One drop of sample which prepared as above was transferred onto the reaction card using a micropipette. One drop of Salmonella latex agent (Oxoid, UK) was added to the sample and mixed. Agglutination was observed after 2 min and compared with negative and positive controls.
2.6. Enumeration and confirmation of E. coli O157:H7
Sorbitol MacConkey agar with supplements (potassium tellurite and cefixime) was used for the detection of E. coli O157:H7 as described by McMahon and Wilson (Citation2001), and the plates were incubated at 37°C for 24 h. For confirmation, we followed the protocol and equipment provided by 3M. Briefly, 25 g of sample was placed in a sterile stomacher bag with 225 g of bufferd peptone water (3M, USA) and incubated at 42°C for 24 h. 20 μl of the sample was then transferred into a lysis tube (3M, USA) and mixed. The lysis tube was kept on a heating block (100°C, 3M, USA) for 15 min, cooled on a chill block (3 M, USA) for 10 min and then left at room temperature for 5 min. 20 μL of each sample lysate was transferred into a reagent tube and mixed with 20 μL of NC lysate (3M, USA). The sample was then transferred onto a speed loader tray (3M, USA) and placed in the instrument (3M™ Molecular Detection System; 3M, USA) for the assay.
2.7. Antibiotic resistance profiling of selected microbial isolates
Disc diffusion tests were conducted on Mueller-Hinton agar plates (Neogen, Citation2011) as described by the Clinical and Laboratory Standards Institute (Clinical & Laboratory Standards Institute, Citation2012). The antibiotic discs used (Oxoid, UK) were: ampicillin (AMP;10 μg), tetracycline (TE; 30 μg), gentamycin (GN; 30 μg), erythromycin (E; 15 μg), trimethoprim–sulfamethoxazole (SXT; 25 μg), nalidixic acid (NA; 30 μg), chloramphenicol (C; 30 μg), ciprofloxacin (CIP; 5 μg), penicillin (P; 10 μg), kanamycin (K; 30 μg), streptomycin (S; 30 μg) and vancomycin (VA; 30 μg). Isolates were classified as susceptible, intermediate or resistant to an antibiotic based on the size of the inhibition zones surrounding the colony.
2.8. Statistical analysis
Statistical analysis was conducted using 2 software programs described below. Geometric mean, standard deviation, ranges and median were calculated using Microsoft Office Excel 2013 (Microsoft, USA) and one-way ANOVA test with Tukey comparisons to derive statistical differences (p < 0.05) of microbial levels by using SPSS version 21 (IBM, USA).
3. Results and discussion
3.1. Microbial quality of farm produce, soil and water
A total of 79 vegetable samples were analysed. The counts of APC, coliform, E. coli, yeast, mould and S. aureus in vegetables, 12 soil and 6 water samples are shown in Table . For Salmonella spp. detection, 79 vegetable and 8 soil samples were analysed. Nine vegetable samples from all farms were positive. Salmonella spp. was present in radish, pak choi (traditional farm), spinach, parsley (compost farm) and pak choi, parsley and lettuce (crop-livestock rotation farm). Two soil samples from the traditional farm and 3 each from the compost and crop-livestock rotation farms were contaminated with Salmonella spp. All soil samples were positive for Salmonella spp. based on the confirmation kit (Oxoid, UK). Twenty-five samples including vegetable and soil samples positive for E. coli were analysed for O157:H7 using the 3M molecular detection system but none were positive.
Table 1. Levels (log10 CFU/g) of APC, coliform, E. coli, yeast and mould and S. aureus in vegetables from different farming systems
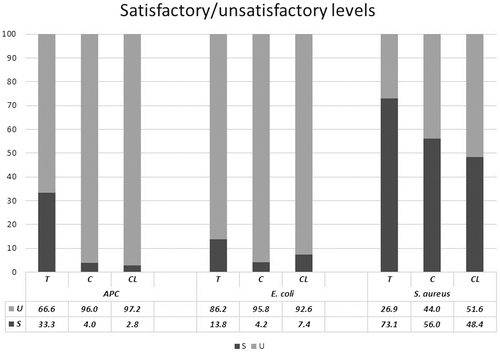
Few studies have compared the microbial quality of fresh produce from different farming systems. Halablab, Sheet, and Holail (Citation2011) reported that APC levels of 63 samples from several riverside farms in Lebanon ranged from 4.3 to 10.4 log10 CFU/g. Oliveira et al. (Citation2010) showed that the aerobic mesophilic count (AMC) of organic and traditional lettuce collected from farms in Spain varied from 0.69–6.35 log10 CFU/g and 0.80–5.67 log10 CFU/g, respectively. Many leafy vegetables have a large, folded surface and higher APC levels are often observed (Aycicek, Oguz, & Karci, Citation2006), because of the contamination of especially leafy vegetables by soil during watering and/or rain (Abadias, Usall, Anguera, Solsona, & Viñas, Citation2008). Although no specific standard has been set for New Zealand fresh produce, according to Food Standards Australia New Zealand (FSANZ) (Citation2001), <4, 4–5 and >5 log10 CFU/g of APC are considered satisfactory, marginal and unsatisfactory respectively for ready-to-eat foods. In general, microbial counts increase markedly during harvesting and processing than at the farm (Sirsat & Neal, Citation2013). In this study, because different vegetables were collected from different farms, average microbial contamination levels for all vegetables taken from each farm were compared. As shown in Figure , the mean APC levels (log10 CFU/ml) was significantly higher (p < 0.05) in the organic farms (the compost farm and the crop-livestock rotation farm) vegetables compared to that of the traditional farm. In addition, as shown in Figure , more than 95% of the samples from the organic farms had unsatisfactory APC levels based on FSANZ (Citation2001) guidelines compared with 66% in the traditional farm.
