Abstract
Background: Diabetes is an enduring condition that causes impaired, peripheral insulin resistance. Curcumin was shown to exert notable anti-diabetic effects, which might be possible by overexpression of certain glucose transporter genes and glycoprotein content within the cells.
Objectives: To investigate the effect of curcumin alone and in concomitant with insulin on glucose translocation from intracellular compartments of nuclear or endoplasmic reticulum membranes into the cytoplasmic membrane (CM) and key kinases involved in insulin signaling pathways.
Materials and Methods: C2C12 myoblast cells were cultured and differentiated to the myotubes. Later, the cells were treated with curcumin alone or a combination of curcumin and insulin, so their viability was measured by MTT assay. The expression level of GLUT 4 gene was examined by Real Time-reverse Transcription Polymerase Chain Reaction (qRT-PCR). To evaluate the activity of curcumin and curcumin/insulin synergistic effect on stimulated GLUT4 translocation, kinases AKT, P-AKT, AMP-activated protein kinase (AMPK) and P-AMPK were assessed and detected via Western Blotting (WB).
Results: Curcumin significantly induced GLUT 4 expression and its translocation from intra-cell space into the cell surface and showed a synergic effect on GLUT 4 translocation in presence of insulin. This synergistic effect was inhibited by the insulin receptor inhibitor AG1024 and the inhibitor of AMPK signaling, compound C.
Conclusion: Curcumin demonstrated a synergistic effect with insulin and could be a choice of type 2 diabetes mellitus (T2DM) treatment, which may be affected by both AKT and AMPK signaling pathways, hereby facilitates glucose uptake into the cells.
Public Interest Statement
In this study we demonstrated the tico-treatment of insulin and curcuminin in C2C12 myotubes exerts synergistic therapeutic effect against diabetes, which at least is partially mediated by insulindependentPI3-K/Akt and the insulin-independent AMPK pathways. Also, curcumin improved insulin sensitivity and up-regulated GLUT4 gene expression and glycoprotein movement from the intra cell pool to the cell membrane. Hence due to the developing interest towards AMPK activators as potential therapies for T2DM, curcumin could be preference.
Competing Interests
The authors declare no competing interests.
1. Introduction
GLUT 4 is a unique member of a facilitative glucose transporter gene family, which is expressed at high levels in cells with insulin-stimulated glucose transport activity (Fereshteh Ahmadipour et al., Citation2012). Several studies demonstrated that tissue-specific GLUT 4 expression plays a crucial role in normal maintenance of glucose homeostasis (Dugani & Klip, Citation2005; Saltiel, Citation2001; Smith & Muscat, Citation2005). The skeletal muscles are known to be responsible for more than 75% of glucose consumption in response to the insulin treatment in post-prandial state (DeFronzo et al., Citation1981; Jarvill-Taylor, Anderson, & Graves, Citation2001).
GLUT 4 is expressed at very high levels in skeletal muscle cells with the highest levels of insulin-stimulated glucose uptake (Koumanov, Jin, Yang, & Holman, Citation2005; Mueckler, Citation1990). Under normal plasma glucose conditions, the GLUT 4 isoform is sequestered to the intracellular membrane compartments. In contrast, stimulation by high glucose levels increases GLUT 4 at the cell surface by promoting its translocation from the intracellular GLUT 4 storage compartments, allowing an enhanced glucose transport within the cells (Watson & Pessin, Citation2001). This insulin-induced GLUT 4 translocation accounts for a significant portion of insulin-induced glucose uptake. Any defects in this particular function of insulin in skeletal muscle cells contribute to the insulin-resistant characteristics of T2DM (Koistinen & Zierath, Citation2002).
Glucose uptake, a rate-limiting step in glucose metabolism, has been shown to be stimulated in skeletal muscle cells by two distinct pathways. One is directly activated by insulin through insulin receptor substrate 1 (IRS 1)/phosphatidylinositol 3 (PI3-kinase)/Akt signaling (IRS 1/PI3-kinase/Akt) pathway and promotes GLUT 4 translocation from an intracellular pool to the plasma membrane. Whereas, the other is being stimulated by AMP-activated protein kinase (AMPK), a phylogenetically conserved intracellular energy sensor that plays a central role in regulation of glucose and lipid metabolism.
Regardless of current treatments that affect proper blood glucose control, natural compounds seem to approach diabetes in variable routes and degrees (Kang et al., Citation2010; Kang & Kim, Citation2010; Liu, Chen, & Hu et al., Citation2010). Preliminary, they would promote glucose transporters and glucose metabolism (Lee et al., Citation2005). In between, turmeric is one of the best known and the most consumed spice in the world that contains several chemical compositions; in particular three polyphenolic curcumin oils, of which curcumin (1,7-bis (4-hydroxy 3-methoxy phenyl)-1,6-heptadiene-3,5-dione or diferuloylmethane) is the principal compound with established anti-diabetic effects (Figure ).
