?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This 125-day experiment evaluated the growth of adult rainbow trout (Oncorhynchus mykiss) fed one of three isonitrogenous and isocaloric diets (46% protein, 16% lipid). Fish meal was the primary protein source for the reference diet, which was compared to two other diets where bioprocessed soybean meal replaced 60% or 80% of the dietary fish meal. At the end of the experiment, there were no significant differences in gain, percent gain, feed conversion ratio, or specific growth rate among the dietary treatments. There were also no significant differences in intestinal morphology, splenosomatic index, hepatosomatic index, and viscerosomatic index among the diets. Based on these results, bioprocessed soybean meal can replace at least 80% of the fish meal in adult rainbow trout diets.
PUBLIC INTEREST STATEMENT
Aquaculture is rapidly growing to feed the increasing number of people in the world. Many farmed fish diets have contained large amounts of fish meal, which is primarily made from small ocean fish like anchovies or menhaden. Because fish meal supplies are limited, feed for some farmed fish can become expensive and limiting. Soybean meal is much more abundant and sustainable than fish meal, but soybean meal cannot be digested by carnivorous fish like trout and salmon. By-processing, such as fermentation, can make soybeans a better food source for fish. This study showed that bioprocessed soybean meal can replace almost all of the fish meal in the diet of rainbow trout.
Competing Interests
The authors declare no competing interests.
1. Introduction
Plant-based proteins, such as soybeans (Glycine max), have been extensively researched as alternatives to dietary fish meal (Gatlin III et al., Citation2007; Li & Robinson, Citation2015; Nordrum, Bakke-McKellep, Krogdahl, & Buddington, Citation2000). Alternative protein sources are needed due to the exponential growth of aquaculture without a corresponding increase in sources of fish meal, which is primarily made from small pelagic fish (Food and Agriculture Organization of the United Nations (FAO), Citation2016). Thus, there needs to be other suitable and cost-effective protein sources to replace dietary fish meal.
Soybean products are one of the leading alternatives to fish meal in aquaculture diets (Li & Robinson, Citation2015; Nordrum et al., Citation2000). Soybeans are highly palatable (Refstie et al., Citation2000; Sugiura, Dong, Rathbone, & Hardy, Citation1998; Watanabe, Citation2002), high in protein, and have a balanced amino acid profile (Gatlin III et al., Citation2007; National Research Council (NRC), Citation2011). However, soybeans also have antinutritional factors that hinder fish digestion (Iwashita, Yamamoto, & Furuita et al., Citation2008; NRC, Citation2011; Teng et al., Citation2012), and can also cause gastrointestinal issues, such as enteritis (Heikkinen et al., Citation2006; Krogdahl, Bakke-McKellep, & Roed et al., Citation2000; Krogdahl et al., Citation2015; Refstie et al., Citation2000). Soybeans also have high levels of carbohydrates (Gatlin III et al., Citation2007; Salunkhe, Adsule, Chavan, & Kadam, Citation1992), which can be deleterious to many carnivorous fish species (NRC, Citation2011). These antinutritional factors and carbohydrates limit the dietary inclusion levels of soybean products for many carnivorous species (Fowler, Citation1980; NRC, Citation2011; Vielma, Makinen, Ekholm, & Koskela, Citation2000).
Nevertheless, there are ways to decrease or eliminate the antinutritional factors in soybeans. Heat occurring during the feed-extrusion process decreases lectins and proteinase inhibitors (Bakke et al., Citation2011; Barrows, Stone, & Hardy, Citation2007; Gatlin III et al., Citation2007; Krogdahl, Penn, Thorsen, Refstie, & Bakke, Citation2010). Saponins, sterols, and oligosaccharides can be decreased by alcohol extraction (Krogdahl et al., Citation2010). Bioprocessing, or fermentation, has also been shown to eliminate or reduce many antinutritional factors (Hong, Lee, & Kim, Citation2004; Refstie, Sahlstrom, Brathen, Baeverfjord, & Krogedal, Citation2005; Yamamoto et al., Citation2010, Citation2012).
Only a limited number of studies have examined soybean meal bioprocessed by fermentation (Barnes, Brown, Bruce, Neiger, & Sindelar, Citation2015b; Barnes, Brown, Bruce, Sindelar, & Neiger, Citation2014; Barnes, Brown, & Neiger, Citation2015a; Barnes, Brown, & Rosentrater et al., Citation2012; Barnes, Brown, Rosentrater, & Sewell, Citation2013, Moniruzzaman et al., Citation2018; Yamamoto et al., Citation2010, Citation2012) or other methods (Bruce, Neiger, & Brown, Citation2017a; Bruce, Sindelar, Voorhees, Brown, & Barnes, Citation2017b) in rainbow trout (Oncorhynchus mykiss) diets. The objective of this study was to examine the effects of a novel bioprocessed soybean meal (BSM) on the rearing performance of rainbow trout.
2. Methods
This feed trial was conducted indoors at McNenny State Fish Hatchery, Spearfish, South Dakota, using degassed and aerated well water at a constant temperature of 11° C (total hardness as CaCO3, 360 mg/L; alkalinity as CaCO3, 210 mg/L; pH, 7.6; total dissolved solids, 390 mg/L) in a single-pass, flow-through system.
Seventy-two Erwin x Arlee strain rainbow trout (initial weight 130.7 ± 4.2 g, length 213.2 ± 2.0 mm, mean ± SE) were randomly selected and stocked into one of 12 semi-circular fiberglass tanks (190-L) on 15 June 2016, at six fish per tank (n = 4). Flow rates were kept constant throughout the 125-day study.