Figure 1. Vertical axis shows average of log10 CFU/g of APC, coliform, E. coli, S. aureus and yeast and mould in vegetables and soil from three farms (crop-livestock rotation farm, compost farm and traditional farm). Horizontal axis shows types of microbes. 79 vegetable and 12 soil samples were analysed.
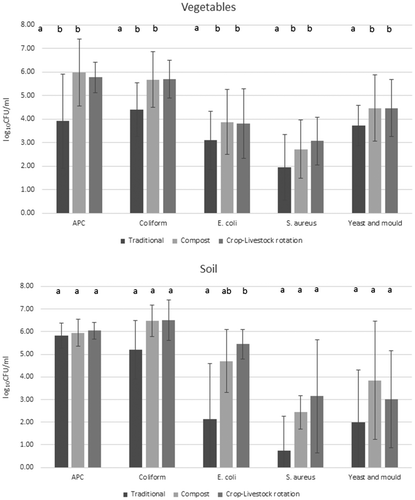
Figure 2. Vertical axis shows percentage of unsatisfactory (U) and satisfactory (S) APC, E. coli, and S. aureus levels of vegetables based on Food Standards Australia New Zealand (FSANZ, Citation2001) or Public Health Laboratory Service (PHLS, Citation2000) guidelines and a comparison between different farm types. Horizontal axis shows types of microbes. T, C and CL in the x-axis refer to the farm types, traditional, compost and crop-livestock rotation farm, respectively.
In general, the total coliform count is not a reliable indicator of harm to human health (British Columbia Centre for Disease Control [BCCDC], Citation2011). However, among the coliforms, E. coli, a faecal coliform, a commensal gut organism can be pathogenic to humans (BCCDC, Citation2011). There was a significant difference (p < 0.05) in the mean coliform levels (log10 CFU/g) of vegetables between organic farms and the traditional farm (Figure ).
E. coli contamination in vegetables reported in the literature is highly variable. Halablab et al. (Citation2011) reported that 1–8.8% of vegetable samples collected from farms in Lebanon contained E. coli. Loncarevic et al. (Citation2005) reported similar results (up to 8.9%) for organic lettuce collected in Norway, while Oliveira et al. (Citation2010) reported values of 22.2% for organic lettuce and 12.5% for lettuce farmed traditionally in Spain. In contrast, Sagoo et al. (Citation2001) found that less than 1.5% of organic vegetables collected from the retail shops in UK contained E. coli. Sirsat and Neal (Citation2013) reported that both traditional lettuce and organic lettuce collected in the USA contained unsatisfactory levels of 2–3.5 log10 CFU/g of E. coli. Ideally, vegetables should not be contaminated with E. coli but < 0.48 log10 CFU/g is considered satisfactory while > 2 log10 CFU/g is unacceptable. In the current study, 92, 96 and 93% of vegetables samples from traditional farm, compost farm and the crop-livestock rotation farm, respectively, contained E. coli (Figure ). Among the soil samples, 50% from the traditional farm, and all from the compost farm and the crop-livestock rotation farm contained E. coli. Both vegetable and soil values are much higher than reported previously (Halablab et al., Citation2011; Loncarevic et al., Citation2005; Oliveira et al., Citation2010; Sagoo et al., Citation2001). As shown in Figure , there was a significant increase (p < 0.05) in the mean E. coli levels (log10 CFU/g) in the vegetables collected from the organic farms compared to the traditional farm. In addition, as shown in Figure , >92% of the samples from the compost and the crop-livestock rotation farms had unsatisfactory E. coli levels based on FSANZ (Citation2001) guidelines compared with 86.2% in the traditional farm.