This compound showed to delay the development of T2DM, improves beta-cell functions and precludes their death, reduces the insulin resistance, hyperglycemia and hyperlipidemia (Jain, Rains, Croad, Larson, & Jones, Citation2009; Shao et al., Citation2012). Besides, curcumin possessed more therapeutic values such as anti-inflammatory, antioxidant, anti-apoptotic, anti-carcinogenic, antiviral and anti-infectious properties (Maheshwari, Singh, Gaddipati, & Srimal, Citation2006; Rashid, Chowdhury, Ghosh, & Sil, Citation2017). Curcumin was shown to augment the specificity and affinity of insulin binding to the insulin receptors, so was capable to retain blood insulin levels (Maheshwari et al., Citation2006). In addition, curcumin activated the adenosine monophosphate (AMP) kinase and inhibited glucose-6-phosphatase and phosphoenolpyruvate carboxykinase activities, exhibiting an inhibitory effect on hepatic gluconeogenesis (Pari & Murugan, Citation2007). In a pre-diabetic population, curcumin treatment improved overall function of beta cells (Chuengsamarn, Rattanamongkolgul, Luechapudiporn, Phisalaphong, & Jirawatnotai, Citation2012) and in this way was able to inhibit hepatic gluconeogenesis and regulated the whole-body glucose metabolism (Meghana, Sanjeev, & Ramesh, Citation2007). Curcumin was also reported to lower the blood levels of tumor necrosis factor alpha (TNF α), interleukin 6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), glucose and glycosylated hemoglobin in diabetic rats (Jain et al., Citation2009). Recently, it was evidenced that curcumin attenuated oxidative stress-induced nuclear factor kappa B (NFκB) mediated inflammatory cytokines, chemokines, adhesion molecules and ER-dependent apoptosis in adipocytes in diabetic animals. Its administration increased the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione-S-transferase (GST) and glutathione peroxidase (GPx) in the spleen tissue, also suppressed the expression of related inflammatory damages and mitochondrial apoptotic parameters including glucose-regulated protein-78 (GRP78), caspase-12, calpain-1, phosphor c-Jun N-terminal kinase (JNK), phosphor p38 and p53 (Rashid et al., Citation2017).
Earlier, we found cinnamon extract enhanced GLUT4 contents in C2C12 cell membrane and facilitated glucose entrance into the cells, while in contrast the level of the transformation was not significant where these cells were exposed to the turmeric water extract (Absalan et al., Citation2012; Absalan, Mohiti-Ardakani, Hadinedoushan, & Khalili, Citation2012).
It is well known that insulin has a major regulatory function in glucose metabolism in various tissues including skeletal muscles and adipocyte by redistributing GLUT 4 from intracellular vesicles to the cell surface. To this end, we hypothesized that whether curcumin alone or in combination with insulin effects on GLUT 4 translocation in membrane fractions of muscle C2C12 cells and also on the other key kinases that deviate in glucose metabolism and/or associated signaling pathways.
2. Materials and methods
2.1. Materials
Akt, AMPK, p-Akt (Ser473), p-AMPK (Thr172) antibodies were purchased from Cell Signaling Technology (MA, USA). Protease and phosphatase inhibitors, commercial curcumin (C7727, Casnumber: 457–37-7), insulin, compound C, dimethylsulfoxide (DMSO), Dulbecco’s modified Eagle’s medium (DMEM) cell culture media, phenylmethylsulfonyl fluoride (PMSF), Bradford reagent, NaHCO3, sucrose and anti β-actin antibody were obtained from Sigma–Aldrich (USA). Horseradish peroxidase-conjugated secondary antibodies were provided by Jackson Immuno Research Laboratories (PA, USA). Tyrphostin AG 1024 came from Alexis Biochemicals (CA, USA). C2C12 cell line (code No. ATCC: CRL-1772) was purchased from National Cell Bank of Iran, Pasteur Institute, Tehran, Iran. Nitrocellulose membrane was obtained from Millipore (UK). ECL Advance Western immunoblotting kit was purchased from Amersham Inc. (UK). Mouse anti-GLUT4 antibody 1F8 and goat anti-mouse IgG-HRP were obtained from Santa Cruz Biotech (USA). All other reagents were provided of the purest grade available.
2.2. Hydro-alcoholic curcumin extract
The extract was prepared as described earlier (Absalan et al., Citation2012)
2.3. C2C12 primary culture and differentiation
C2C12 cells were cultured as the author’s previous study (Absalan et al., Citation2012). Briefly, 8 × 104 myoblast cells were seeded in DMEM medium containing 4 mmol/l glutamine, 0.025 mol/l glucose, 1 mmol/l sodium pyrovate, 0.018 mol/l NaHCO3, 100 U/ml Penicillin G, 100 μg/ml streptomycin and 10% fetal bovine serum (FBS) to nearly confluent. Myoblasts differentiation to the myotubes was induced by addition of DMEM medium supplemented with 2% heat inactivated horse serum (HS). Differentiation medium was replaced each 48 h (22) and the establishment of polynucleotide myotubes was assessed microscopically until day 5 of differentiation induction.
2.4. Sample grouping
Sample groups were added to the related culture flasks containing differentiated myotubes 6 days after differentiation initiation and were ordered as; 1) DMEM + 2% HS containing 40 µmolar curcumin; 2) DMEM + 2% HS containing 30 µmolar curcumin; 3) DMEM + 2% HS containing 40 µmolar curcumin + 100 nm insulin; 4) DMEM + 2% HS containing 30 µmolar curcumin + 100 nm insulin; and 5) 1% DMSO treated cells were considered as the control group. All experiments were conducted in triplicates.