Three different diets were used (Table ), with a modified soybean meal replacing 0%, 60%, or 80% of the fish meal as the primary protein source. The modified soybean meal was produced using a proprietary microbial conversion process (SDSU, Brookings, SD, USA). All of the diets were isocaloric and isonitrogenous, and were all manufactured by cooking extrusion (ExtruTech model 325, Sabetha, KS) at Prairie Aquatech (Brookings, SD). Feed was analyzed according to Association of Official Analytical Chemists (AOAC) (Citation2009) method 2001.11 for protein, 2003.5 (modified by substituting petroleum ether for diethyl ether) for crude lipid, and American Association of Cereal Chemists (AACC) (Citation2000) method 08–03 for ash content.
Table 1. Diet formulation and composition analyses of the diets used in the 125-day trial. Analysis conducted on post-extrusion feed pellets
The individual fish weights were combined to obtain total tank weight. Fish were individually weighed and measured approximately every four weeks. Weight gain, percent gain, feed conversion ratio (FCR), and specific growth rate (SGR) were calculated for each individual tank. Individual fish weights and total lengths were used to calculate Fulton’s condition factor (K).
Fish were fed by hand daily, except on the days they were weighed and measured (days 30, 61, 92, and 125). Feeding amounts were initially determined by the hatchery constant method (Butterbaugh & Willoughby, Citation1967), with planned feed conversion rates of 1.1 and maximum growth rate of 0.08 cm/day, which was based on historical maximum growth rate of Erwin x Arlee strain rainbow trout at McNenny State Fish Hatchery (Barnes et al., Citation2011), and then adjusted daily to be at or near satiation. Total feed fed and mortalities were recorded daily.
To collect weight and length data on 30-day intervals, the fish were anesthetized using 60 mg/L MS-222 (Tricaine-S, tricaine methanesulfonate, Syndel USA, Ferndale, Washington). On day 125, fish were euthanized using a lethal dose of 250 mg/L MS-222 (American Veterinary Medical Association (AVMA), Citation2013). In addition to weight and length measurements, fin lengths to the nearest 1.0 mm, and organ (spleen, liver, and visceral) weights to the nearest 1.0 mg, were recorded from three randomly selected trout per tank. Fin indices, hepatosomatic index (HSI) (Strange, Citation1996), splenosomatic index (SSI) (Goede & Barton, Citation1990), and viscerosomatic index (VSI) (Goede & Barton, Citation1990) were calculated for individual fish.
The following equations were used:
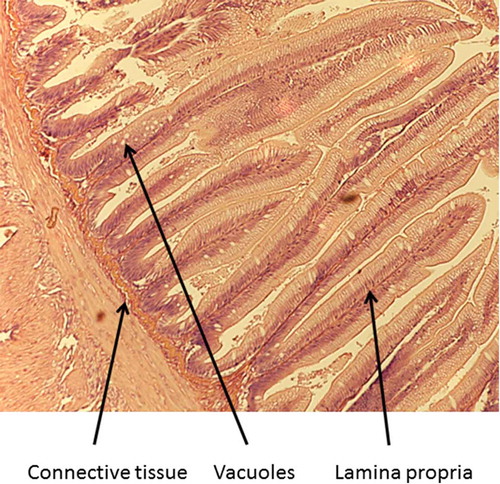
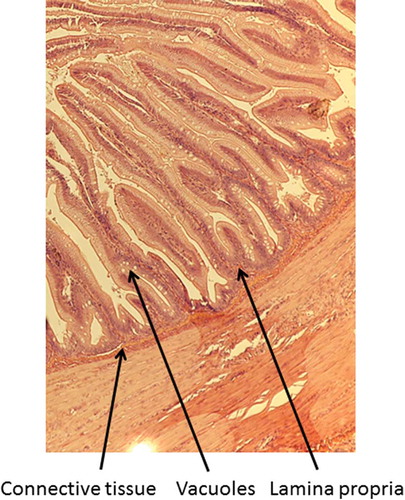
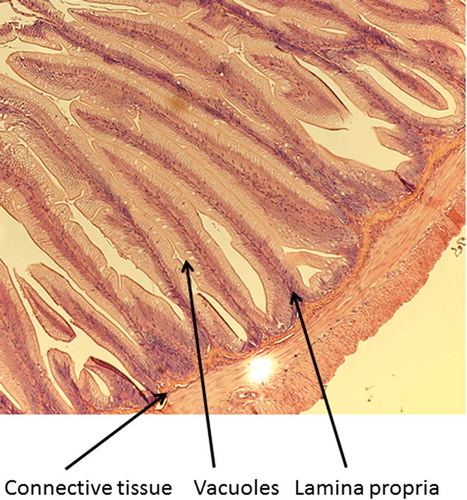
A 2-mm wide section of the distal intestine was removed from three randomly selected fish per tank to assess any soy-induced enteritis (Booman, Forster, Vederas, Groman, & Jones, Citation2018; Gu et al., Citation2017; Novriadi, Rhodes, Powell, Hanson, & Davis, Citation2017; Wang, Wang, Zhang, & Song, Citation2017). After dissection, the intestinal tissue was immediately placed in 10% buffered formalin, and stained with hematoxylin and eosin using standard histological techniques (Bureau, Harris, & Cho, Citation1998; Burrells, Williams, Southgate, & Crampton, Citation1999). Intestinal inflammation was assessed using an ordinal scoring system (Table ) based on lamina propria thickness and cellularity, submucosal connective tissue width, and leukocyte distribution (Barnes et al., Citation2014; Colburn et al., Citation2012; Knudsen, Urán, Arnous, Koppe, & Frøkiær, Citation2007).