Yeast is widespread in the environment and on rare occasions can contaminate and cause vegetable spoilage and colour change in the vegetables (Corato, Citation2012) or produce volatile metabolites that can lower the quality of vegetables (Ragaert et al., Citation2006). Moulds can affect human health via production of harmful mycotoxins (Kovacs, Citation2004). Some mycotoxins, such as aflatoxins can cause carcinogenesis, immunosuppression and developmental abnormalities in the reproductive and nervous systems. The yeast and mould concentration in the vegetables between the different farming systems were significantly different (p < 0.05) (Figure ). There is a lack of data on yeast and mould contamination of different vegetable farming systems in New Zealand. Values reported from other countries, are quite variable with 5.5 and 5 log10 CFU/g of yeast in traditional and organic lettuce respectively in the USA (Sirsat & Neal, Citation2013); 2–8.6 log10 CFU/g of yeast and 2–4.6 log10 CFU/g of mould in cut and whole vegetables in retail shops in the USA (Tournas, Citation2005); 1.6–3.8 log10 CFU/g for moulds in mixed cut vegetables in Zambia (Nguz, Shindano, Samapundo, & Huyghebaert, Citation2005); 3.91–5.57 and 3.25–5.17 log10 CFU/g for organic and traditional lettuce respectively in Spain (Oliveira et al., Citation2010); and 2.7–6.3 log10 CFU/g of yeast and 2.3–4.2 log10 CFU/g of mould in whole vegetables in Turkey (Erkan & Vural, Citation2008). Other studies have reported combined yeast and mould levels with values of 4–7 log10 CFU/g from Brazil (Maffei et al., Citation2013), 2–2.6 log10 CFU/g from Spain (Abadias et al., Citation2008), 4–7 log10 CFU/g also from Spain (Badosa, Trias, Parés, Pla, & Montesinos, Citation2008), 4–7 log10 CFU/g for whole vegetables purchased from markets in Singapore (Seow, Ágoston, Phua, & Yuk, Citation2012), and 5.68 log10 CFU/g for cut vegetables and 5.78 log10 CFU/g for herbs collected in Iran (Mohammad & Bahreini, Citation2012). In the current study, the level of yeast and mould was similar to the above mentioned reports with values ranging from 1.78 to 6.39 log10 CFU/g. There was a significant increase (p < 0.05) in the mean yeast and mould levels (log10 CFU/g) in the vegetables collected from the organic farms compared to that from the traditional farm (Figure ). Because there is a lack of data on the yeast and mould levels of fresh produce collected from New Zealand farms, further studies are required.
3.2. Microbial quality of soil and water
According to Johannessen et al. (Citation2004), microbial contamination of soil varies markedly between the different soil enrichment types. No significant difference was observed in the lettuce grown in the different soils in Norway at harvest (Johannessen et al., Citation2004). In the soils with no added fertilizer, APC and E. coli levels were 6.16–6.24 log10 CFU/g and <1–2.14 log10 CFU/g respectively. However, in soils with added inorganic fertilizer, APC and E. coli levels were 5.83–6.16 log10 CFU/g, and <1–1.89 log10 CFU/g (Johannessen et al., Citation2004). In soils with added compost fertilizer, APC and E. coli levels were 6.53–7.06 and 1–2.07 log10 CFU/g respectively (Johannessen et al., Citation2004). In soils with added manure, APC and E. coli values were 6.17–6.73 and 1 - 2.44 log10 CFU/g respectively (Johannessen et al., Citation2004). In the current study, the range of APC levels in soil for each farm was less than those reported by Johannessen et al. (Citation2004) but E. coli levels were higher. In the current study, E. coli levels (log10 CFU/g) were significantly higher (p < 0.05) in the soil samples collected from the organic farms compared to the crop-livestock rotation farms (Figure ).
The APC, coliform, yeast, mould and S. aureus levels in soils from the different farms varied with value ranges of 0–7.12, 2.37–7.78, 1.78–6.39 and 0–5.58 log10 CFU/g but were not significantly different (p < 0.05). Since there has been only a very few studies on relating the pathogen contamination of soils to soil enrichment types in the world, further studies are required before any firm conclusions can be made.
Most of the water samples were free of microbes. Two water samples from the traditional farm were significantly (p < 0.05) contaminated with APC, coliform, E. coli and yeast with maximum values of 3.72, 4.34, 2.18, and 3.76 log10 CFU/g, respectively (not shown). It was suspected that the contamination may have been from the water hose and not from the source. The water collected from the compost farm and crop-livestock rotation farm was from a tap (without hoses attached) but in the traditional farm water was collected from a long hose. Since the water was used for washing the harvested vegetables before distribution, it is important to check the pathogen contamination arising from the hoses but probably rarely practiced.
3.3. Pathogen detection and confirmation in farm produce, soil and water
S. aureus usually exists in the mucosa of nasal passages and the skin of animals and humans. In most instances it is not harmful to human health because it is vulnerable to ‘hurdles’ (a combination of technologies used in the food industry to eliminate bacteria such as heat, acidity, water activity, preservatives) that are used to reduce the microbial load (Maistro, Miya, Sant’Ana, & Pereira, Citation2012). The existence of S. aureus in farm produce, soil or water indicates lack of proper hygienic measures. Halablab et al. (Citation2011) reported S. aureus levels in whole vegetables collected in Lebanon as 1.47–8.77 log10 CFU/g. According to PHLS (Citation2000), S. aureus contamination is graded as satisfactory (<2 log10 CFU/g), fairly satisfactory (2–3), unsatisfactory (3–4) and unacceptable (>4). There was a significant increase (p < 0.05) in the mean S. aureus levels in the organic farms compared to the traditional farm (Figure ). About 44% of the vegetable samples from the organic farms were in the unsatisfactory range for S. aureus contamination compared to 27% in the traditional farm based on PHLS (Citation2000) guidelines.
