Abstract
The effects of different priming techniques were evaluated to improve the dormancy and germination of wild seeds of “Piquín” chili pepper (Capsicum annuum var. glabriusculum). Three experiments were designed for pre-sowing treatment of seeds: (a) chemical seeds digestion; (b) halopriming (with K+ or NH4+ of NO3−, SO42- or Cl−) at different priming times (24, 48 or 72 h) and osmotic potential (−5, −10 or −15 atm) and (c) previously selected halopriming (KNO3 and NH4NO3) + Gibberellic acid (GA3, at 100 or 200 mg·L−1) were tested. Digestion treatments did show any effect on seed germination. Recommended values of osmotic potential (Ψs), to improve Piquín chili seed germination, must be between −10 and −15 atm (−1.0 and −1.5 MPa) and the priming time must be between 48 and 72 h. Priming techniques can considerably reduce Capsaicinoids content on seeds, improve dormancy, seed germination performance, and increase the rate and uniformity of seedling establishment. KNO3 and secondly GA3 treatments may improve rapid and uniform germination and seedling emergence. The results provide basic information to develop guidelines for commercial establishment of Piquín pepper crops.
PUBLIC INTEREST STATEMENT
Piquín chili pepper has an appreciable culinary tradition in Mexico and the United States. Currently, wild populations of this specie have decreased significantly due to anthropogenic pressure and inadequate management of natural resources. To reduce this pressure, it is necessary to have techniques that allow to regeneration of wild populations and to improved cultivar’s crop. The first problem to solve is the improvement of its low germination and to obtain seeds that acclimatize to both situations. Priming is recognized a technique that improves germination. The work aims to improve wild seeds germinability and reduce seedlings mortality. Priming with osmotically active fertilizer and gibberellic acid solutions has been effective for these purposes. Future research will aim, to maintain biodiversity, by adapting the technique to wild seeds for high risk ecotypes regeneration, and on the other hand to reduce the pressure on wild populations, selecting cultivars with greater domestication possibilities and the study of the proper crop’s conditions.
1. Introduction
Chili “Piquín” or “chiltepín”, [Capsicum annuum var. glabriusculum (Dunal) Heiser & Pickersgill); syn. C. annuum var. aviculare (Dierb.) D’Arcy & Eshbaugh)], is distributed from Colombia, Central America, and Mexico to the southwestern United States. The natural populations of “chiltepín” are considered an important genetic resource for pepper crop improvement (Hayano, Gamez, & Medina, Citation2016). This species is of great significance in the culture and identity of indigenous peoples of Mexico who usually harvested its fruits of wild plants (Guerrero Velázquez, Citation2015). The heat of chili pepper is due to the accumulation of capsaicinoids, a group of related alkaloids unique to Capsicum. Capsaicinoids are produced in the fruit placenta and transferred to the seeds during fruit maturation (Barchenger & Bosland, Citation2016). In highland regions where it occurs, is an important part of the local economy, especially in the time of harvest, generating employment and income for rural communities. This activity might threaten the genetic diversity in this species, affecting habitat degradation of natural populations of wild pepper. This problem could be solved by limiting the collection of wild populations and increasing their cultivation as a crop, in turn generating economic resources derived from this activity (Kim, Citation2016). While there is basic information that allows for developing guidelines for its cultivation, more research, related to germination, stand establishment and crop development and productivity, is necessary to develop commercial Piquín pepper crops.
Domestication of Piquín pepper plants have not been fully developed because problems are encountered related to low and erratic seed germination, morphologic and genetic variability, and limited environmental physiology information (Rodriguez-Uribe, Hernandez, Kilcrease, Walker, & O’Connell, Citation2014). Some authors suggest that germination of its seeds is restricted by physiological dormancy and is achieved after passing through the digestive tract of certain birds. Seeds of many species remain viable after passing through the digestive tracts of animals, with varying effects on germination (Cano-Vazquez et al., Citation2015). Seed dormancy is generally an undesirable characteristic in agricultural crops, where rapid germination and growth are required. Extensive domestication and breeding of crop species have ostensibly removed most dormancy mechanisms present in the seeds of their wild ancestors. Studies have reported a myriad of methods to break seed dormancy, including chemical, mechanical, thermal, and hormonal seed treatments (Paparella et al., Citation2015).