2.5. Cell viability
The effect of curcumin on cell viability was measured to determine the maximum concentration of curcumin which is not toxic to the cells. The cell morphology was studied by phase-contrast microscopy, and the viability was evaluated with MTT assay. Following the treatment of cells with curcumin, 100 µl of 0.45 g/L, the MTT solution was added to each well of a 96-well culture plate. Cells were incubated at 37°C for 60 min, allowing the color to appear and 100 µl of 20% SDS in DMF: H2O (1:1) solution was added to each well to stop the reaction. The plates were then incubated overnight at 37°C to dissolve the formazan products (Kang & Kim, Citation2010). The metabolized MTT product was quantified by reading the absorbance at 570 nm on a microplate reader.
2.6. Real time-reverse transcription polymerase chain reaction (qRT-PCR)
To investigate the molecular mechanisms involved in anti-diabetic activities of curcumin, insulin and their combination in C2C12 cells, the expression level of GLUT 4 gene was examined by quantitative qRT-PCR. In this regard, C2C12 cells were grown for 24 h, trypsinized, centrifuged and pelleted. The pellets were washed three times with sterile PBS for further RNA isolation. Total RNA was extracted with the TaKaRa Fast Pure RNA kit (#TAK-9190) according to the manual guidelines. The concentration of RNA was quantified by Thermo Scientific NanoDrop 2000c UV-Vis spectrophotometer (Thermo Scientific, USA) (Table ). Then, the cDNA was reverse transcribed by MMLV Reverse Transcriptase and Oligo (dT) Primers according to the manufacturer’s protocol (TaKaRa reverse transcription reagents kit, #6130) at 25°C for 10 min, 50°C for 60 min and 70°C for 15°C (Schmittgen & Livak, Citation2008).
Table 1. Effect of curcumin on mitochondrial activity in C2C12 cells
Table 2. The effects of curcumin (40 μM) and insulin and their combination on mRNA levels of GluT 4 in c2c12 muscle cells. Results represent from triplicate determinations, representative of 3 independent experiments compared with control. Fold changes were calculated using the 2−∆∆CT
The sense and antisense oligonucleotide sequences of GLUT 4 gene were designed as: GLUT4-F:5ʹ-CAACTGGACCTGTAACTTCATTGT-3ʹandGLUT4-R:5ʹACGGCAAATAGAAGGAAGACGTA-3ʹ, and the ones for endogenous gene, β- Actin, wereActin-F:5ʹCGTTGACATCCGTAAAGACCTC-3ʹandActin-R: 5ʹ- AGCCACCGATCCA CACAGA-3ʹ, respectively.
The experiment was carried out in triplicates under RT condition; 10 min at 95ºC, followed by 40 cycles at 95ºC for 15 s and 60ºC for 60 s. The expression level of the target GLUT 4 gene was calculated by using the comparative cycle threshold method (El-Moselhy et al., Citation2011), after being normalized to the CT value of the β-Actin housekeeping gene.
2.7. Subcellular fractionation
After 3 h exposure to curcumin (40 µM); curcumin (40 µM) + insulin (100 nM) or DMSO; the myotubes were washed with ice-cold phosphate buffer trice (pH 7.4) and were homogenized for 3 min in HES buffer. To obtain crude plasma membrane or N/ER, the homogenate cells were centrifuged (Tortorella & Pilch, Citation2002) and the resulted pellet was homogenized in HES buffer and layered on a buffer (1.12 M sucrose in 20 mM HEPES and 1 mM Na2 EDTA buffer), 0.2% protease inhibitor cocktail, then centrifuged at 100,000 g for 1 h. The interphase portion was contained plasma membrane particles floating on HCS and the remained pellet was consisting of N/ER particles. Both layers were aspirated and the interphase membrane particles were centrifuged at 40,000 g for additional 20 min to be pelleted. The pellet was re-suspended in PBS (pH 7.4) containing protease inhibitors. N/ER particles were re-suspended in a buffer solution containing protease inhibitor cocktail. All centrifugation steps were performed at 4°C with Beckman Coulter Ultracentrifuge and Type 90 Ti rotor (Absalan et al., Citation2012).
2.8. WB analysis
Total protein concentrations were analyzed using Bradford reagent and proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) Overall, 100 μg protein was resolved in SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes via electrophoretic transfer system prior to WB analysis. The membranes were blocked using 2% blocking agent for 2 h, while gently shaking. Later, the blotted papers were immersed in blocking agent containing specific primary antibodies (1:200) for 4 h. Primary antibodies were included mouse anti-GLUT 4, p-Akt, Akt, p-AMPK and AMPK as well as mouse monoclonal antibodies to β-actin to observe loading. The membranes were thoroughly washed with PBS, 0.05% Tween-20 (PBS-T) and incubated with goat anti-mouse IgG-HRP conjugated secondary antibodies (1:1000) at 4°C for 4 h (Baeeri et al., Citation2017). The blots were exposed to chemiluminescent detection reagents for detection of target antigens. Spots were visualized with ECL-Plus detection kit or Gel documentation and were compared together for percent determination (syngeneGel documentation; www.syngene.com). Data were described as a percent that divided between intervention group and its equal vehicle group.