The main source of E. coli O157:H7 is from cattle (Himathongkham et al., Citation1999) and contamination of vegetables is not common. No E. coli O157:H7 was detected in 3,200 and 400 whole organic vegetables sampled in the UK (Sagoo et al., Citation2001), and in the USA (Johnston et al., Citation2005), and 44 lettuce samples taken from university restaurants in Spain (Soriano, Rico, Moltó, & Mañes, Citation2000). However, there is potential for contamination of vegetables by E. coli O157:H7 during application of manure, irrigation or from “contaminated surface runoff” (Ackers et al., Citation1998; Hilborn et al., Citation1999). E. coli O157:H7 can survive in manure for long periods depending on the environmental conditions (Kudva, Blanch, & Hovde, Citation1998; Wang, Zhao, & Doyle, Citation1996). Times taken to reduce E. coli O157:H7 numbers in cow faeces depend on a variety of conditions. It takes 105 days at 4°C or 45 days at 37°C to achieve a more than 5 log10 CFU/g reduction (Himathongkham et al., Citation1999). In contrast, it was reported that E. coli O157:H7 survived for 77 days at 5°C, 226 days at 15°C, 231 days at 21°C in manure-amended autoclaved soil (Jiang et al., Citation2002), 21 months at 23°C in air-devoid manure, and for 100 days at −200°C and at 4 or 10°C in bovine and ovine manure, respectively (Kudva et al., Citation1998). Similarly, according to Wang et al. (Citation1996) 3 log10 CFU/g of E. coli O157:H7 could survive for 42 days at 37°C, 63 days at 5°C, 49 days at 22°C, and 5 log10 CFU/g for 49 days at 37°C, 70 days at 5°C and 56 days at 22°C. E. coli O157:H7 levels in raw cow faeces is 2–5 log10 CFU/g (but lower inside than in the outer layers) and levels could potentially decline by 5 log10 CFU/g in 15 days at 4°C (Himathongkham et al., Citation1999). According to Buck, Walcott, and Beuchat (Citation2003) composting or aging is therefore important if manure is to be used as fertilizer and animals should not come in contact with fresh vegetable produce at any time during its production. According to FSANZ (Citation2001), E. coli O157:H7 should not be present in ready-to-eat foods. In the current study, though many E. coli O157:H7 suspected colonies were observed in the MacConkey agar with sorbitol, cefixime and tellurite plates (all 52 samples). For confirmation purpose, colonies from selected 25 fresh vegetable and soil samples from the 3 different farms were analysed using the 3M molecular detection system and all samples showed negative results for E. coli O157:H7 detection. This could be due to a limitation of the MacConkey agar with sorbitol, cefixime and tellurite plates (HAEDY Diagnostics, Citation1996). It has been reported that sometimes sorbitol negative colonies are produced underneath the sorbitol positive colonies (which are the majority of colonies) and therefore it is difficult to obtain an accurate estimation of the exact number of colonies (HAEDY Diagnostics, Citation1996). Hence, it is recommended to test the sample using biochemical and serological tests also. Furthermore, according to Lauri and Mariani (Citation2009), the 3M molecular detection system sometimes may give false positive results because of contamination from the environment and/or equipment, a PCR inhibitor in the sample, or the DNA in vegetable and soil samples because of its size (larger than bacteria). Since the results obtained were all negative, this limitation of the technique is not applicable to this study.
No Salmonella have either been detected or confirmed in the organic fresh produce in most previous studies. For example, no Salmonella spp. have been detected in any organic produce collected in Spain, Norway and the UK (Loncarevic et al., Citation2005; Oliveira et al., Citation2010; Sagoo et al., Citation2001), including 86 organic vegetable samples from retail shops and farms in the UK (McMahon & Wilson, Citation2001). Even when present, composting reduced the concentration from 7 log10 CFU/g S. enteritidis to zero within 48 h (Lung et al., Citation2001). According to van Diepeningen, de Vos, Korthals, and van Bruggen (Citation2006), soil in organic farms contain less nitrate and total soluble nitrogen and more non-pathogenic bacteria than in traditional farms. It is suggested that these differences affect the “resistance to colonization by microorganisms” (Gu et al., Citation2013). “Organic soil” also has a greater capacity to suppress Salmonella Typhimurium growth than soil in traditional farms (He et al., Citation2010), probably by bacteria acting synergistically to protect plants from pathogens (Mendes et al., Citation2011) because of microbial diversity (Wetzel, Lee, Lee, & Binkley, Citation2010) and a higher bacterial population (Gu et al., Citation2013) in the soil of organic than in traditional farming systems. In support is an inverse relationship between microbial diversity and S. Typhimurium population (Klerks, Franz, van Gent-Pelzer, Zijlstra, & van Bruggen, Citation2007). According to FSANZ (Citation2001) guidelines, no Salmonella spp. should be present in ready-to-eat foods. In the current study, even though Salmonella spp. were confirmed in all soil samples, only 11.4% of vegetable samples from all farms (2 from the traditional farm, 3 from the compost farm and 4 from the crop-livestock rotation farm) were contaminated. There were no significant differences (p < 0.05, results not shown) in S. Typhimurium levels between the three farms. According to Joseph and Carlos (Citation2012), the Reveal kit method which is a lateral flow immunoassay, sometimes gives false positive results because antibodies that are used for the detection may bind to denatured, captured antibodies which may be produced during the reaction. No further confirmatory analysis was conducted in this study.
