Abstract
The giant African snail, Lissachatina fulica, is a known agricultural pest with some public health concern; it was introduced in Trinidad in 2008. The purpose of this study is to highlight results obtained from the evaluation of the attractiveness of L. fulica to selected food baits in a bucket trap set up. Selected food baits (banana, breadfruit, cabbage and papaya) were evaluated for their use as a potential attractant for L. fulica in two different locations in Orange Grove, Trinidad. All food baits used in the traps showed some level of attraction. Data suggests that small and medium sized snails appeared to be associated with a more significant contribution to total snail counts than large snails for the food baited traps used in the study. Being an agricultural and public health pest, the management of giant African snail is a serious concern at the farm and national levels. Thus it is important that various management strategies are explored whether intended to complement current baiting strategies or to assist in the monitoring of populations in infested areas over time.
Public Interest Statement
The giant African snail was introduced in Trinidad in 2008. It has a voracious appetite and has a strong liking for most agricultural crops. Additionally, the giant African snail is of significant public health concern since it is known to be a vector of the rat lungworm which is linked to meningitis. In this study, an attempt was made to assess options using locally sourced crop material and buckets to capture and kill the giant African snail. The results obtained show promise for use as a complementary activity to the application of snail baits in the field and also for the purpose of in-field monitoring for the presence of the snail. Additionally, the trap can be easily adopted by the average small farmer and homeowner in an attempt to manage and monitor the giant African snail.
Competing interests
The authors declare no competing interest.
1. Introduction
Over the last 20 years, Lissachatina fulica (Bowdich) (also known as Achatina fulica) has become well established in the new world (Ciomperlik et al., Citation2013). The earliest record of the giant African snail in the Caribbean region is 1984 in Guadeloupe (Pollard, Fields, & Taylor, Citation2008). Since then, L. fulica has been reported in Anguilla (Connor, Citation2006), Antigua, Dominica, Martinique, St Martin (Pollard et al., Citation2008), St. Lucia, Barbados (Ciomperlik et al., Citation2013) and, Trinidad (IPPC, Citation2009). L. fulica has not been reported in Tobago. Ciomperlik et al. (Citation2013) suggests that the spread of L. fulica in many areas globally are primarily as result of intentional introductions and accidental hitch hiking.
L. fulica is polyphagous and has been recorded on numerous different plant species; it seems to have a preference for papaya, cassava, breadfruit, cocoa, papaya, peanut, rubber and most species of legumes and cucurbits (Mead, Citation1961). According to Tomiyama and Nakane (Citation1993), L. fulica is primarily active during the night time; however, under wet conditions, some activity may be observed during the day. Not only is L. fulica an agricultural pest in areas where it is found, but it is also a pest of public health concern due to it being a known intermediate host for rat lungworm, Agiostrongylus cantonensis, a parasitic nematode linked to eosinophilic meningitis in humans in the Pacific Basin (Kliks & Palumbo, Citation1992) and Brazil (Thiengo et al., Citation2010). The purpose of this paper is to highlight results obtained from evaluation of the attractiveness of L. fulica to selected food baits in a bucket trap set up.
2. Methodology
The experiment was conducted in Orange Grove, Trinidad. Bucket traps were arranged in a complete random design in two different locations where the L. fulica population was previously observed. The two locations (Location A and B) were 150 metres apart. Bucket traps were placed on the ground in shaded areas within each location and arranged in a “W” pattern with each bucket trap at 5 metres apart. The predominant crop in both locations was coconut; however, location B was next to a small tributary. In location B, the furthest line of traps was located 6 metres away while the nearest line of traps was located 5 metres from the small tributary. Each treatment was replicated 4 times and randomly placed within each of the 2 locations.
Forty (40) bucket traps were constructed using dark green, covered plastic buckets with the following dimensions: 10 inches (25.4 cm) cm diameter and 11.5 inches (27.94 cm) cm height. Windows 6 inches (15.24 cm) wide by 3 inches (7.62 cm) high were cut out on either sides of the bucket at a height of 1.5 inches (3.81 cm) from the top of the bucket.