Table 2. Histological scoring system used on rainbow trout fed fish meal or incremental amounts of bioprocessed soybean meal in diets (Barnes et al., Citation2014, modified from; Goede & Barton, Citation1990; Barton et al., Citation2002)
Data were analyzed using the SPSS (9.0) statistical analysis program (SPSS, Chicago Illinois), with significance predetermined at P < 0.05. One-way analysis of variance (ANOVA) was conducted, and if treatments were significantly different, post hoc mean separation tests were performed using Tukey’s HSD test.
3. Results
At the end of this experiment, there were no significant differences in gain, percent gain, feed fed, feed conversion ratios, specific growth rates, or percent mortality among the tanks of fish being fed the three different diets (Table ). Overall mean (± SE) feed conversion ratios were not significantly different at 1.30 (± 0.04), 1.14 (± 0.03), and 1.25 (± 0.07) for the 0%, 60%, and 80% BSM diets, respectively. There was no mortality observed in any treatments.
Table 3. Mean (± SE) gain, percent gain, food fed, feed conversion ratio (FCRa), specific growth rate (SGRb), and mortality of rainbow trout receiving fish meal or incremental levels of bioprocessed soybean meal (BSM) as the main protein ingredient. Overall means with different letters in the same row differ significantly (P< 0.05)
There were no significant differences among the diets in gain, percent gain, or SGR overall and after the first rearing period (days 31–125). However, during the first rearing period, the fish in the tanks that were fed the reference (fish meal) diet had significantly higher gain, percent gain, and SGR than the fish in the tanks receiving the 80% BSM diet, but were not significantly different than the fish receiving the 60% BSM diet. Mean (± SE) percent gain at the end of the first rearing period was 26.3 (± 1.7) %, 18.8 (± 3.3) %, and 9.9 (± 2.6) % for the fish being fed the 0%, 60%, and 80% diets, respectively.
Similarly, there were no significant differences overall in individual fish weight, length, or condition factor (Table ). However, during rearing period 3, the mean (± SE) condition factor of the fish in tanks fed the fish meal reference diet was 1.38 (± 0.01), which was significantly different from the fish in tanks being fed the 80% bioprocessed soybean meal diet at 1.28 (± 0.02). The condition factor of the fish in the tanks that were fed the 60% BSM was 1.31 (± 0.02), which was not significantly different from the other two diets.
Table 4. Mean (± SE) condition factor (Ka), fin indicesb, hepatosomatic index values (HSIc), splenosomatic index (SSId), viscerosomatic index (VSIe), and histology scores for lamina propria, connective tissue, and vacuoles of rainbow trout fed one of three diets containing either fish meal or incremental amounts of bioprocessed soybean meal (BSM) as the primary protein source. Means with different letters in the same row differ significantly (P< 0.05)
Fish receiving the 80% bioprocessed diets had significantly longer dorsal fins than those receiving the 60% diet, but were not significantly different from those fed the reference diet. No significant differences were observed among the dietary treatments for the pectoral and pelvic fin indices. There were also no significant differences in any of the organosomatic indices (HSI, SSI, and VSI), nor any of the histological scores (lamina propria, connective tissue, and vacuoles). Representative images of the distal intestines from fish fed each diet used for the scoring are shown in Figures –.
4. Discussion
The lack of significant differences in gain, percent gain, and feed conversion ratios indicates that at least 80% of the fish meal can be replaced by BSM in adult rainbow trout diets. NRC (Citation2011) states that most fish species exhibit reduced feed intake for a short period when their diets are changed. The initial rearing performance differences between the fish receiving the fish meal diet compared to the fish receiving the 80% BSM diet could be due to the relative novelty of the 80% diet. Because the pre-trial feed was a commercial diet that did not contain any BSM, the relatively large amount of soy in the 80% fish meal replacement diet likely required an acclimation period.