3.4. Bacterial resistance to antibiotics
The bacterial isolates were classified as susceptible (≥20 mm; S), intermediate (15–19 mm; I) or resistant (≤14 mm; R) based on the size of the inhibition zone at 24 h. All Salmonella spp. positive vegetable samples showed resistance to at least 3 antibiotics and all Salmonella spp. positive soil samples were resistant to at least two antibiotics. Eighty-one percent of S. aureus positive vegetables and 60% of soil samples were resistant to at least 1 antibiotic (Figure ). All Salmonella spp. positive vegetable samples showed resistance to vancomycin, ampicillin and penicillin. All Salmonella spp. positive soil samples showed resistance to penicillin and more than 80% of samples showed resistance to ampicillin. Similarly, more than 80% of S. aureus positive vegetable samples showed resistance to penicillin. In the soil, 40% of the samples positive for S. aureus were resistant to chloramphenicol, erythromycin and penicillin (Figure ).
Figure 3. Vertical axis shows levels (%) of antimicrobial susceptibility for S. aureus and Salmonella spp. with selected antibiotics (ampicillin, tetracycline, gentamycin, erythromycin, trimethoprim–sulfamethoxazole, nalidixic acid, chloramphenicol, ciprofloxacin, penicillin, kanamycin, streptomycin and vancomycin). T, C and CL in the x-axis refer to the farm types, traditional, compost and crop-livestock rotation, respectively.
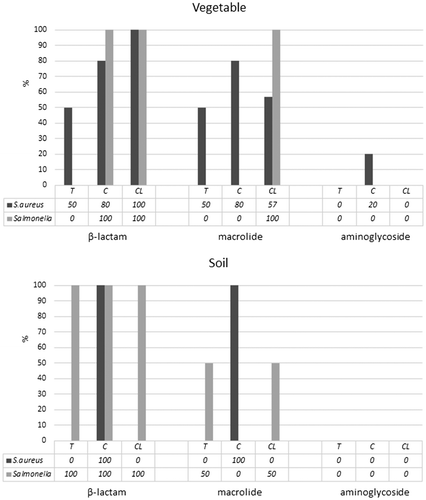
Bacterial resistance to antibiotics is a major concern worldwide. In the current study, all Salmonella spp. positive strains from vegetable samples and most of the soil samples showed resistance to vancomycin (aminoglycoside), erythromycin (macrolide), ampicillin and penicillin (β-lactam). The results for Salmonella positive strains were similar to the study by Yildirim, Gonulalan, Pamuk, and Ertas (Citation2011) who reported resistance of Salmonella spp. from raw chicken carcasses to penicillin, oxacillin, clindamycin, vancomycin, erythromycin and ampicillin. Similar resistance to antibiotics has been reported for Salmonella spp. from retail chicken and beef to nalidixic acid, tetracycline, trimethoprim and streptomycin (Dallal et al., Citation2010). In this study, all Salmonella spp. positive strains from vegetables samples in three different farms did not show resistance to nalidixic acid nor tetracycline but all strains from the crop-livestock rotation farm showed resistance to streptomycin. In addition, 50% of Salmonella spp. positive strains from soil samples in the traditional farm showed resistance to nalidixic acid, and that in the crop-livestock rotation farm, were resistant to tetracycline. S. aureus positive strain from vegetable samples and soil samples were not resistant to ampicillin or vancomycin but S. aureus positive strains from vegetables were resistant to penicillin. This is in agreement with Gündoğan, Citak, and Turan (Citation2006) who reported resistance of S. aureus to β-lactams such as penicillin and ampicillin. Further studies are required to fully understand the extent of antibiotic resistance of bacteria in fresh farm produce and there is an urgent need to develop strategies to prevent this because bacterial resistance to antibiotics is widespread and spreading fast and this is a major human food safety issue.
3.5. Limitations
Our study enables us to compare the microbiological risks of fresh produce grown in three different farming systems. However, there were some limitations faced when work was being conducted.
We aimed to collect samples of similar fresh produce from each farm weekly; however, it was not practically possible due to harvesting variations and availability of some produce on the farms. Therefore, comparison of the results for each fresh produce sample between farms was not possible to be made. We made efforts to conduct statistical analysis where enough data was available to do so. Results of all samples tested in this study are reported here to provide an overall snapshot of the microbiological safety of fresh produce samples. A total of 79 samples were tested due to short season of the fresh produce availability and time constraints to complete this project. Despite these limitations, this is an informative preliminary report on the microbiological safety of fresh produce grown in Canterbury, New Zealand.
4. Conclusion
In conclusion, based on the FSANZ (Citation2001) or PHLS (Citation2000) guidelines for ready-to-eat foods, the microbiological status of fresh produce from different farms in Canterbury, New Zealand was acceptable for E. coli O157:H7 but not for APC, E. coli, S. aureus or Salmonella spp. The yeast and mould levels were similar to studies previously reported for samples purchased in retail shops. S. aureus and Salmonella spp. showed higher resistance to some antibiotics such as vancomycin, erythromycin, ampicillin and penicillin.
There were significant (p < 0.05) increases in the levels of APC, coliform, E. coli, yeast and mould and S. aureus in the vegetable samples collected from the organic farms compared to the traditional farm. The fresh produce collected from the organic farms was more contaminated with a wider range of pathogens than those from the traditional farm. Resistance of some bacteria to selected antibiotics in the farming environments is a concern. Although organically farmed produce appears attractive because of its pesticide-free status, public health may be at risk due to a higher microbial load. Future studies should be aimed to develop strategies to minimise microbial contamination of organic produce in Canterbury, New Zealand and address bacterial resistance to antibiotics in the farming environments.
Funding
This research was provided by the Faculty of Agriculture and Life Science, Lincoln University New Zealand.