2.9. Akt and AMPK signaling pathway
To evaluate the molecular mechanism of curcumin and insulin-induced synergistic effect on stimulated GLUT4 translocation, the insulin receptor inhibitor AG1024 as inhibitor of Akt kinase and Compound C (Com.C) as an inhibitor of AMPK were studied. C2C12 cells were serum-starved for 16 h. For Akt inhibitor, cells were pretreated with insulin receptor inhibitor AG1024 (60 μM) for 2 h. In AMPK case, 200 mM of Com.C was contacted with myoblasts for 20 min. Thereafter, the medium was replaced with a fresh medium containing 100 nM insulin or 40 µM curcumin for both inhibitors and was stimulated for another 30 min. Cell lysates were analyzed by WB (as described in the previous section) using anti-phospho-Akt, anti- phospho-AMPK, anti-AMPK and anti-Akt antibodies.
2.10. Quantifications of bands and statistical analysis
Comparative histograms were plotted in Excel and data were analyzed by SPSS 11.5 software with two independent samples t-test.
3. Results
3.1. Curcumin viability assay
As curcumin concentration was increased, the cell viability was decreased dose-dependently in C2C12 cells. Curcumin appeared to be toxic at higher doses comparing to the DMSO group. Cell viability decreased to 1.5% and 2% at concentration of 200 µg/ml (Table ).
3.1. Real Time-PCR (qRT-PCR)
To determine the in vitro effect of curcumin and insulin on expression of GluT 4, Quantitative RT-PCR experiment was performed by using Glut4-specific primers. Table shows more than six times fold change the mRNA levels of Glut4 in the cells treated with insulin (100 nM) plus curcumin (40 μM) compared to the controls (P ≤ 0.05). No distinct changes were observed in the expression level of GLUT 4 by either insulin or curcumin (40 μM) treated groups alone or when compared to each other. Relative expression level was quantified based on the cycle threshold (CT) values and normalized against the internal control β-Actin. Each sample was measured in triplicate, and gene expression levels were calculated using the 2-ΔΔct method.
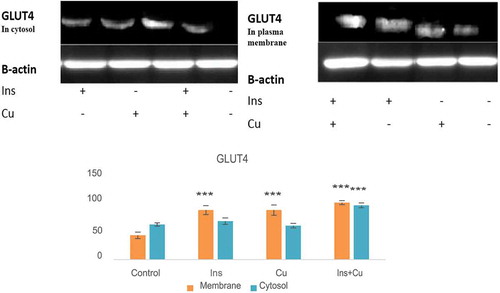
3.2. Co-treatment of insulin and curcumin-induced GLUT 4 translocation
Co-treatment of insulin and curcumin increased the plasma membrane abundance of GLUT 4 significantly (P < 0.05). Curcumin and insulin alone could also elevate the GLUT 4 translocation to the membrane (Figure ). Data suggested that there can be a synergistic interaction between curcumin and insulin in GLUT 4 translocation from intracellular space to the cell membrane.
Figure 2. Effect of curcumin and insulin on cytosolic GLUT 4. Mutual synergism between curcumin and insulin enhances GLUT 4 translocation to the membrane. C2C12 cells were treated with 40 µM curcumin for 2 h in the presence or absence of insulin (100 nM).* P < 0.05; as compared with the control value.
Ins: insulin, Cu: curcumin
3.3. Insulin receptors, Akt and AMPK signaling kinase inhibition
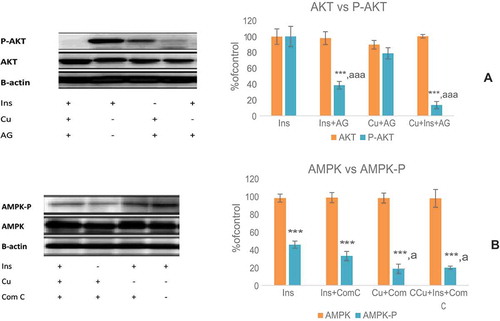
As shown in Figure ), pretreatment of C2C12 cells with AG1024 has led to the blockage of phosphorylation of Akt at its Ser473 in curcumin (40 μM) + insulin (100 nM) group or without curcumin. Curcumin (40 μM) alone was less effective on p-Akt. The involvement of curcumin in AMPK signaling was indicated by exploring the contribution of AMPK to curcumin stimulated phosphorylation of AMPK, which was significantly attenuated by preincubation of C2C12 cells with Com.C, indicating the participation of the AMPK pathway in curcumin-induced glucose uptake (Figure )).
Figure 3. Effect of the inhibitors of insulin signaling kinases on curcumin-mediated phosphorylation of AKT (a) and AMPK (b). (Ins; insulin, Cu; curcumin 40 μM). The results are expressed as mean ± S.D for three independent experiments, * P < 0.05; compares each group with its own phosphorylated form. * P < 0.05; as compared with the control value.
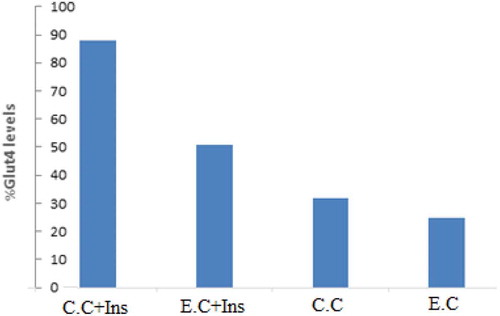
3.4. Comparison of the effect of curcumin extracted from curcuma longa and commercial curcumin on the expression of GLUT 4 in C2C12 cells
WB analysis was conducted to compare the effect of the curcumin isolated from Curcuma longa and our experimental commercial curcumin (C.C) on the expression level of GLUT 4 in C2C12 cells. Comparison of the GLUT 4 arbitrary levels obtained from the extracted curcumin (E.C) (40 µM), C.C (40 µM) and their combination with insulin (100 nM) demonstrated that the GLUT 4 expression level was significantly higher in C.C (both in insulin and non-insulin treated groups) as compared to the E.C groups. Percentage of the GLUT 4 expression level for the samples tested was ordered as; C.C + Ins (88%), E.C + Ins (51%), C.C (32%) and E.C (25%) (Figure ).