The beneficial effects of priming on the vigour, germination of seeds and establishment of the seedlings is known since the times of Pliny the elder (A.D. 23–79) (Gaius, Citation1949). Seed priming is a pre-sowing treatment that involves the controlled hydration of seeds, sufficient to allow pre-germinative metabolic events to take place but insufficient to allow primary root protrusion through the seed coat (Dutta, Citation2018; Paparella et al., Citation2015). It also involves complex physiological and biochemical process which offers an effective means to improve seed quality, seed germination and vigour (Bose, Kumar, Singhal, & Mondal, Citation2018; Siri, Vichitphan, Kaewnaree, Vichitphan, & Klanrit, Citation2013). Priming treatments are widely applied by seed companies to increase the germination rate and uniformity of seedling establishment of commercial vegetable and flower seeds. The benefits, associated with certain physiological, biochemical, cellular and molecular changes, include rapid, uniform and increased germination, improved seedling vigour and growth under a broad range of environments resulting in better stand establishment (Demir & Mavi, Citation2004). Different priming treatments can be effectively employed to prime many hot pepper seeds at one time (Ozbay, Citation2018; Paparella et al., Citation2015). Halopriming can affect osmoregulation in seeds by the active uptake of inorganic ions, promoting K+ and Ca2+ absorption and decreasing Na+ and Cl− accumulation. Potassium plays an important role in balancing membrane potential and turgor, activating enzymes, and regulating osmotic pressure in cells (Ibrahim, Citation2016). Some authors hypothesized that capsaicinoids could have some allelopathic effect on pepper seed germination (Barchenger & Bosland, Citation2016). Capsaicinoids are a well-established allelochemical and has been shown to reduce root and shoot growth or suppress germination in several plant species (Kato-Noguchi & Tanaka, Citation2004). The effects of incorporating plant growth regulators into the priming solution have also been indicated to improve the germination and the growth of pepper seedlings (García, Montes, Rangel, García, & Mendoza, Citation2010; Quintero et al., Citation2017), and other vegetables.
The objective of this study was to evaluate both the response rate of wild seeds of Chili Piquín (Capsicum annuum var. glabriusculum) to break dormancy and improve germination rate through seed priming and halopriming integrated with gibberellic acid (GA3) treatments. This information is needed to help in the development of sound and reliable guidelines for seedling production of Piquín pepper and contribute to its domestication.
2. Materials and methods
2.1. Plant materials
Fruit of Chili Piquín were collected from different wild populations in the States of Tamaulipas and San Luis Potosí, in North-eastern Mexico. Seed extraction was carried out manually, macerating fruits of each wild populations and dipping them in water to separate the pure seed from impurities. Seeds from different wild population were disinfected, as separate seed lot, in 1% sodium hypochlorite solution for 15 min to eliminate seed borne microorganisms. After disinfection, seeds were dried on shade for two weeks, then stored for three month, at laboratory hot and dry conditions (25ºC, 50% RH), before giving the different treatments (Aloui, Souguir, & Hannachi, Citation2014).
2.2. Seed treatments
To achieve the proposed objective, a series of three consecutive experiments were designed for pre-sowing treatment of seeds. Each series of experiments was three times replicated for each stored seed lot. Following every treatment all seeds were rinsed under running tap water for 3 min and then with distilled deionized water for 1 min. After rinsing, seeds were surface dried by placing them between paper towels for 30 min. at room temperature. The seeds were then slowly dried at 25ºC for 2 days until they reached their original moisture content (∼7–9%) and stored until capsaicinoids content determinations and germination test were carried out (Aloui et al., Citation2014). Untreated seeds were used as control and subjected to the same disinfection, rinsing and drying conditions.
2.2.1. Digestion treatments
To simulate the effect of the digestive tract of birds on breaking dormancy on Piquín chili seeds, a group of seeds were subjected to a chemical wet digestion process using HCl and H2O2. Seeds were dipped in 0.2 N HCl for 5 min., and rinsed with ddH2O for 2 min. Subsequently were oxidized with 0.5 N hydrogen peroxide for 5 min and newly rinsed with ddH2O for 2 min. These redox sequences were repeated three times alternating both treatments (Jaganathan, Yule, & Liu, Citation2016).
2.2.2. Priming treatments
Factorial halopriming was accomplished by imbibing 5 g of seed at 25°C in darkness for (24, 48 or 72 h) under an aerated solution of (KNO3, K2SO4, NH4NO3, KCl, (NH4)2SO4 or NH4Cl) at −5, −10 or −15 atm (−0.5; −1.0 or −1.5 MPa respectively) of osmotic potential (Ψs) to prevent seeds from entering the phase III of hydration (growth) (Aloui et al., Citation2014). Solutions were prepared by dissolving different salts in 250 ml Erlenmeyer glasses containing 100 mL of distilled water (Marín Sánchez., Mejía Contreras, Hernández Livera, Peña Lomeli, & Carballo Carballo, Citation2007). Untreated seeds were also used as control.