Acknowledgments
We thank Mr Omega Amoafo for his helpful contribution.
Additional information
Notes on contributors
Malik Altaf Hussain
Our group has been examining the food industry supply chain and applying recent scientific advancements to identify the role microbes and mycotoxins play in food spoilage and food safety and security. Dr Malik Altaf Hussain is a microbiologist, Dr Ravi Gooneratne is a toxicologist and Ms Yukiko Wadamaori is a food scientist. Our focus has been on the potential sources of microbiological contamination in food supply chain. Our research group has international reputation and expertise in food science, safety and security, novel methodologies in the monitoring of food and environmental contaminants, and how microbes and contaminants impinge on food quality and safety. We have conducted several technical workshops and short courses both in New Zealand and China on food safety and security in the past 3 years.
References
- Abadias, M., Usall, J., Anguera, M., Solsona, C., & Viñas, I. (2008). Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. International Journal of Food Microbiology, 123, 121–129.10.1016/j.ijfoodmicro.2007.12.013
- Ackers, M.-L., Mahon, B. E., Leahy, E., Goode, B., Damrow, T., Hayes, P. S., … Griffin, P. M. (1998). An outbreak of Escherichia coli O157:H7 infections associated with leaf lettuce consumption. The Journal of Infectious Diseases, 177, 1588–1593.10.1086/jid.1998.177.issue-6
- Anwar, M., Patra, D. D., Chand, S., Alpesh, K., Naqvi, A. A., & Khanuja, S. P. S. (2005). Effect of organic manures and inorganic fertilizer on growth, herb and oil yield, nutrient accumulation, and oil quality of French Basil. Communications in Soil Science and Plant Analysis, 36, 1737–1746.10.1081/CSS-200062434
- Aycicek, H., Oguz, U., & Karci, K. (2006). Determination of total aerobic and indicator bacteria on some raw eaten vegetables from wholesalers in Ankara, Turkey. International Journal of Hygiene and Environmental Health, 209, 197–201.10.1016/j.ijheh.2005.07.006
- Badosa, E., Trias, R., Parés, D., Pla, M., & Montesinos, E. (2008). Microbiological quality of fresh fruit and vegetable products in Catalonia (Spain) using normalised plate-counting methods and real time polymerase chain reaction (QPCR). Journal of the Science of Food and Agriculture, 88, 605–611.10.1002/(ISSN)1097-0010
- British Columbia Centre for Disease Control (BCCDC). (2011). Food Quality Check Program. Vancouver: Author.
- Buck, J. W., Walcott, R. R., & Beuchat, L. R. (2003). Recent trends in microbiological safety of fruits and vegetables. Plant Health Progress. doi:10.1094/PHP-2003-0121-01-RV
- Chapman, P. A., Siddons, C. A. J. M., Manning, J. (1997). An outbreak of infection due to verocytotoxin-producing Escherichia coli O157 in four families: the influence of laboratory methods on the outcome of the investigation. Epidemiology and Infection, 119, 113–119.10.1017/S0950268897007991
- Clinical and Laboratory Standards Institute. (2012). Performance standards for antimicrobial disk susceptibility tests; approved standard (11th ed.). Wayne, PA: Author.
- Corato, U. D. (2012). Fungal population dynamics in ready-to-eat salads during a shelf-life in Italy. Journal of Agricultural Science and Technology, A2, 569–576.
- Dallal, M. M. S., Doyle, M. P., Rezadehbashi, M., Dabiri, H., Sanaei, M., Modarresi, S., … Sharifi-Yazdi, M. K. (2010). Prevalence and antimicrobial resistance profiles of Salmonella serotypes, Campylobacter and Yersinia spp. isolated from retail chicken and beef, Tehran, Iran. Food Control, 21, 388–392.10.1016/j.foodcont.2009.06.001
- Erkan, M. E., & Vural, A. (2008). Investigation of microbial quality of some leafy green vegetables. Journal of Food Science and Technology, 6, 285–288.
- European Union. (2013). Facts and figures on organic agriculture in the European Union. Brussels: Agriculture and Rural Development.
- Fang, M., Wong, J. W. C., Li, G. X., & Wong, M. H. (1998). Changes in biological parameters during co-composting of sewage sludge and coal ash residues. Bioresource Technology, 64, 55–61.10.1016/S0960-8524(97)00156-9
- FiBL and IFOAM. (2013). The world of organic agriculture statistics and emerging trends 2013. Frick: Author.
- Food and Agriculture Organization of the United Nations [FAO]. (1998). Evaluating the potential contribution of organic agriculture to sustainability goals. FAO's technical contribution to IFOAM's Scientific Conference (16–19 November), Mar del Plata.
- Food Standards Australia New Zealand (FSANZ). (2001). Guidelines for microbiological examination of ready-to-eat foods. Canberra: Author.
- Ge, C., Lee, C., & Lee, J. (2013). Localization of viable Salmonella typhimurium internalized through the surface of green onion during preharvest. Journal of Food Protection, 76, 568–574.10.4315/0362-028X.JFP-12-374
- Gu, G., Cevallos-Cevallos, J. M., Vallad, G. E., & van Bruggen, A. H. (2013). Organically managed soils reduce internal colonization of tomato plants by Salmonella enterica Serovar Typhimurium. Phytopathology, 103, 381–388.10.1094/PHYTO-04-12-0072-FI
- Gündoğan, N., Citak, S., & Turan, E. (2006). Slime production, DNase activity and antibiotic resistance of Staphylococcus aureus isolated from raw milk, pasteurised milk and ice cream samples. Food Control, 17, 389–392.