Each trap consisted of 1 litre total amount of liquid solution mixed in a ratio of 10 grams copper sulphate (99%) (CuSo4) to 1 litre of water. Food baits were added to treatments 1–4 while treatment 5 was the negative control which consisted only of a 1 litre total solution of 10 grams copper sulphate (99%) (CuSo4): 1 litre of water. In a pre-test done to observe the effects on snails immersed in 10 g/L copper sulphate solution, snails collected from the field and placed in the copper sulphate solution remained inactive and were tightly retracted in the shells while in submersion for the 24 h period under observation. Therefore, in this study, the copper sulphate and water solution was selected for the bucket traps in an attempt to immobilize snails after they entered the traps.
Bait treatments in each bucket trap consisted of one of the following:
| • | Treatment 1: 150 grams fruit portion of ripe banana, Musa accuminata | ||||
| • | Treatment 2: 150 grams fruit portion of breadfruit, Artocarpus altilis | ||||
| • | Treatment 3: 150 grams of cabbage, Brassica oleracea | ||||
| • | Treatment 4: 150 grams fruit portion of papaya, Carica papaya | ||||
| • | Treatment 5 (negative control): water and copper sulphate solution only | ||||
The traps were placed in the field on 14 February 2017 and the monitoring period extended from February 17 to 3 April 2017 where the number of snails in each trap was recorded as the weather permitted. For counts, snails were categorized as small, medium and large based on the following categories: shell length of <20 mm = small (neonates); shell length of 20–45 mm = medium (juveniles); shell length of >45 mm = large (adults) (Ciomperlik et al., Citation2013). The number of snails in each trap was recorded 18 times across the monitoring period between the hours of 5:30 and 7:00 am (See supplement table of means for the dates that data was collected). Treatment baits were changed twice on 23 February 2017 and 20 March 2017 during the monitoring period.
Data were analyzed using Genstat through the use of Analysis of Variance (ANOVA) for a repeated measurement. Significance in the main effects of treatment and location as well as the interaction of treatment and location was determined at the 5% level of significance. The effect of time was investigated for significance at the 5% level as well as the interaction of time with the above main and interaction effects.
3. Results and discussion
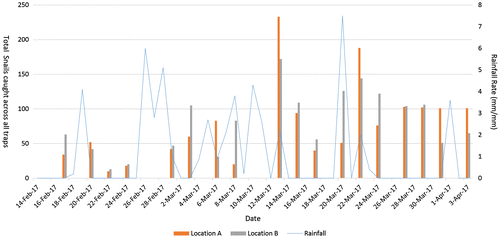
During the study period, minimum temperature was 19.6°C and maximum temperature was 34.9°C. Minimum daily rainfall rate over the period was 0 mm/mm which occurred 30 out of the 49 day study period while the maximum rainfall rate was 7.5 mm/mm (See Figure ).
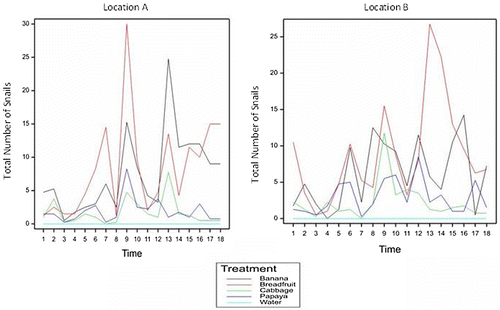
Figure 1. Total number of snails caught in the baited traps over the period.
All food baits used in the experiment showed some level of attraction to L. fulica except for the negative control (treatment 5). During the study period, L. fulica were not observed to be attracted to treatment 5 (negative control) which suggests that L. fulica found in food baited traps were attracted to the bucket traps due to the presence of one of the food bait evaluated during the monitoring period. Snails collected from the baited bucket traps were dead in all instances and exuded yellow mucus. This mucus secretion associated with CuSo4 was also reported by Vanitha, Karuppuchamy, and Sivasubramanian (Citation2013) who evaluated CuSo4 as one of the substance tested as a barrier to the movement of L. fulica onto vanilla plants.