4. Discussion and conclusion
Today, DM is the fastest growing health threat and emerges urgent concerns. The pathogenesis of T2DM and obesity is known to be contributed to inflammation; thus, curcumin, a reputed anti-inflammatory compound has been assumed to be a potential source for diabetic and obesity pharmacology (Maheshwari et al., Citation2006). A 16-week in vivo study revealed that curcumin treatment significantly reduced the induction of diabetic retinopathy in rats (Gupta et al., Citation2011). Oral administration of curcumin to rats fed with a high-fat diet for 75 days lowered hyperglycemia, improved the insulin sensitivity and exhibited an anti-lipolytic effect via attenuating TNF-α and plasma free fatty acid levels (El-Moselhy et al., Citation2011). Several studies showed curcumin might promote the GLUT4 genes and glycoprotein contents in muscle cells. To investigate this possibility, our study aimed to perceive if curcumin is capable to modulate the amount of membrane GLUT4. As well, we investigated the synergic effect of insulin and curcumin on GLUT 4 translocation from the intracellular vesicular pool to the membrane in C2C12 muscle cells. We found that curcumin and insulin caused overexpression of GLUT4 mRNA in differentiated C2C12 skeletal muscle cells, showing that curcumin can augment the insulin sensitivity by promoting GLUT4 protein translocation to the plasma membrane. In addition, insulin and curcumin combination strongly induced the GLUT4 translocation in these cells, indicating the possible regulatory role of curcumin in glucose metabolism in C2C12cells.
In insulin signaling pathway, AMPK and PI 3-kinase are key molecules that play imperative roles in insulin-stimulated glucose transport and metabolism. Thus, WB analyses of the inhibitors of these kinases indicated that the phosphorylation of both p-Akt (Ser473), p-AMPK (Thr172) were inhibited by AG1024 and Com.C, respectively, which clarifies there is a correlation between curcumin-activated glucose metabolism and the insulin-independent glucose transport. Once C2C12 cells were exposed to curcumin and insulin simultaneously, both of the AMPK and PI 3-kinase/Akt pathways were more stimulated, when compared with each one alone. The activation of AMPK induces the recruitment of GLUT4 to the plasma membrane, resulting in up-regulated glucose uptake (Amitani et al., Citation2013, Russell III, Bergeron, Shulman, & Young, Citation1999); like those of cacao extract, resveratrol and anthocyanin (Kurimoto et al., Citation2013; Yamashita, Okabe, & Natsume et al., Citation2012). In accordance, we found curcumin stimulated GLUT4 translocation to the plasma membrane and increased glucose uptake in the presence of both curcumin and insulin.
The activation of AMPK and PI3-kinase/Akt pathways by natural compounds has been reported repeatedly, while the detailed mechanism(s) still remains unclear. For instance, berberine and S-allyl cysteine (compounds present in garlic) ameliorated diabetes and obesity via stimulation of AMPK activity; however, the mechanism for AMPK phosphorylation or its upstream signaling has not been verified (Cheng et al., Citation2006; Hwang et al., Citation2013; Lee et al., Citation2006; Baeeri M et al., Citation2017). The hypoglycemic effect of isoeugenol, a structurally analog of curcumin, was shown to be exerted through AMPK activation in the skeletal muscle cells. This compound also increased the GLUT4 expression and translocation to the plasma membrane (Kim et al., Citation2016). Kang and colleagues showed that curcumin can promote PI3-kinase activation with increased the insulin sensitivity in muscle cells (Kang & Kim, Citation2010). Also, curcumin stimulated glucose uptake in L6 cells AMPK dependently by the activation of AMPK-p38 MAPK pathways (Kim et al., Citation2010). Current studies suggested that there is a crosstalk between insulin signaling pathways (Kovacic et al., Citation2003; Park et al., Citation2007). In a study, once L6 rat skeletal muscle cells were exposed to the insulin and curcumin simultaneously, the PI3-kinase signaling pathway was activated. Moreover, co-treatment of insulin and curcumin may activate both PI3 and AMP kinases and a possible synergistic interaction between them. On the other hand, such synergistic effect was not perceived in insulin and epigallocatechingallate (EGCG) co-treatment (Jung et al., Citation2008). Of note, a very same stimulus may trigger the GLUT4 activation signaling pathways via different mechanisms in different experimental conditions. Another research team has reported EGCG increased GLUT 4 translocation from cytosolic compartments to the cell membrane in L6 cells, which was considered as an AMPK and PI3-K dependent event (Zhang et al., Citation2010). In skeletal muscles of mice in vivo condition, procyanidins promoted the GLUT4 translocation through both PI3K/Akt and AMPK dependent signaling pathways, while in L6 cells; it was shown that procyanidins only stimulated the AMPK dependent pathway (Yamashita et al., Citation2016). Similarly, it was evidenced that resveratrol activated the Akt pathway in patients with T2DM (Brasnyó et al., Citation2011); while in L6 myotubes, AMPK-dependent pathway was engaged to transfer GLUT4 (Park et al., Citation2007).