The overall results of this study are similar to other experiments feeding BSM to rainbow trout (Barnes et al., Citation2014, Citation2015a, Citation2012, Citation2013; Bruce et al., Citation2017a, Citation2017b; Yamamoto et al., Citation2010, Citation2012). Yamamoto et al. (Citation2010, Citation2012) noted similar results replacing 100% of the fish meal with fermented soybean meal, while Barnes et al. (Citation2012, Citation2013, Citation2014, Citation2015a) found that a maximum of approximately 70% fish meal substitution was possible without any deleterious effects. Bruce et al. (Citation2017a, Citation2017b) reported a 65% replacement of fish meal by BSM could be attained with no effect on growth. Moniruzzaman et al. (Citation2018) found only 30% of the fish meal could be replaced with fermented protein concentrates. Other species where BSM has been evaluated include: Atlantic cod (Gadus morhua) (Refstie et al., Citation2006; Ringø, Sperstad, Myklebust, Refstie, & Krogdahl, Citation2006), Atlantic salmon (Salmo salar) (Refstie et al., Citation2005), black sea bream (Acanthopagrus schlegeli) (Azarm & Lee, Citation2014; Zhou et al., Citation2011), brown trout (Salmo salar) (Sotoudeh, Moghaddam, Shahhosseini, & Aramli, Citation2016), chinese sucker (Myxocyprinus asiaticus) (Yuan et al., Citation2012), Florida pompano (Trachniotus carolinus) (Novriadi et al., Citation2017), gilthead sea bream (Sparus aurata L.) (Kokou, Rigos, Henry, Kentouri, & Alexis, Citation2012), Japanese flounder (Paralichthys olivaceus) (Kader et al., Citation2012), largemouth bass (Micropterus salmoides) (Jiang et al., Citation2018), orange-spotted grouper (Epinephelus coioides) (Shiu et al., Citation2015), olive flounder (Paralichthys olivaceus) (Seong et al., Citation2018), rockfish (Sebastes schlegeli) (Lee, Mohammadi Azarm, & Chang, Citation2016), white seabass (Atractosion nobilis) (Trushenski, Rombenso, Page, Jirsa, & Drawbridge, Citation2014), whiteleg shrimp (Litopenaeus vannamei) (Chiu et al., Citation2016; Van Nguyen, Hoang, Khanh, Duy Hai, & Hung, Citation2018), and yellowtail jack (Seriola lalandi) (Trushenski et al., Citation2014).
At 125 days, this experiment should have lasted long enough to determine any differences in fish rearing performance among the diets (Weathercup & McCraken, Citation1999). NRC (Citation2011) recommends feed trial durations of 56–84 days, with larger fish attaining at least a 200–300% gain. This experiment provided fish weight gains of approximately 250%, thereby meeting both requirements.
The FCR observed in this experiment was slightly higher than that reported in some other experiments involving rainbow trout (Barnes et al., Citation2012, Citation2013), but were also similar to other studies (Barnes et al., Citation2014, Citation2015a; Bruce et al., Citation2017b; Yamamoto et al., Citation2010, Citation2012). The SGR was slightly lower in this experiment (0.9–1.0) compared to the 1.0 to 1.3 reported by Bruce et al. (Citation2017b) in a similar study, but were extremely low compared to 1.8 to 3.0 reported by Yamamoto et al. (Citation2010, Citation2012) and Bruce et al. (Citation2017a). The slower growth rate could possibly be due to the size of the fish or water temperatures differences. Yamamoto et al. (Citation2010, Citation2012) and Bruce et al. (Citation2017a, Citation2017b) used juvenile fish, while this study used adult rainbow trout, which have slower growth rates (Stickney, Citation1994).
The condition factors observed in this experiment were higher than many other rainbow trout experiments (Barnes et al., Citation2012, CitationCitation2013, Citation2014, Citation2015a, 2015b; Bruce et al., Citation2017b). This could possibly be because the fish in this experiment were older and larger, and closer to sexual maturity (Barton, Morgan, & Vijayan, Citation2002).
Relative fin length can be influenced by several factors, including tank-induced abrasions (Bosakowski & Wagner, Citation1995), rearing unit size and type (Bosakowski & Wagner, Citation1994), aggressive behavior (Latremouille, Citation2003), feeding rates (Wagner, Intelmann, & Routledge, Citation1996), rearing densities (Miller, Wagner, & Bosakowski, Citation1995; North et al., Citation2006; Wagner, Jeppsen, Arndt, Routledge, & Bradwisch, Citation1997), dietary nutritional differences (Kindschi, Shaw, & Bruhn, Citation1991; Lemm, Rottiers, Dropkin, & Dennison, Citation1988), environmental stress (Latremouille, Citation2003), and fish health (Devesa, Barja, & Toranzo, Citation1989). The lack of difference between the pectoral and pelvic fin indices in this experiment could be attributed to similar environmental stressors and adequate feeding rates. However, the significant difference in dorsal fin relative length between the 60% and 80% BSM may not be biologically different, as both lengths are normal for rainbow trout (Arndt, Routledge, & Wagner et al., Citation2001, Citation2002). Kindschi et al. (Citation1991) found a significant difference in the dorsal fin measurement of steelhead trout fed diets containing either menhaden or herring oil. The overall pectoral fin values observed in this experiment are similar to those reported by Parker and Barnes (Citation2015).
The lack of any differences in HSI between the dietary treatments indicates similar energy partitioning. HSI is an indirect measure of glycogen and carbohydrate levels, and can be used to indicate the nutritional state of the fish (Barton et al., Citation2002; Daniels & Robinson, Citation1986; Kim & Kaushik, Citation1992). The HSI levels observed in this study were similar to those reported by Barnes et al. (Citation2013, Citation2014, Citation2015b), and slightly higher than those reported by Yamamoto et al. (Citation2010, Citation2012), Barnes et al. (Citation2012), and Bruce et al. (Citation2017a). Differences in HSI among the studies could be related to fish age. Barton et al. (Citation2002) noted that organosomatic indices can vary depending on life stage, and the rainbow trout used in this study were much larger and older than those used in other experiments.
The VSI indicates how lipids are being used or partitioned with VSI and lipids positively related (Company, Calduch-Giner, Kaushik, & Pérez-Sánchez, Citation1999; Jobling, Koskela, & Savolainen, Citation1998; Yildiz, Sener, & Timur, Citation2006). Thus, similar VSI values among the dietary treatments are likely due to similar dietary lipid levels. At 13.0 to 13.7 the VSI values are similar to the 12.0 to 13.8 values reported by Barnes et al. (Citation2014, Citation2015a), but higher than those reported by Barnes et al. (Citation2013, Citation2015b), Parker and Barnes (Citation2014, Citation2015), Kientz and Barnes (Citation2016), and Bruce et al. (Citation2017a).