2.2.3. Priming integrated with gibberellic acid treatments
Priming, integrated with GA3 treatment, was performed using two of the priming treatments [KNO3 (−15 atm) and NH4NO3 (−10 atm)], which further increased the germination parameters of the previous experiments. These priming treatments were supplemented with gibberellic acid (GA3) at 100 or 200 mg·L−1. Both controls (unprimed and without GA3) were used as absolute and relative control respectively. Indices were calculated referring to absolute control (untreated seeds) and to their respective relative control (priming treatments) and these denoted with the subscript.
2.3. Capsaicinoids determination
To test whether seeds capsaicinoids could be a contributor to seed germination, capsaicinoids content was determined on all seeds (primed and untreated) after treatments. Five-gram whole dry seeds were ground with a home blender for 3 min and then a fivefold volume of acetone was added, respectively, to the extract at 50ºC for 1 h in triplicate. Centrifuged supernatant was taken for colorimetric analysis, following the methods proposed by Wang-Kyun, Hee-Woong, Geun-Dong, and Hae-Ik (Citation2017).
2.4. Germination tests
These were carried out in darkness in a temperature-controlled incubator held at 25 ± 0.5°C and 100% relative humidity (Guerrero Velázquez, Citation2015). Seeds were placed on two layers of filter paper moistened with 3 mL of distilled water in covered 10 cm petri dishes. Germination values were recorded daily for 28 days to establish statistical data. From the total number of germinated seeds, final germination percentage (FGP) was calculated. For ungerminated seeds, tetrazolium chloride tests were conducted to differentiate between dormant and dead seeds (ISTA, Citation2017). Final latent percentage (FLP) and final mortality percentage (FMP) of seeds were calculated accordingly.
Primary root protrusion to 1 mm was scored as germinated seed. To evaluate root growth, a network of fiberglass of 1 mm2 was placed under seeds. Primary root length (PRL) was measured in mm. Development germination index (DGI) allows to quantify effects (including FGP and PRL) of treatments (t) respect to control (o) on germination development. DGI was calculated by Zucconi tests (Selim, Zayed, & Atta, Citation2012) by following the formula:
[DGI = 100·(FGP(t)/FGP(o)·(PRL(t)/PRL(o)].
Days to 50% of FGP (T50) and days between 10% and 90% of FGP (G10–90) were also calculated. T50 is an inverse measure of mean germination rate, while G10–90 is an estimate of germination uniformity (GU) or germination synchrony. To contrast the behaviour of treatments(t) to control(o), these parameters were transformed in their respective indices, according to the following formulas: Rate germination index [RGI = 100·(T50(t)/T50(o))]; synchrony germination index [SGI = 100·(G10–90(t)/G10–90(o))].
After germination testing, germinated seeds were transplanted to conventional seedling trays inside a greenhouse to evaluate the number of abnormal seedlings generated by each treatment. Abnormal seedling percentage (ASP) and its corresponding abnormality seedling index [ASI = 100·(ASP(t)/ASP(o))], were calculated from abnormal plantlets.
2.5. Experimental design and statistical analysis
Treatments were arranged in completely randomized factorial design with four replications of 25 seeds. Data were subjected to multifactorial ANOVA. Mean separation was performed by Fisher’s least significant difference (LSD0.05) test if F test was significant at p ≤ 0.05 (*).
3. Results and discussion
Capsaicinoids contents, germination parameters, primary root growth and transplant abnormality for each seed treatment are shown in Tables – respectively. No differences were found between seeds lot or replications. The average daily percent germination values for treatments and control over a 28-day germination period are shown in Figure .
Table 1. Average values, ANOVA significance and LSD0.05 values of Capsicum annuum var. glabriusculum/aviculare seeds and seedless, germinated in darkness at 25 °C following digestion treatments
Figure 1. Cumulative germination percentage of Piquín pepper after seeds treatments (digested or primed) monitored for 28 days. Error bars are presented only for control, digested and KNO3 treatments.