Generally, the treatment effect was found to be significant at the 5% level (p-value 0.001) for small, medium, large and total number of snails (See Appendix A). Data also suggests that the number of L. fulica found in the bucket traps varied based on the food bait found in the traps (See Figure ). Overall, traps baited with breadfruit were associated with higher snail counts followed by traps baited with banana, papaya and cabbage respectively. Mean number of total snails caught per trap count over the duration of the experiment from highest to lowest were as follows: breadfruit (8.63 ± 0.693); banana (6.88 ± 0.693); papaya (2.44 ± 0.693) and cabbage (1.95 ± 0.693) (See Table and Appendix A). By comparing the differences in means to the Least Significant Difference at 5% level (See Table ), it can be assumed that both the banana and breadfruit baits performed similarly in terms of their high level of attraction to small, medium and large L. fulica when compared to the other food baits used in the study.
Table 1. Mean number of snails caught in each trap
Overall, a total of 2,865 snails were captured (See Appendix B) across all the traps over the duration of the experiment (49 days). Results from the study also suggests that over time, with the exception of the large (adult) snails, there was some significance shown in the number of small (neonates) (p-value 0.003), medium (juveniles) (p-value 0.001), and total number of snails (p-value 0.001), caught in the bucket traps (See Table and Appendix A). It was assumed that the snail population would always come towards the bait therefore a decrease in the snail population was not investigated.
Table 2. Mean number of snails caught in each trap per collection
Overtime, there also appeared to be significant interaction between treatment and time for small (p-value 0.036), medium (p-value 0.002) and total (p-value 0.001) number of snails caught but not for the number of large (p-value 0.065) snails caught (See Appendix A).
Location was significant at the 5% level for large snails (p-value 0.028) only (See Appendix A and Table ). Mean trap counts associated with adult snails over the duration of the study were as follows: Location B, located next to a small tributary (0.489 ± 0.0597) and location A (0.294 ± 0.0597). This observation suggests that the start of L. fulica infestation in location B may have been at an earlier time than the start of infestation in location A.
Table 3. Mean number of snails caught at each location
Generally, food baited bucket traps appeared to attract more neonates (small) and juvenile snails (medium) than adults (large) in both locations (See Table ). Mean trap counts over the duration of the study, associated with small and medium snails respectively were as follows: location B (1.59 ± 0.196; 1.97 ± 0.258) and location A (1.88 ± 0.196; 1.74 ± 0.258) respectively. No significant differences were observed for trap counts of small (neonates) (p-value 0.300) and medium (juvenile) (p-value 0.528) snails based on amount caught per location thus implying that these snails were generally equally distributed across both locations (See Appendix A).
Additionally, there appeared to be no significant interaction between treatment and location for small (p-value 0.257), medium (p-value 0.458), large (p-value 0.758) and total (p-value 0.798) number of snails caught (See Appendix A). However, the means of the interaction of treatment and location for total number of snails caught was found to be significant at the 5% level for each level of time (p-value 0.004) (See Appendix A). This implies that collectively, the total snail population was changing across time at each location for each individual treatment. Further analysis done to determine whether small (p-value 0.068), medium (p-value 0.082) and large (p-value 0.334) snails contributed to the change observed in the total number of snails caught showed that the means for each were similar. Therefore, the sizes of the snail did not seem to influence the change in means of the total number of snails caught over time.
4. Conclusion
Being an agricultural and public health pest, the management of giant African snail is a serious concern at the farm and national levels. Thus it is important that various management strategies are explored whether intended to complement current baiting strategies or to assist in the monitoring of populations in infested areas over time. The bucket trap setup evaluated in this experiment showed potential for use on a wider scale by small farmers and homeowners who may use the system to compliment active baiting programs for the snail. The system may also be used to monitor for the presence of L. fulica in new areas. The food baits used are readily available locally and can be adopted by the average small farmer and or homeowner.
Further studies will be needed to evaluate suitable placement of the bucket traps regarding distance range for attraction to L. fulica. This study did not evaluate the distances travelled by L. fulica caught in the baited traps.
Funding
The authors received no direct funding for this research.