SSI measures both immune status and hematopoietic capacity of fish (Barton et al., Citation2002; Smith, Citation1991). Similar SSI values indicate that fish health was likely unaffected by dietary treatment. The SSI values observed were within the range reported by other studies (Bruce et al., Citation2017b; Kientz & Barnes, Citation2016; Parker & Barnes, Citation2015).
Enteritis was not observed in this study, despite the well-documented and potentially negative effects of soybean products to the distal intestine of rainbow trout (Romarheim et al., Citation2008; Merrifield, Dimitroglou, Bradley, Baker, & Davies, Citation2009; Sealey, Barrows, Smith, Overturf, & LaPatra, Citation2009). The BSM used in this study likely decreased or eliminated the saponins (Krogdahl et al., Citation2015) and other antinutritional factors responsible for such enteritis (Barnes et al., Citation2012, Citation2013; Yamamoto et al., Citation2010, Citation2012). The absolute intestinal scores observed in this study tended to be lower than those reported by Barnes et al. (Citation2014, Citation2015a, Citation2015b) for rainbow trout fed different fermented soybean meal diets. This could be due to the dietary differences among the studies or scoring difference between readers.
In conclusion, this study indicates that at least 80% of the dietary fish meal can be directly replaced by BSM in diets of adult rainbow trout. It is unknown if the suitability of dietary BSM extends further during the trout life cycle, prior to spawning. Additional research is needed to determine if this BSM can replace all of the fish meal in adult rainbow trout diets.
Abbreviations: bioprocessed soybean meal (BSM), feed conversion ratio (FCR), specific growth rate (SGR), centimeter (cm), millimeter (mm), milligram (mg), gram (g), kilogram (kg), degree Celsius (°C), Fulton’s condition factor (K), standard error (SE), analysis of variance (ANOVA), hepatosomatic index (HIS), splenosomatic index (SSI), viscerosomatic index (VSI), second (s), liter (L), parts per million (ppm), Calcium Carbonate (CaCO3), tricaine methanesulfonate (MS-222)
Conflict of Interest and Ethical Approval
The authors state that they have no conflict of interest. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Acknowledgements
Thanks to Cody Treft, Tabor Martin, Brian Fletcher, Patrick Nero, Eric Krebs, and the numerous hatchery interns who assisted with this study. The South Dakota Cooperative Fish and Wildlife Research Unit is jointly sponsored by the US Geological Survey, South Dakota Department of Game, Fish and Parks, South Dakota State University, the Wildlife Management Institute, and the US Fish and Wildlife Service. Any use of trade names is for descriptive purposes only and does not imply endorsement by the US Government.
Additional information
Funding
Notes on contributors
Jill M. Voorhees
Jill M. Voorhees is a research biologist with the South Dakota Department of Game, Fish and Parks. She conducts aquaculture and fisheries research on numerous trout and salmon species.
Michael E. Barnes
Michael E. Barnes is the manager at McNenny State Fish Hatchery in South Dakota. He has a has a wide variety of research interests.
Steven R. Chipps
Steven R. Chipps is the Unit Leader of the U.S. Geological Survey, South Dakota Cooperative Fish and Wildlife Research Unit and Professor in the Department of Natural Resource Management at South Dakota State University where he teaches graduate classes in Aquatic Ecology and Fish Energetics.
Michael L. Brown
Michael L. Brown is a professor of fisheries science and the Wildlife & Fisheries Sciences program coordinator at South Dakota State University. His current research focus is the development of sustainable finfish feed ingredients.
References
- American Association of Cereal Chemists (AACC). (2000). Approved methods of the American Association of Cereal Chemists (10th ed.). St. Paul Minnesota: American Association of Cereal Chemists.
- American Veterinary Medical Association (AVMA). (2013). AVMA guidelines for the euthanasia of animals: 2013 edn. Schaumburg, Illinois: American Veterinary Medical Association.
- Arndt, R. E., Routledge, M. D., Wagner, E. J., et al. (2001). Influence of raceway substrate and design on fin erosion and hatchery performance of rainbow trout. North American Journal Of Aquaculture, 63, 312–15. doi:10.1577/1548-8454(2001)063<0312:IORSAD>2.0.CO;2
- Arndt, R. E., Routledge, M. D., Wagner, E. J., et al. (2002). The use of AquaMats to enhance growth and improve fin condition among raceway cultured rainbow trout Oncorhynchus mykiss (Walbaum). Aquaculture Research, 33, 359–367. doi:10.1046/j.1365-2109.2002.00670.x
- Association of Official Analytical Chemists (AOAC). (2009). Official methods of analysis. Gaithersburg Maryland. Retrieved from http://www.eoma.aoac.org/
- Azarm, H. M., & Lee, S.-M. (2014). Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, amino acid and biochemical parameters of juvenile black sea bream Acanthopagrus schlegeli. Aquaculture Research, 45, 994–1003. doi:10.1111/are.12040
- Bakke, A. M. (2011). Pathophysiological and immunological characteristics of soybean meal-induced enteropathy in salmon: Contribution of recent molecular investigations. In Cruz-Suárex L. E., Ricque-Marie D., Tapia-Salazar M., Nieto-López M. G., Villarreal-Cavazos D. A., Gamboa-Delgado J., Hernández-Hernández L. (Eds.) Proceedings of International Symposium Aquaculture Nutrition XI (pp. 345–372). Monterrey, Mexico.