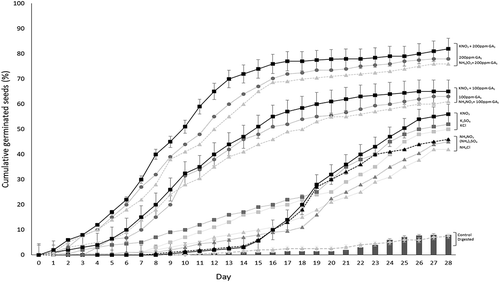
3.1. Digestion treatments
Table shows germination parameters of seeds digested with HCl and H2O2. Average values show no significant difference for CC, FLP, FMP, FGP, PRL, T50, G10–90, or ASP, while significant differences for DGI, RGI, SGI and ASI indices were found, indicating that these indices, are more sensitive to detect the treatment effects referred to control than the proper parameters. The chemical digestion of Piquín pepper seeds does not affect capsaicinoids content (CC) on seeds. The lower FGP and PRL of digested seeds lead to a strong reduction on DGI (−33%) indicating a marked detrimental effect on germination development. Digestive treatments only increase mean germination rate (+11% RGI) and could contribute to break dormancy or latency reducing FLP (Table ), but also reduces synchrony (−9% SGI), increases FMP, does not improve FGP, and strongly worsen early developmental stage of seedling and abnormality of transplants (+9% ASI).
3.2. Priming treatments
Average values of germination parameters and DGI are presented on Table . Significant differences were found in all factors of priming treatment (salt, time and Ψs) for all parameters and indices. Different behaviour was observed for different salts, showing differences between K+- and NH4+- salts on FGP (Figure ) and between NO3− and SO42-or Cl− on synchrony (Table ). All treatment reduces capsaicinoids content on primed seeds. Highest CC reduction were obtained (Table ) on seeds primed with NO3− salts (more than SO42- or Cl−) and at −10 or −15 atm (more than −5), for 48 or 72 h (more than 24).
Table 2. Average values, ANOVA significance and LSD0.05 values of Capsicum annuum var. glabriusculum seeds and seedling, germinated in darkness at 25°C following priming (Pr) treatments
FGP was increased four to five times for Cl- and SO42-. NO3− salts (of NH4+ or K+) increased FGP (6 times) and reduced to 25% seeds mortality (Table ). A higher final percent of germinated seeds was also obtained for K+ rather than NH4+ containing salts (Figure ). Highest FGP (together with low effect on PRL reduction) of NO3− primed seeds lead to a strong increase on DGI, indicating a clear improvement on germinative process. DGI increases three to four times for Cl− and SO42- and by five times for NO3−. KNO3 increased DGI reducing T50 and G10–90 more than NH4NO3, whereas NH4NO3 reduced ASP more than KNO3. An incremental effect was observed for priming time and Ψs on FGP, DGI and T50. Increments on germination rate were 6–12% higher using K+ than NH4+ containing salts (Table ). Latent seeds were only significantly reduced for K2SO4 or NH4Cl salts at −10 or −15 atm for 48 or 72 h. Radicle length was only significantly reduced on KCl primed seeds under Ψs −5 atm for 24 or 48 h.
A differential effect was observed on germination synchrony for different factors. Germination synchrony increases on nitrate primed seeds (lowest G10–90), whereas was reduced on seeds primed with sulphate or chloride. Priming times shorter than 72 h, or lower than −10 atm of Ψs on priming solution, reduces synchrony (highest G10–90 on Table ). Figure shows the average percentage germination values over time for all priming and digestion treatments. A different behaviour appears on the germination process for each treatment during 28 days of germination. Germination synchronies (G10–90 on Table ) were expanded by Cl− and SO42- whereas reduced by NO3−. Seeds primed with nitrate containing salts clearly increases germination synchrony and mean germination speed, but the effect not appears to be responsible for breaking of dormancy. Seeds latency (FLP) could probably be improved by including GA3 in priming solutions (Figure ).
Abnormality of plantlets was reduced as priming time increases and was lower for Ψs −10 atm. ASP reduced 38% for Cl−, 62% for SO42- and 70% for NO3−. Graphic analysis of interactions indicated that 72 h priming treatments with NH4NO3 (−15 atm) and KNO3 (−10 atm) are optimum regarding the improvement of FGP, PRL, DGI and ASP.
3.3. Priming integrated with gibberellic acid treatments
Average values of germination parameters and indices are presented on Table . All treatment significantly reduces capsaicinoids content on primed seeds. Highest CC reduction were obtained on seeds primed with NO3− salts and at 200 mg·L−1 of AG3. Combined effects of nitrate priming and AG3 reduces initial capsaicinoids contents to 10%. A high correlation between DGI and CC were found (DGI = (60.07–1.71*sqrt (CC)) ^2; R2 = 96.91%).