We observed that curcumin increased GLUT 4 contents of the cell membrane in C2C12 myotubes. Consequently, we hypothesize that curcumin may have the same effect alike EGCG on GLUT 4 intracellular compartments movements/translocation and this movement may be PI3-K dependent with a direction from cytosol to the cytoplasmic membrane. In the same manner, the co-treatment of saffron and insulin improved the insulin sensitivity via insulin-independent (AMPK/ACC and MAPKs) and insulin-dependent (PI3-K/Akt and mTOR) pathways (Kang, Lee, & Jung et al., Citation2012). In a similar way, resveratrol and procyanidins (isolated from black soybean) provoked GLUT4 translocation through the activation of AMPK signaling pathways. However, the presence of insulin potentiated the effect of resveratrol on AMPK activation and further stimulated PI3-K/Akt signaling pathway (Park et al., Citation2007; Yamashita et al., Citation2016).
In summary, the current study showed that the co-treatment of insulin and curcumin in C2C12 myotubes exerts a synergistic therapeutic effect against diabetes, which at least is partiallymediatedbytheinsulin-dependentPI3-K/Aktandtheinsulin-independentAMPKpathways. Also, curcumin improved the insulin sensitivity and up-regulated GLUT4 gene expression and glycoprotein movement from the intracellular pool to the cell membrane. Regarding the growing interests towards the AMPK activators as potential therapies for T2DM, curcumin could be a preference.
Abbreviations
| DM | = | diabetes mellitus |
| T2DM | = | type 2 diabetes mellitus, N, nuclear |
| ER | = | endoplasmic reticulum |
| IRS 1 | = | insulin receptor substrate 1 |
| PI3-kinase | = | phosphatidylinositol 3 |
| GLUT 4 | = | Glucose transferases4 |
| AMPK | = | AMP-activated protein kinase |
| TNF α | = | tumor necrosis factor alpha |
| IL-6 | = | interleukin 6 |
| MCP-1 | = | monocyte chemoattractant protein-1 |
| NF-kB | = | nuclear factor kappa B |
| CAT | = | catalase; SOD, superoxide dismutase |
| GST | = | glutathione-S-transferase; GPx, glutathione peroxidase |
| GR | = | glutathione reductase |
| JNK | = | phosphor c-Jun N-terminal kinase |
| PMSF | = | phenylmethylsulfonyl fluoride |
| SDS-PAGE | = | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| DMEM | = | Dulbecco’s Modified Eagle’s medium |
| PVDF | = | transferred to polyvinylidene fluoride |
| WB | = | western blot |
| Com.C | = | Compound C |
| EGCG | = | epigallocatechingallate |
| Ins | = | insulin |
| MTT | = | dimethylsulfoxide |
| GRP-78 | = | glucose-regulated protein-78 |
Acknowledgements
This work is supported by research grants from Iran National science foundation (INSF), so we thank the presidency of Islamic Republic of vice-presidency for Science and technology and all University authors, professors and staffs who helped us especially Professor Ali Akbar Mosavi-Movahedi for his kindly guidelines.
Additional information
Funding
Notes on contributors
Javad Mohiti-Ardekani
This work has done in our laboratory by my Ph.D student following previous work on C2C12 cells. In this study we demonstrated the current study established that co-treatment of insulin and curcuminin in C2C12 myotubes exerts synergistic therapeutic effect against diabetes, which at least is partially mediated by insulindependentPI3-K/Akt and the insulin-independent AMPK pathways. Also, curcumin improved insulin sensitivity and up-regulated GLUT4 gene expression and glycoprotein movement from the intra cell pool to the cell membrane. Hence due to the developing interest towards AMPK activators as potential therapies for T2DM, curcumin could be preference. I hope that the paper conforms to the rigorous standards of your journal, and I look forward to hearing your comments and decision concerning the publication of our paper.
References
- Absalan, A., etal (2012). C2C12 cellline is a good model to explore the effects of herbal extracts on GLUT4 expression and translocation.
- Absalan, A., Mohiti-Ardakani, J., Hadinedoushan, H., & Khalili, M. A. (2012). Hydro-Alcoholic cinnamon extract, enhances glucose transporter isotype-4 translocation from intracellular compartments into the cytoplasmic membrane of C2C12 myotubes. Indian Journal of Clinical Biochemistry, 27(4), 351–356. doi:10.1007/s12291-012-0214-y
- Ahmadipour, F., Vakili, T., & Absalan, A., et al. (2012). C2C12 cell line is a good model to explore the effects of herbal extracts on GLUT4 expression and translocation. Iranian Journal of Diabetes and Obesity, 4(4), 143-151.