- HAEDY Diagnostics. MacConkey agar with sorbitol, cefixime and tellurite [Internet]. Retrieved from https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/CT-SMAC.htm
- Halablab, H. A., Sheet, I. H., & Holail, H. M. (2011). Microbiological quality of raw vegetables grown in bekaa valley, Lebanon. American Journal of Food Technology, 6, 129–139.
- He, M., Ma, W., Tian, G., Blok, W., Khodzaeva, A., Zelenev, V. V., Semenov, A. M., & van Bruggen, A. H. (2010). Daily changes of infections by Pythium ultimum after a nutrient impulse in organic versus conventional soils. Phytopathology, 100, 593–600.10.1094/PHYTO-100-6-0593
- Hilborn, E. D., Mermin, J. H., Mshar, P. A., Hadler, J. L., Voetsch, A., … Glynn, M. K. (1999). A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Archives of Internal Medicine, 159, 1758–1764.10.1001/archinte.159.15.1758
- Hilimire, K. E. (2011). Pastured poultry/crop systems and their effect on soil and plant quality, food safety, and farmer experience (p. 116). Santa Cruz: University of California.
- Himathongkham, S., Bahari, S., Riemann, H., & Cliver, D. (1999). Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiology Letters, 178, 251–257.10.1111/fml.1999.178.issue-2
- Jiang, X., Morgan, J., & Doyle, M. P. (2002). Fate of Escherichia coli O157:H7 in manure-amended soil. Applied and Environmental Microbiology, 68, 2605–2609.10.1128/AEM.68.5.2605-2609.2002
- Johannessen, G. S., Froseth, R. B., Solemdal, L., Jarp, J., & Wasteson, Y. L. M. R. (2004). Influence of bovine manure as fertilizer on the bacteriological quality of organic Iceberg lettuce. Journal of Applied Microbiology, 96, 787–794.10.1111/jam.2004.96.issue-4
- Johnston, L. M., Jaykus, L. A., Moll, D., Martinez, M. C., Anciso, J., Mora, B., & Moe, C. L. (2005). A field study of the microbiological quality of fresh produce. International Journal of Food Microbiology, 68, 1840–1847.
- Joseph, A. O., & Carlos, G. L.-V. (2012). Salmonella detection methods for food and food ingredients. In S. M. M. Barakat (Ed.). Salmonella - A Dangerous Foodborne Pathogen (pp. 373–392). Guelph: InTech.
- Klerks, M. M., Franz, E., van Gent-Pelzer, M., Zijlstra, C., & van Bruggen, A. H. C. (2007). Differential interaction of Salmonella enterica serovars with lettuce cultivars and plant-microbe factors influencing the colonization efficiency. The ISME Journal, 1, 620–631.10.1038/ismej.2007.82
- Kovacs, M. (2004). Nutritional health aspects of mycotoxins. Orvosi Hetilap, 145, 1739–1746.
- Kudva, I. T., Blanch, K., & Hovde, C. J. (1998). Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Applied and Environmental Microbiology, 64, 3166–3174.
- Lauri, A., & Mariani, P. O. (2009). Potentials and limitations of molecular diagnostic methods in food safety. Genes & Nutrition, 4(1), 1–12.10.1007/s12263-008-0106-1
- Loncarevic, S., Johannessen, G. S., & Rorvik, L. M. (2005). Bacteriological quality of organically grown leaf lettuce in Norway. Letters in Applied Microbiology, 41, 186–189.10.1111/lam.2005.41.issue-2
- Lung, A. J., Lin, C. M., Kim, J. M., Marshall, M. R., Nordstedt, R., Thompson, N. P., & Wei, C. I. (2001). Destruction of Escherichia coli O157:H7 and Salmonella enteritidis in cow manure composting. Journal of Food Protection, 64, 1309–1314.10.4315/0362-028X-64.9.1309
- Maffei, D. F., Silveira, N. F. A., & Catanozi, M. P. L. M. (2013). Microbiological quality of organic and conventional vegetables sold in Brazil. Food Control, 29, 226–230.10.1016/j.foodcont.2012.06.013
- Maistro, L. C., Miya, N. T. N., Sant’Ana, A. S., & Pereira, J. L. (2012). Microbiological quality and safety of minimally processed vegetables marketed in Campinas, SP – Brazil, as assessed by traditional and alternative methods. Food Control, 28, 258–264.10.1016/j.foodcont.2012.05.021
- McMahon, M. A. S., & Wilson, I. G. (2001). The occurrence of enteric pathogens and Aeromonas species in organic vegetables. International Journal of Food Microbiology, 70, 155–162.10.1016/S0168-1605(01)00535-9
- Mendes, R., Kruijt, M., de Bruijn, I., Dekkers, E., van der Voort, M., Schneider, J. H., … Raaijmakers, J. M. (2011). Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science, 332, 1097–1100.10.1126/science.1203980
- Mohammad, B. H. N., & Bahreini, M. (2012). Microbiological quality of mixed fresh-cut vegetable salads and mixed ready- to-eat fresh herbs in Mashhad, Iran. In 2012 International Conference on Nutrition and Food Sciences. Singapore: IACSIT Press.