Table 3. Average values, ANOVA significance and LSD0.05 values of Capsicum annuum var. glabriusculum seeds and seedling, germinated in darkness at 25 °C following pre-sowing with gibberellic acid treatments and priming integrated with gibberellic acid (GA3) treatments
Pre-sowing with gibberellic acid treatments (Control +100 or +200 mg·L−1 GA3) also shows (Figure ) a positive effect on germination respective to absolute control for all evaluated parameters (Table ), except PRL (100 and 200 mg·L−1) and G10–90 (100 mg·L−1).
GA3 significantly reduces latency (FLP) in Piquín chili seeds (Table ) referred to the absolute control and maintains this effect when it is added to priming solutions (Figure ). The addition of GA3 (at 100 or 200 mg·L−1) activates dormant seeds to a rate between 73 and 84% respectively. This latency inhibition causes an increase in FGP of between 30 and 60%. However, GA3 additions to priming solutions increases FMP respect to controls.
GA3 significantly increases mean germination rate (reducing T50 on Table ) compared to absolute or relative controls. At 200 mg·L−1 this T50 decrease by 2.5 times. However, the effect of GA3 on synchrony is different. While additions of 100 mg·L−1 has no effect, addiction of 200 mg·L−1 double the synchrony, reducing germination time (G10–90) from 15 to 8 days. These synergic effects of the addition of GA3 to priming solutions is clearly show for germination percentages on Figure . Conversely, 200 mg·L−1 GA3 has no effect on ASP, while 100 mg·L−1 GA3 significantly increases the presence of abnormal seedlings in primed seeds.
Gibberellic acid applied alone, significantly reduces the length of the primary root with respect to the absolute control. However, the integrated priming treatment with GA3, practically duplicate PRL for GA3 (200 mg·L−1) and increases it by between 50 and 70% for GA3 (100 mg·L−1). These increases in PRL together with the originated in FGP lead to double or triple values of DGI (associated with GA3) compared to their respective relative controls. On the other hand, the reduction in PRL (associated with the application of GA3) regarding the absolute control, neutralizes the positive impact generated on FGP and originates DGI increases, on relative control, like those produced by the halopriming without GA3.
3.4. Digestion treatment
While some authors argue that Piquín chili seed germination increases after passage through the digestive tract of birds (Arcia González, Citation1985; Bañuelos, Salido, & Gardea, Citation2008), evidence of this fact has not been provided (Araiza Lizarde, Araiza Lizarde, & Martínez Martinez, Citation2011). Digestive treatments could contribute to breaking dormancy, increasing mean germination speed, but do not improve germination percentage or synchrony and strongly worsen early developmental stage of seedlings. The positive effects on germination related to birds appear to be more associated to the dispersal and deposition of seeds in favourable environments that on stimulate further germination. Digestion treatments have not shown any positive effect on the germination of Piquín chili seeds of. Authors have presented both similar results (Reid & Armesto, Citation2011), and have also found large differences (Guerrero Velázquez, Citation2015; Jaganathan et al., Citation2016) in the behaviour of different accessions of plants.
3.5. Priming treatments
Priming has been proposed as a mechanism of invocation of different stress tolerance of germinating seeds. Seed priming treatments have been applied to various crops under saline conditions (Ruttanaruangboworn, Chanprasert, Tobunluepop, & Onwimol, Citation2017). Some authors find that a specific ion or salt is not essential to priming pepper seed (Smith & Cobb, Citation1991), or other horticultural crop (Siri, Vichitphan, Kaewnaree, Vichitphan, & Klanrit, Citation2013). Nitrate enhanced germination and seedling establishment rates under adverse conditions, of onion, tomato, asparagus, melon, watermelon, husk tomato and pepper (Marín Sánchez, Rivas Jacobo, Flores Cano, Rojas Velazquez, & Jarquin Gálvez, Citation2013). Our results also indicate that nitrate-containing salts are more efficient than nitrate-free salts on promoting germination of primed seeds (except breaking dormancy). In addition, the effects of priming with KNO3 seem to be more positive than NO4NO3 on main germination and seedling establishment parameters (except for seed mortality and seedling abnormality). Seed priming stimulates pre-germinative metabolic processes and prepares seeds for primary root protrusion. Priming increases antioxidant system activity and repair membranes, moreover the reduction of capsaicinoids on seeds during priming, could contribute to break dormancy and stimulate germinative process. These changes promote seed vigour during germination and emergence (Ibrahim, Citation2016).