- Barnes, M. E., Brown, M. L., Bruce, T., Sindelar, S., & Neiger, R. (2014). Rainbow trout rearing performance, intestinal morphology, and immune response after long-term feeding of high levels of fermented soybean meal. The North American Journal Aquace, 76, 333–345. doi:10.1080/15222055.2014.920748
- Barnes, M. E., Brown, M. L., Bruce, T. J., Neiger, R., & Sindelar, S. (2015b). Effects of fermented soybean meal diet on rainbow trout mortality and immune function during a disease outbreak. Journal of Aquaculture Feed Science and Nutrition, 7, 6–15. doi:10.3923/joafsnu.2015.6.15
- Barnes, M. E., Brown, M. L., & Neiger, R. (2015a). Comparative performance of two rainbow trout strains fed fermented soybean meal. Aquaculture International, 23, 1227–1238. doi:10.1007/s10499-015-9879-6
- Barnes, M. E., Brown, M. L., Rosentrater, K. A., et al. (2012). An initial investigation replacing fish meal with a commercial fermented soybean meal product in the diets of juvenile rainbow trout. Veterinary and Animal Science, 2, 234–243. doi:10.4236/ojas.2012.24033
- Barnes, M. E., Brown, M. L., Rosentrater, K. A., & Sewell, J. R. (2013). Preliminary evaluation of rainbow trout diets containing PepSoyGen, a fermented soybean meal product, and additional amino acids. The Open Fish Science Journal, 6, 19–27. doi:10.2174/1874401X01306010019
- Barnes, M. E., Wintersteen, K., Krebs, E., Nero, P., Tycz, J., Reichert, S., & Zimmerman, S. (2011). 2010 McNenny state fish hatchery annual production report. South Dakota Game, Fish and Parks Annual Report 11-03. Pierre, South Dakota.
- Barrows, F. T., Stone, D. A. J., & Hardy, R. W. (2007). The effects of extrusion conditions on the nutritional value of soybean meal for rainbow trout (Oncorhynchus mykiss). Aquaculture, 265, 244–252. doi:10.1016/j.aquaculture.2007.01.017
- Barton, B. A., Morgan, J. D., & Vijayan, M. M. (2002). Physiological and condition-related indicators of environmental stress in fish. In S. M. Adams (Ed.), Biological indicators of aquatic ecosystem stress (pp. 111–148). Bethesda Maryland: American Fisheries Society.
- Booman, M., Forster, I., Vederas, J. C., Groman, D. B., & Jones, S. R. M. (2018). Soybean meal-induced enteritis in Atlantic salmon (Salmo salar) and Chinook salmon (Oncorhynchus tshawytscha) but not in pink salmon (O. gorbuscha). Aquaculture, 483, 238–243. doi:10.1016/j.aquaculture.2017.10.025
- Bosakowski, T., & Wagner, E. J. (1994). Assessment of fish erosion by comparison of relative fin length in hatchery and wild trout in Utah. Canadian Journal of Fisheries and Aquatic, 51, 636–641. doi:10.1139/f94-064
- Bosakowski, T., & Wagner, E. J. (1995). Experimental use of cobble substrates in concrete raceways for improving fin condition of cutthroat (Oncorhynchus clarki) and rainbow trout (O. mykiss).. Aquaculture, 130, 159–165. doi:10.1016/0044-8486(94)00223-B
- Bruce, T. J., Neiger, R. D., & Brown, M. L. (2017a). Gut histology, immunology and the intestinal microbiota of rainbow trout, Oncorhynchus mykiss (Walbaum), fed process variants of soybean meal. Aquaculture Research, 2017, 1–13. doi:10.1111/are.13480
- Bruce, T. J., Sindelar, S. C., Voorhees, J. M., Brown, M. L., & Barnes, M. E. (2017b). Performance and immunological responses of rainbow trout (Oncorhynchus mykiss) fed bioprocessed plant-based proteins. Aquaculture Nutrition, 23, 1160–1168. doi:10.1111/anu.12485
- Bureau, D. P., Harris, A. M., & Cho, C. Y. (1998). The effects of purified alcohol extracts from soy products on feed intake and growth of chinook salmon (Oncorhynchus tshawytscha) and rainbow trout (Oncorhynchus mykiss). Aquaculture, 72, 27–43. doi:10.1016/S0044-8486(97)00254-8
- Burrells, C., Williams, P. D., Southgate, P. J., & Crampton, V. O. (1999). Immunological, physiological and pathological responses of rainbow trout (Oncorhynchus mykiss) to increasing dietary concentrations of soybean proteins. Veterinary Immunology and Immunopathology, 72, 277–288. doi:10.1016/S0165-2427(99)00143-9
- Butterbaugh, G. L., & Willoughby, H. (1967). A feeding guide for brook, brown and rainbow trout. The Progressive Fish-Culturist, 29, 210–215. doi:10.1577/1548-8640(1967)29[210:AFGFBB]2.0.CO;2
- Chiu, S.-T., Wong, S.-L., Shiu, Y.-L., Chiu, C.-H., Guei, W.-C., & Liu, C.-H. (2016). Using a fermented mixture of soybean meal and earthworm meal to replace fish meal in the diet of white shrimp, Penaeus vannamei (Boone). Aquaculture Research, 47, 3489–3500. doi:10.1111/are.12799