- Amitani, H., Asakawa, A., Cheng, K., Amitani, M., Kaimoto, K., Nakano, M., … Sanchez-Margalet, V. (2013). Hydrogen improves glycemic control in type1 diabetic animal model by promoting glucose uptake into skeletal muscle. PloS One, 8(1), e53913. doi:10.1371/journal.pone.0053913
- Baeeri, M., Momtaz, S., Navaei-Nigjeh, M., Niaz, K., Rahimifard, M., Ghasemi-Niri, S. F., … Abdollahi, M. (2017). Molecular evidence on the protective effect of ellagic acid on phosalone-induced senescence in rat embryonic fibroblast cells. Food and Chemical Toxicology, 100, 8–23. doi:10.1016/j.fct.2016.12.008
- Brasnyó, P., Ga, M., Mohás, M., Markó, L., Laczy, B., Cseh, J., … Wittmann, I. (2011). Resveratrolimprovesinsulinsensitivity,reducesoxidativestress and activates the Akt pathway in type 2 diabetic patients. British Journal of Nutrition, 106(3), 383–389. doi:10.1017/S0007114511000316
- Burattini, S., Ferri, P., Battistelli, M., Curci, R., & Luchetti, F. (2004). E FalcieriC2C12 murine myoblasts as a model of skeletal muscle development: Morpho-functional characterization. European Journal of Histochemistry, 48(3), 213–222.
- Cheng, Z., Pang, T., Gu, M., Gao, A.-H., Xie, C.-M., Li, J.-Y., … Li, J. (2006). Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochimica Et Biophysica Acta (BBA)-General Subjects, 1760(11), 1682–1689. doi:10.1016/j.bbagen.2006.09.007
- Chuengsamarn, S., Rattanamongkolgul, S., Luechapudiporn, R., Phisalaphong, C., & Jirawatnotai, S. (2012). Curcumin extract for prevention of type 2 diabetes. Diabetes Care, 35(11), 2121–2127. doi:10.2337/dc12-0116
- DeFronzo, R., Jacot, E., Jequier, E., Maeder, E., Wahren, J., & Felber, J. P. (1981). The effect of insulin on the disposal of intravenous glucose: Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes, 30(12), 1000–1007.
- Dugani, C. B., & Klip, A. (2005). Glucose transporter 4: Cycling, compartments and controversies: Third in the cycles review series. EMBO Reports, 6(12), 1137–1142. doi:10.1038/sj.embor.7400584
- El-Moselhy, M. A., Taye, A., Sharkawi, S. S., El-Sisi, S. F. I., & Ahmed, A. F. (2011). The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-α and free fatty acids. Food and Chemical Toxicology, 49(5), 1129–1140. doi:10.1016/j.fct.2011.02.004
- Gupta, S. K., Kumar, B., Nag, T. C., Agrawal, S. S., Agrawal, R., Agrawal, P., … Srivastava, S. (2011). Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. Journal of Ocular Pharmacology and Therapeutics, 27(2), 123–130. doi:10.1089/jop.2010.0123
- Hwang, Y. P., Kim, H. G., Choi, J. H., Do, M. T., Chung, Y. C., Jeong, T. C., & Jeong, H. G. (2013). S-allyl cysteine attenuates free fatty acid-induced lipogenesis in human HepG2 cells through activation of the AMP-activated protein kinase-dependent pathway. The Journal of Nutritional Biochemistry, 24(8), 1469–1478. doi:10.1016/j.jnutbio.2012.12.006
- Jain, S. K., Rains, J., Croad, J., Larson, B., & Jones, K. (2009). Curcumin supplementation lowers TNF-α, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-α, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxidants & Redox Signaling, 11(2), 241–249. doi:10.1089/ars.2008.2140
- Jarvill-Taylor, K. J., Anderson, R. A., & Graves, D. J. (2001). A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. Journal of the American College of Nutrition, 20(4), 327–336.
- Jung, K. H., Choi, H. S., Kim, D. H., Han, M. Y., Chang, U. J., Yim, S.-V., … Kang, S. A. (2008). Epigallocatechin gallate stimulates glucose uptake through the phosphatidylinositol3-kinase-mediatedpathwayinL6ratskeletalmusclecells. Journal of Medicinal Food, 11(3), 429–434. doi:10.1089/jmf.2007.0107
- Kang, C., Jin, Y. B., Lee, H., Cha, M., Sohn, E.-T., Moon, J., … Kim, E. (2010). Brown alga Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt signaling pathways. Food and Chemical Toxicology, 48(2), 509–516. doi:10.1016/j.fct.2009.11.004
- Kang, C., & Kim, E. (2010). Synergisticeffectofcurcuminandinsulinonmusclecellglucosemetabolism. Foodand Chemical Toxicology, 48(8–9), 2366–2373. doi:10.1016/j.fct.2010.05.073
- Kang, C., Lee, H., Jung, E.-S., et al. (2012). Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food chemistry, 135(4), 2350–2358.
- Kim, J. H., Park, J. M., Kim, E.-K., Lee, J. O., Lee, S. K., Jung, J. H., … Kim, H. S. (2010). Curcumin stimulates glucose uptake through AMPK‐p38 MAPK pathways in L6 myotube cells. Journal of Cellular Physiology, 223(3), 771–778. doi:10.1002/jcp.22093
- Kim, N., Lee, J. O., Lee, H. J., Lee, Y. W., Kim, H. I., Kim, S. J., … Kim, H. S. (2016). AMPK, a metabolic sensor, is involved in isoeugenol-induced glucose uptake in muscle cells. Journal of Endocrinology, 228(2), 105–114. doi:10.1530/JOE-15-0302
- Koistinen, H. A., & Zierath, J. (2002). Regulation of glucose transport in human skeletal muscle. Annals of Medicine, 34(6), 410–418.