Time-course experiments show that effective priming is strongly dependent on both the osmotic potential of the priming solution and the duration of the treatment to avoid “over priming” (Smith & Cobb, Citation1991). Accordingly, the recommended values of osmotic potential to improve germination of Piquín chili seed must be between −10 and −15 atm (−1.0 and −1.5 MPa) and the treatment time must be between 48 and 72 h.
A small number of studies were developed on Piquín pepper germination. All of them are heavily dependent on the origin of seeds accessions and on genetic diversity and present conflicting results (Cano-Vázquez et al., Citation2015; Guerrero Velázquez, Citation2015; Rodriguez-Uribe et al., Citation2014). Authors do not find positive effects of KNO3 priming, whereas only positive effects with GA3 at extremely high doses (5000 mg·L−1) were observed. However, none of these studies combine priming with GA3 at low doses. The undesirable observed effects of seed latency mean germination time (T50) and synchrony (G10-90) could be improved by including gibberellic acid (GA3) in osmotically active priming solutions (as shown in Figure ).
3.6. Priming integrated with gibberellic acid treatments
Halopriming with the addition of plant growth regulators may be an effective way to shorten emergence time and increase stand establishment in watermelon and pepper at low temperatures (Korkmaz, Citation2005). Halo-priming using KNO3 or a growth regulator like GA3 improves germination rate and reduces the mean germination time (Tzortzakis, Citation2009).
The integration of priming with GA3 was effective in improving germination and establishment of pepper and tomato seeds. Priming, during which germination is suspended, provides a unique way to rapid and efficiently digest the endosperm by GA-induced enzymes and reduce the mechanical restraints of endosperm thus providing energy to start and sustain embryo growth. Studies of genetics and physiology have shown the important roles of the plant hormones such as abscisic acid and gibberellin in the regulation of seed dormancy and germination (Koornneef, Bentsink, & Hilhorst, Citation2002).
Considerable improvements on germination performance (rate and uniformity) and seedlings establishment are shown for KNO3 and GA3 treatments, in agreement with Tzortzakis (Citation2009). The lowest values of capsaicinoids found on KNO3 + 200 mg·L−1 GA3 primed seeds could reduce their allelopathic effect on germination. Since high concentrations of capsaicin inhibit the germination of chili seeds (Barchenger & Bosland, Citation2016), the positive effects on germination may be due to the elimination of these as germination inhibitors (Cano-Vazquez et al., Citation2015).
4. Conclusions
This study showed that it is possible to relieve dormancy and improve germination performance on Piquín chili seeds by priming techniques. Wild Piquín chili seed primed with KNO3 (−10 atm; 72 h) + GA3 (200 mg·L−1) improved germination (percentages and synchronicities) and reduced the start-up time of germination and seedling abnormality. Moreover, the results of current study provide essential information needed for the development of guidelines for the domestication and cultivation of Piquín chili plants.
Competing interests
The authors have declared that no competing interests exist
Author contributions
Conceived and designed the experiments: MFQ and MG. Performed the experiments: OG, AGC and AGS. Analysed the data: MFQ, PD, AGS and MG. Contributed reagents/materials/analysis tools: PD, and AGS. Wrote the paper: MFQ and MG
Additional information
Funding
Notes on contributors
Maria Fernanda Quintero C
Maria Fernanda Quintero C, Oscar Guillen Castillo, Pablo Delgado Sánchez, José Marín-Sánchez, Ana Isabel Guzmán, Agustín Sánchez and José Miguel Guzmán The paper is the result of a multidisciplinary work between the research groups: UASLP-CA-236 (UASLP) and RNM 151 PAIDI-UAL (CEI A3- UAL). It is a part of a master’s thesis in agricultural production. Dra Quintero (UASLP-CA-236) and Dr Guzmán (RNM 151 PAIDI-UAL) are the main researchers, they carried out the experimental design, methodology and the final writing of work. Dr Delgado and Dr. Sánchez organized laboratory work and provided additional resources for their development. Postgraduate students Guillen and Guzmán, carried out the experiments and laboratory tasks, collaborated in information treatment writing of the paper. The work constitutes the preliminary stage of a broad project on “contribution to the domestication and production of Chile Piquín (Capsicum annuum L. var. Glabriusculum) in Mexico“. The project has been recently positively evaluated by the National Council of Science and Technology of Mexico, subject to budget availability.