- Neogen. (2011). Mueller Hinton Agar (7101). Lansing, MI: Author.
- Nguz, K., Shindano, J., Samapundo, S., & Huyghebaert, A. (2005). Microbiological evaluation of fresh-cut organic vegetables produced in Zambia. Food Control, 16, 623–628.10.1016/j.foodcont.2004.07.001
- Norwegian Food Safety Authority. (2000). Microbiological Guidelines. Retrieved from http://www.mattilsynet.no
- Oliveira, M., Usall, J., Viñas, I., Anguera, M., Gatius, F., & Abadias, M. (2010). Microbiological quality of fresh lettuce from organic and conventional production. Food Microbiology, 27, 679–684.10.1016/j.fm.2010.03.008
- Pell, A. N. (1997). Manure and microbes: Public and animal health problem? Journal of Dairy Science, 80, 2673–2681.10.3168/jds.S0022-0302(97)76227-1
- Public Health Laboratory Service (PHLS). (2000). Microbiological guidelines for some ready-to-eat foods sampled at the point of sale. Communicable Disease and Public Health, 3, 163–167.
- Ragaert, P., Devlieghere, F., Devuyst, E., Dewulf, J., Van Langenhove, H., & Debevere, J. (2006). Volatile metabolite production of spoilage micro-organisms on a mixed-lettuce agar during storage at 7°C in air and low oxygen atmosphere. International Journal of Food Microbiology, 112, 162–170.10.1016/j.ijfoodmicro.2006.06.018
- Raviv, M., Chen, Y., & Inbar, Y. (1986). Peat and peat substitutes as growth media for container-grown plants. In Y. Chen & Y. Avnimelech (Eds.), The Role of Organic Matter in Modern Agriculture (pp. 257–287). Springer Netherlands.10.1007/978-94-009-4426-8
- Sagoo, S. K., Little, C. L., & Mitchell, R. T. (2001). The microbiological examination of ready-to-eat organic vegetables from retail establishments in the United Kingdom. Letters in Applied Microbiology, 33, 434–439.10.1046/j.1472-765X.2001.01026.x
- Seow, J., Ágoston, R., Phua, L., & Yuk, H.-G. (2012). Microbiological quality of fresh vegetables and fruits sold in Singapore. Food Control, 25, 39–44.10.1016/j.foodcont.2011.10.017
- Sirsat, S., & Neal, J. (2013). Microbial profile of soil-free versus in-soil grown lettuce and intervention methodologies to combat pathogen surrogates and spoilage microorganisms on lettuce. Foods, 2, 488–498.10.3390/foods2040488
- Solomon, E. B., Yaron, S., & Matthews, K. R. (2002). Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Applied and Environmental Microbiology, 68, 397–400.10.1128/AEM.68.1.397-400.2002
- Soriano, J. M., Rico, H., Moltó, J. C., & Mañes, J. (2000). Assessment of the microbiological quality and wash treatments of lettuce served in University restaurants. International Journal of Food Microbiology, 58, 123–128.10.1016/S0168-1605(00)00288-9
- Suarez-Estrella, F., Vargas-Garcia, M. C., Elorrieta, M. A., Lopez, M. J., & Moreno, J. (2003). Temperature effect on Fusarium oxysporum f. sp. melonis survival during horticultural waste composting. Journal of Applied Microbiology, 94, 475–482.10.1046/j.1365-2672.2003.01854.x
- Tournas, V. H. (2005). Moulds and yeasts in fresh and minimally processed vegetables, and sprouts. Journal of Applied Microbiology, 99, 71–77.
- Trevor, S. (2011). Risk assessment of Salmonella preharvest internalization in relation to irrigation water quality standards for melons and other cucurbits. Woodland, CA: Center for Produce Safety.
- van Diepeningen, A. D., de Vos, O. J., Korthals, G. W., & van Bruggen, A. H. C. (2006). Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. Applied Soil Ecology, 31, 120–135.10.1016/j.apsoil.2005.03.003
- Vilrla, L., Macedo, M. C. M., Júnior, G. B. M., & Kluthcouski, J. (2003). Crop-livestock integration benefits. In J. Kluthcouski, L. F. Stone, & H. Aidar (Eds.), Integração lavoura-pecuária. Santo Antônio de Goiás: Embrapa Arroz e Feijão. Retrieved from http://www.fao.org/ag/AGP/AGPC/doc/integration/papers/integration_benefits.htm
- Wang, G., Zhao, T., & Doyle, M. P. (1996). Fate of Enterohemorrhagic Escherichia coli O157:H7 in Bovine Feces. Applied and Environmental Microbiology, 62, 2567–2570.
- Wetzel, K., Lee, J., Lee, C. S., & Binkley, M. (2010). Comparison of microbial diversity of edible flowers and basil grown with organic versus conventional methods. Canadian Journal of Microbiology, 56, 943–951.10.1139/W10-082
- Yildirim, Y., Gonulalan, Z., Pamuk, S., & Ertas, N. (2011). Incidence and antibiotic resistance of Salmonella spp. on raw chicken carcasses. Food Research International, 44, 725–728.10.1016/j.foodres.2010.12.040