- Colburn, H. R., Walker, A. B., Breton, T. S., Stilwell, J. M., Sidor, I. F., Gannam, A. L., & Berlinsky, D. L. (2012). Partial replacement of fishmeal with soybean meal and soy protein concentrate in diets of Atlantic cod. North American Journal Of Aquaculture, 74, 330–337. doi:10.1080/15222055.2012.676008
- Company, R., Calduch-Giner, J. A., Kaushik, S., & Pérez-Sánchez, J. (1999). Growth performance and adiposity in gilthead sea bream (Sparus aurata): Risks and benefits of high energy diets. Aquaculture, 171, 279–292. doi:10.1016/S0044-8489(98)00495-5
- Daniels, W. H., & Robinson, E. H. (1986). Protein and energy requirements of juvenile red drum (Sciaenops ocellatus). Aquaculture, 53, 243–252. doi:10.1016/0044-8486(86)90354-6
- Devesa, S., Barja, J. L., & Toranzo, A. E. (1989). Ulcerative skin and fin lesions in reared Turbot, Scopthalmus maximus (L.). Journal of Fish Diseases, 12, 323–333. doi:10.1111/j.1365-2761.1989.tb00321.x
- Food and Agriculture Organization of the United Nations (FAO). (2016). The state of world fisheries and aquaculture 2016: Contributing to food security and nutrition for all. Rome Italy: Food and Agriculture Organization. Retrieved from http://www.fao.org/3/a-i5555e.pdf
- Fowler, L. G. (1980). Substitution of soybean and cottonseed products for fish meal in diets fed to chinook and coho salmon. The Progressive Fish-Culturist, 42, 87–91. doi:10.1577/1548-8659(1980)42[87:SOSACP]2.0.CO;2
- Gatlin III, D. M., Barrows, F. T., Brown, P., Dabrowski, K., Gaylord, T. G., Hardy, R. W., … Wurtele, E. (2007). Expanding the utilization of sustainable plant products: A review. Aquaculture Research, 38, 551–579. doi:10.1111/j.1365-2109.2007.01704.x
- Goede, R. W., & Barton, B. A. (1990). Organismic indices and an autopsy-based assessment as indicators of health and condition in fish. In S. M. Adam Symposium 8 (Ed.), Biological indicators of stress in fish (pp. 93–108). Bethesda Maryland: American Fisheries Society.
- Gu, M., Bai, N., Xu, B., Xu, X., Jia, Q., & Zhang, Z. (2017). Protective effect of glutamine and arginine against soybean meal-induced entreritis in juvenile turbot (Scophthalmus maximusi). Fish & Shellfish Immunology, 70, 95–105. doi:10.1016/j.fsi.2017.08.048
- Heikkinen, J., Vielma, J., Kemilainen, O., Tiirola, M., Eskelinen, P., Kiuru, T., … von Wright, A. (2006). Effects of soybean meal based diet on growth performance, gut histopathology and intestinal microbiota of juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture, 261, 259–268. doi:10.1016/j.aquaculture.2006.07.012
- Hong, K.-J., Lee, C.-H., & Kim, S. W. (2004). Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. Journal of Medicinal Food, 7, 430–435. doi:10.1089/jmf.2004.7.430
- Iwashita, Y., Yamamoto, T., Furuita, H., et al. (2008). Influence of certain soybean antinutritional factors supplemented to a casein-based semipurified diet on intestinal and liver morphology in fingerling rainbow trout Oncorhynchus mykiss. Fisheries Science: FS, 74, 1075–1082. doi:10.1111/j.1444-2906.2008.01627.x
- Jiang, Y., Zhao, P.-F., Lin, S.-M., Tang, R.-J., Chen, Y.-J., & Luo, L. (2018). Partial substitution of soybean meal with fermented soybean residue in diets for juvenile largemouth bass, Micropterus salmoides. Aquac Nutr Early View. doi:10.1111/anu.12659
- Jobling, M., Koskela, J., & Savolainen, R. (1998). Influence of dietary fat level and increased adiposity on growth and fat deposition in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture Research, 29, 601–607. doi:10.1046/j.1365-2109.1998.00251.x
- Kader, M. A., Koshio, S., Ishikawa, M., Yokoyama, S., Bulbul, M., Nguyen, B. T., … Laining, A. (2012). Can fermented soybean meal and squid by-product blend be used as fishmeal replacements for Japanese flounder (Paralichthys olivaceus)? Aquaculture Research, 43, 1427–1437. doi:10.1111/j.1365-2109.2011.02945.x
- Kientz, J. L., & Barnes, M. E. (2016). Structural complexity improves the rearing performance of rainbow trout in circular tanks. North American Journal Of Aquaculture, 78, 203–207. doi:10.1080/1522055.2016.1159629
- Kim, J. D., & Kaushik, S. J. (1992). Contributions of digestible energy from carbohydrates and estimation of protein/energy requirements for growth of rainbow trout (Oncorhynchus mykiss). Aquaculture, 106, 161–169. doi:10.1016/0044-8486(92)90200-5
- Kindschi, G. A., Shaw, H. T., & Bruhn, D. S. (1991). Effect of diet on performance, fin quality, and dorsal skin lesions in steelhead. J Appl Aquac, 1, 113–120. doi:10.1300/J028b01n01_01
- Knudsen, D., Urán, P., Arnous, A., Koppe, W., & Frøkiær, H. (2007). Saponin-containing subfractions of soybean molasses induce entritis in the distal intestine of Atlantic salmon. Journal of Agricultural and Food Chemistry, 55, 2261–2267. doi:10.1021/jf0626967