References
- Aloui, H., Souguir, M., & Hannachi, C. (2014). Determination of an optimal priming duration and concentration protocol for pepper seeds (Capsicum annuum L.). Acta Agriculturae Slovenica, 103(2), 213–221. doi:10.14720/aas
- Araiza Lizarde, N., Araiza Lizarde, E., & Martínez Martinez, J. G. (2011). Evaluación de la Germinación y Crecimiento de Plántula de Chiltepín (Capsicum annuum L. var. glabriusculum) en invernadero. Revista Colombiana de Biotecnología, 13(2), 170–175.
- Arcia González, D. I. (1985). Evaluación financiera y económica de un sistema agroforestal en el estado de Quintana Roo. Montecillo, Mex: Tesis Maestría. Colegio de Postgraduados.
- Bañuelos, N., Salido, P. L., & Gardea, A. (2008). Etnobotánica del Chiltepín. Pequeño gran señor en la cultura de los sonorenses. Estudios Sociales: Revista de Investigación Científica, 16(32), 177–206.
- Barchenger, D. W., & Bosland, P. W. (2016). Exogenous applications of capsaicin inhibits seed germination of Capsicum annuum. Scientia horticulturae, 203, 29–31. doi:10.1016/j.scienta.2016.03.009
- Bose, B., Kumar, M., Singhal, R. K., & Mondal, S. (2018). Impact of seed priming on the modulation of physico-chemical and molecular processes during germination, growth, and development of crops. In Advances in seed priming (pp. 23–40). Singapore: Springer Singapore. doi:10.1007/978-981-13-0032-5_2
- Cano-Vazquez, A., Lopez-Peralta, M., Zavaleta-Mancera, H. A., Cruz-Huerta, N., Ramirez, R. I., Gardea, B. A., & Gonzalez, H. V. A. (2015). Variation in seed dormancy among accessions of chile piquin (Capsicum annuum var. glabriusculum). Botanical Sciences, 93(1), 175–184.
- Cano-Vázquez, A., López-Peralta, M. C., Zavaleta-Mancera, H. A., Cruz-Huerta, N., Ramírez-Ramírez, I., Gardea-Béjar, A., & González-Hernández, V. A. (2015). Variación en grados de latencia en semillas entre colectas de chile piquín (Capsicum annuum var. glabriusculum). Botanical Sciences, 93(1), 175. doi:10.17129/botsci.138
- Demir, I., & Mavi, K. (2004). The effect of priming on seedling emergence of differentially matured watermelon (Citrullus lanatus (Thunb.) Matsum and Nakai) seeds. Scientia horticulturae, 102(4), 467–473. doi:10.1016/j.scienta.2004.04.012
- Dutta, P. (2018). Seed priming: new vistas and contemporary perspectives. In Advances in seed priming (pp. 3–22). Singapore: Springer Singapore. doi:10.1007/978-981-13-0032-5_1
- Gaius, PS (1949–1954). Naturalis historia, vol. IV–VII, Books 12–27 (trans: Rackham H, Jones WHS, Eichholz DE). Harvard University Press, Massachussets and William Heinemann, London.
- García, F. A., Montes, H. S., Rangel, L. J. A., García, M. E., & Mendoza, E. M. (2010). Respuesta fisiológica de la semilla chile piquín [Capsicum annuum var. glabriusculum (Dunal) Heiser & Pickersgill] al ácido giberélico e hidrotermia. Revista Mexicana de Ciencias Agrícolas, 1(2), 203–216.
- Guerrero Velázquez, R. (2015). Niveles de dormancia en semillas de chile silvestre de diferentes ecorregiones y desarrollo de protocolos para la germinación y regeneración de accesiones. ( MsC Thesis). Universidad Autónoma de Aguascalientes.
- Hayano, C., Gamez, N., & Medina, L. A. (2016). Wild pepper capsicum annuum L. var. glabriusculum: Taxonomy, plant morphology, distribution, genetic diversity, genome sequencing, and phytochemical compounds. Crop Science, 56(1), 1–11. doi:10.2135/cropsci2014.11.0789
- Ibrahim, E. A. (2016). Seed priming to alleviate salinity stress in germinating seeds. Journal of Plant Physiology, 192(3), 38–46. doi:10.1016/j.jplph.2015.12.011
- ISTA. (2017). Changes to the ISTA rules for 2017. International Rules for Seed Testing. Zurich, Switzerland, 12, 345. doi: 10.15258/istarules.2017.i.