Acknowledgement
The authors would like to thank Mr Nazir Ali and Mr Lawrence Mahabir for assistance in data collection and trap maintenance as well as the farmers for allowing access to the sites for the evaluation. The authors would also like to thank Mr Nazir Ali for allowing us to use the cover image.
Additional information
Notes on contributors
Annika T. Minott
Annika T. Minott is a scientist at the Caribbean Agricultural Research and Development Institute (CARDI). Research/work interests relate to crop science and pest management. Minott obtained her Doctor of Plant Medicine (DPM) degree at the University of Florida (UF), USA.
Videsh S. Jagroo
Videsh S. Jagroo is a biometrician at the Caribbean Agricultural Research and Development Institute (CARDI). Research/work areas relate to statistical inputs on experimental designs and data analyses. Jagroo obtained his MSc in Statistics at the University of the West Indies, St. Augustine, Trinidad and Tobago.
Marcus Ramdwar
Marcus Ramdwar is an assistant professor at the Eastern Caribbean Institute of Agriculture and Forestry, within the Biosciences Agriculture & Food Technology Unit of the University of Trinidad and Tobago (UTT). His current research interests are within the areas of crop science, food security, agricultural education and agricultural extension.
References
- Ciomperlik, M. A., Robinson, D. G., Gibbs, I. H., Fields, A., Stevens, T., & Taylor, B. M. (2013). Mortality to the giant african snail, Lissachatina fulica (Gastropoda: Achatinidae), and non-target snails using select molluscicides. Florida Entomologist, 96(2), 370–379.10.1653/024.096.0257
- Connor, R. (2006). Distribution, habitat association, species abundance and perceptions of residents towards Achatina fulica in Anguilla (MSc thesis, pp. 41), University of Exeter, Devon, UK.
- IPPC. (2009). Giant african snail, Achatina fulica bowdich ( IPPC Official Pest Report, No. TTO03/2). Rome: FAO. Retrieved from https://www.ippc.int/en/countries/trinidad and https://www.ippc.int/en/countries/trinidad-and-tobago/pestreports/2009/01/giant-african-snail-achatina-fulica-bowdich/tobago/pestreports/2009/01/giant-african-snail-achatina-fulica-bowdich/
- Kliks, M. M., & Palumbo, N. E. (1992). Eosinophilic meningitis beyond the Pacific Basin: The global dispersal of a peridomestic zoonosis caused by angiostrongylus cantonensis, the nematode lungworm of rats. Social Science & Medicine, 34(2), 199–212.10.1016/0277-9536(92)90097-A
- Mead, A. R. (1961). The giant African snail: A problem in economic malacology. Chicago: University of Chicago Press.
- Pollard, G. V., Fields, A., & Taylor, B. (2008). Giant African snail in the Caribbean sub-region. Proceedings of the Caribbean Food Crops Society, 44, 126–134.
- Thiengo, S. C., Maldonado, A., Mota, E. M., Torres, E. J. L., Caldeira, R., Carvalho, O. D. S., … Lanfredi, R. M. (2010). The giant African snail Achatina fulica as natural intermediate host of Angiostrongylus cantonensis in Pernambuco, northeast Brazil. Acta Tropica, 115(3), 194–199.10.1016/j.actatropica.2010.01.005
- Tomiyama, K., & Nakane, M. (1993). Dispersal patterns of the giant african snail, Achatina fulica (ferussac) (stylommatophora: Achatinidae), equipped with a radiotransmitter. Journal of Molluscan Studies, 59(3), 315–322.10.1093/mollus/59.3.315
- Vanitha, K., Karuppuchamy, P., & Sivasubramanian, P. (2013). Feeding preference of Achatina fulica attacking vanilla and its management through barrier substances. Pest Management in Horticultural Ecosystems, 17(1), 38–41.
Appendix A
Table A1. Average small and medium sized snails caught in each trap at two locations in Orange Grove, Trinidad
**Significance at 5% level
Table A2. Average large sized and overall total number of snails caught in each trap at two locations in Orange Grove, Trinidad
**Significance at 5% level