- Koumanov, F., Jin, B., Yang, J., & Holman, G. D. (2005). Insulin signaling meets vesicle traffic of GLUT4 at a plasma- membrane-activated fusion step. Cell Metabolism, 2(3), 179–189. doi:10.1016/j.cmet.2005.08.007
- Kovacic, S., Soltys, C.-L. M., Barr, A. J., Shiojima, I., Walsh, K., & Dyck, J. R. B. (2003). Akt activity negatively regulates phosphorylation ofAMPactivatedproteinkinaseintheheart. Journal of Biological Chemistry, 278(41), 39422–39427. doi:10.1074/jbc.M305371200
- Kurimoto, Y., Shibayama, Y., Inoue, S., Soga, M., Takikawa, M., Ito, C., … Tsuda, T. (2013). Blacksoybeanseedcoatextractameliorateshyperglycemiaand insulin sensitivity via the activation of AMP-activated protein kinase in diabetic mice. Journal of Agricultural and Food Chemistry, 61(23), 5558–5564. doi:10.1021/jf401190y
- Lee, W. J., Song, K.-H., Koh, E. H., Won, J. C., Kim, H. S., Park, H.-S., … Park, J.-Y. (2005). α-Lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochemical and Biophysical Research Communications, 332(3), 885–891. doi:10.1016/j.bbrc.2005.05.035
- Lee, Y. S., Kim, W. S., Kim, K. H., Yoon, M. J., Cho, H. J., Shen, Y., … Kim, J. B. (2006). Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes, 55(8), 2256–2264. doi:10.2337/db06-0006
- Liu, Q., Chen, L., Hu, L., et al. (2010). Small molecules from natural sources, targeting signaling pathways indiabetes.Biochimicaet BiophysicaActa(BBA)-Gene Regulatory Mechanisms, 1799(10), 854–865.
- Maheshwari, R. K., Singh, A. K., Gaddipati, J., & Srimal, R. C. (2006). Multiplebiologicalactivitiesofcurcumin: Ashortreview. Life Sciences, 78(18), 2081–2087. doi:10.1016/j.lfs.2005.12.007
- Meghana, K., Sanjeev, G., & Ramesh, B. (2007). Curcumin prevents streptozotocin-induced islet damage by scavengingfreeradicals:Aprophylacticandprotectiverole. European Journal of Pharmacology, 577(1–3), 183–191.
- Mueckler, M. (1990). Family of glucose-transporter genes: Implications for glucose homeostasis and diabetes. Diabetes, 39(1), 6–11.
- Pari, L., & Murugan, P. (2007). Antihyperlipidemiceffectofcurcuminandtetrahydrocurcumininexperimentaltype 2 diabetic rats. Renal Failure, 29(7), 881–889. doi:10.1080/08860220701540326
- Park, C. E., Kim, M.-J., Lee, J. H., Min, B.-I., Bae, H., Choe, W., … Ha, J. (2007). Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Experimental & Molecular Medicine, 39(2), 222. doi:10.1038/emm.2007.25
- Rashid, K., Chowdhury, S., Ghosh, S., & Sil, P. C. (2017). Curcumin attenuates oxidative stress induced NFκB mediated inflammation and endoplasmic reticulum dependent apoptosis of splenocytes in diabetes. Biochemical Pharmacology, 143, 140–155. doi:10.1016/j.bcp.2017.07.009
- Russell III, R. R., Bergeron, R., Shulman, G. I., & Young, L. H. (1999). Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. American Journal of Physiology-Heart and Circulatory Physiology, 277(2), H643–H649. doi:10.1152/ajpheart.1999.277.2.H643
- Saltiel, A. R. (2001). New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell, 104(4), 517–529.
- Schmittgen, T. D., & Livak, K. J. (2008). Analyzing real-time PCR data by the comparative C T method. Nature Protocols, 3(6), 1101. doi:10.1038/nprot.2008.73
- Shao, W., Yu, Z., Chiang, Y., Yang, Y., Chai, T., Foltz, W., … Schneider-Stock, R. (2012). Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PloS One, 7(1), e28784. doi:10.1371/journal.pone.0028784
- Smith, A., & Muscat, G. (2005). Skeletal muscle and nuclear hormone receptors: Implications for cardiovascular and metabolic disease. International Journal of Biochemistry and Cell Biology, 37, 2047–2063. doi:10.1016/j.biocel.2005.03.002
- Tortorella, L. L., & Pilch, P. F. (2002). Dexamethasone markedly induces glut4 expression in C2c12 Myocytes without concomitant formation of an intracellular, insulin responsive vesicular compartment. Diabetes, 51, A312.
- Watson, R. T., & Pessin, J. E. (2001). Intracellular organization of insulin signaling and GLUT4 translocation. Recent Progress in Hormone Research, 56, 175–193.
- Yamashita, Y., M., et al. (2012). Cacao liquor procyanidin extract improves glucose tolerance by enhancing GLUT4 translocation and glucose uptake in skeletal muscle. Journal of Nutritional Science, May 31;1:e2.1.
- Yamashita, Y., Wang, L., Nanba, F., Ito, C., Toda, T., Ashida, H., & Kanzaki, M. (2016). Procyanidin promotes translocation of glucose transporter 4 in muscle of mice through activation of insulin and AMPK signaling pathways. PLoS One, 11(9), e0161704. doi:10.1371/journal.pone.0161704
- Zhang, Z., Li, Q., Liang, J., Dai, X. Q., Ding, Y., Wang, J. B., & Li, Y. (2010). Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phyto Medicine, 17(1), 14–18. doi:10.1016/j.phymed.2009.09.007