- Jaganathan, G. K., Yule, K., & Liu, B. (2016). On the evolutionary and ecological value of breaking physical dormancy by endozoochory. Perspectives in Plant Ecology, Evolution and Systematics, 22, 11–22. doi:10.1016/j.ppees.2016.07.001
- Kato-Noguchi, H., & Tanaka, Y. (2004). Effects of capsaicin on plant growth. Biologia Plantarum, 47(1), 157–159. doi:10.1023/A:1027317906839
- Kim, H. J. (2016). Opportunities and challenges of alternative specialty crops: The global picture. HortScience : A Publication of the American Society for Horticultural Science, 51(11), 1316–1319. doi:10.21273/HORTSCI10659-16
- Koornneef, M., Bentsink, L., & Hilhorst, H. (2002). Seed dormancy and germination. Current Opinion in Plant Biology, 5(1), 33–36. doi:10.1016/S1369-5266(01)00219-9
- Korkmaz, A. (2005). Inclusion of acetyl salicylic acid and methyl jasmonate into the priming solution improves low-temperature germination and emergence of sweet pepper. HortScience : A Publication of the American Society for Horticultural Science, 40(1), 197–200.
- Marín Sánchez, J., Mejía Contreras, J. A., Hernández Livera, A., Peña Lomeli, A., & Carballo Carballo, A. (2007). Osmotic conditioning of Husk tomato seeds. Agricultura Técnica En México, 33, 115–123.
- Marín Sánchez, J., Rivas Jacobo, M., Flores Cano, J., Rojas Velazquez, A., & Jarquin Gálvez, R. (2013). Efecto del priming sobre la calidad fisiológica de semilla criolla de chile ancho (Capsicum annuum L.). Corpoica Ciencia y Tecnología Agropecuaria, 1(1), 1–6.
- Ozbay, N. (2018). Studies on seed priming in pepper (Capsicum annuum L.). In Advances in seed priming (pp. 209–239). Singapore: Springer Singapore. doi:10.1007/978-981-13-0032-5_12
- Paparella, S., Araujo, S. S., Rossi, G., Wijayasinghe, M., Carbonera, D., & Balestrazzi, A. (2015). Seed priming: State of the art and new perspectives. Plant Cell Reports, 34(8), 1281–1293. doi:10.1007/s00299-015-1784-y
- Quintero, M. F., Guerrero-Gonzalez, M. L., Guillen Castillo, O., Marín-Sanchez, J., Delgado-Sánchez, P., & Guzmán, M. (2017). Osmo-priming improves germinability on piquín chili seeds. In C. N. D. P. de Chile (Ed.), World pepper convention (pp. 300–307). Aguascalientes, México: SAGARPA.
- Reid, S., & Armesto, J. J. (2011). Avian gut-passage effects on seed germination of shrubland species in mediterranean central chile. Plant Ecology, 210, 1–10. doi:10.1007/s11258-010-9796-8
- Rodriguez-Uribe, L., Hernandez, L., Kilcrease, J. P., Walker, S., & O’Connell, M. A. (2014). Capsaicinoid and carotenoid composition and genetic diversity of Kas I and Ccs in New Mexican capsicum annuum L. Landraces. HortScience : A Publication of the American Society for Horticultural Science, 49(11), 1370–1375.
- Ruttanaruangboworn, A., Chanprasert, W., Tobunluepop, P., & Onwimol, D. (2017). Effect of seed priming with different concentrations of potassium nitrate on the pattern of seed imbibition and germination of rice (Oryza sativa L.). Journal of Integrative Agriculture, 16(3), 605–613. doi:10.1016/S2095-3119(16)61441-7
- Selim, S. M., Zayed, M. S., & Atta, H. M. (2012). Evaluation of phytotoxicity of compost during composting process. Nature and Science, 12(2), 69–77.
- Siri, B., Vichitphan, K., Kaewnaree, P., Vichitphan, S., & Klanrit, P. (2013). Improvement of quality, membrane integrity and antioxidant systems in sweet pepper (Capsicum annuum Linn.) Seeds affected by osmopriming. Australian Journal of Crop Science, 7(13), 2068–2073.
- Smith, P. T., & Cobb, B. G. (1991). Accelerated germination of pepper seed by priming with salt solutions and water. HortScience : A Publication of the American Society for Horticultural Science, 26(4), 417–419.
- Tzortzakis, N. G. (2009). Effect of pre-sowing treatment on seed germination and seedling vigour in endive and chicory. Horti Science, 36(3), 117–125.
- Wang-Kyun, R., Hee-Woong, K., Geun-Dong, K., & Hae-Ik, R. (2017). Rapid determination of capsaicinoids by colorimetric method. Journal of Food and Drug Analysis, 25(4), 798–803. doi:10.1016/J.JFDA.2016.11.007