- Kokou, F., Rigos, G., Henry, M., Kentouri, M., & Alexis, M. (2012). Growth performance, feed utilization and non-specific immune response of gilthead sea bream (Sparus aurata L.) fed graded levels of a bioprocessed soybean meal. Aquaculture, 364–365, 74–81. doi:10.1016/j.aquaculture.2012.08.009
- Krogdahl, Å., Bakke-McKellep, A. M., Roed, K. H., et al. (2000). Feeding Atlantic salmon Salmo salar L. soybean products: Effects on disease resistance (furunculosis), and lysozyme and IgM levels in the intestinal mucosa. Aquaculture Nutrition, 6, 77–84. doi:10.1046/j.1365-2095.2000.00129.x
- Krogdahl, Å., Gajardo, K., Kortner, T. M., Penn, M., Gu, M., Berge, G. M., & Bakke, A. M. (2015). Soya Saponins Induce Enteritis in Atlantic Salmon (Salmo salarL.). Journal of Agricultural and Food Chemistry, 63, 3887–3902. doi:10.1021/jf506242t
- Krogdahl, Å., Penn, M., Thorsen, J., Refstie, S., & Bakke, A. M. (2010). Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquaculture Research, 41, 333–344. doi:10.1111/j.1365-2109.2009.02426.x
- Latremouille, D. N. (2003). Fin erosion in aquaculture and natural environments. Reviews in Fisheries Science & Aquaculture, 11, 315–335. doi:10.1080/10641260390255745
- Lee, S.-M., Mohammadi Azarm, H. M., & Chang, K. H. (2016). Effects of dietary inclusion of fermented soybean meal on growth, body composition, antioxidant enzyme activity and disease resistance of rockfish (Sebastes schlegeli). Aquaculture, 459, 110–116. doi:10.1016/j.aquaculture.2016.03.036
- Lemm, C. A., Rottiers, D. V., Dropkin, D. S., & Dennison, B. A. (1988). Growth, composition, and fin quality of Atalantic salmon fed different diets at seasonal temperatures in a laboratory and hatchery. U.S. Fish and Wildlife Service Biological Report 88, 1–12.
- Li, M. H., & Robinson, E. H. (2015). Complete feeds – Intensive systems. In D. A. Davis (Ed.), Feed and feeding practices in aquaculture (pp. 112–126). Sawston Waltham Maryland: Woodhead.
- Merrifield, D. L., Dimitroglou, A., Bradley, G., Baker, R. T. M., & Davies, S. J. (2009). Soybean meal alters autochthonous microbial populations, microvilli morphology and compromises intestinal enterocyte integrity of rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Fish Diseases, 32, 755–766. doi:10.1111/j.1365-2761.2009.01052.x
- Miller, S. A., Wagner, E. J., & Bosakowski, T. (1995). Performance and oxygen consumption of rainbow trout reared at two densities in raceways with oxygen supplementation. The Progressive Fish-Culturist, 57, 206–212. doi:10.1577/1548-8640(1995)057<0206:PAOCOR>2.3.CO;2
- Moniruzzaman, M., Bae, J. H., Won, S. H., Cho, S. J., Chang, K. H., & Bai, S. C. (2018). Evaluation of solid-state fermented protein concentrates as a fish meal replacer in the diets of juvenile rainbow trout, Oncorhynchus mykiss. Aquac Nutr Early View. doi:10.1111/anu.12658
- National Research Council (NRC). (2011). Nutrient requirements of fish and shirmp. Washington D. C: National Academy.
- Nordrum, S., Bakke-McKellep, A. M., Krogdahl, Å., & Buddington, R. K. (2000). Effects of soybean meal and salinity on intestinal transport of nutrients in Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology B, 125, 317–335. doi:10.1016/S0305-0491(99)00190-X
- North, B. P., Turnbull, J. F., Ellis, T., Porter, M. J., Migaud, H., Bron, J., & Bromage, N. R. (2006). The impact of stocking density on the welfare of rainbow trout (Oncorhynchus mykiss). Aquaculture, 255, 466–479. doi:10.1016/j.aquaculture.2006.01.004
- Novriadi, R., Rhodes, M., Powell, M., Hanson, T., & Davis, D. A. (2017). Effects of soybean meal replacement with fermented soybean meal on growth, serum biochemistry, and morphological condition of liver and distal intestine of Florida pompano Trachinotus carolinus. Aquac Nutr Early View, doi:10.1111/anu.12645
- Parker, T. M., & Barnes, M. E. (2014). Rearing velocity impacts on landlocked fall Chinook salmon (Oncorhynchus tshawytscha) growth, condition, and survival. Journal of Animal Science, 4, 244–252. doi:10.4236/ojas.2014.45031
- Parker, T. M., & Barnes, M. E. (2015). Effects of different water velocities on hathcery rearing performance and recovery from transportation of rainbow trout fed two different rations. Transactions of the American Fisheries Society, 144, 882–890. doi:10.1080/00028487.2015.1047533
- Refstie, S., Korsoen, O. J., Storebakken, T., Baeverfjord, G., Lein, I., & Roem, A. J. (2000). Differing nutritional responses to dietary soybean meal in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Aquaculture, 190, 49–63. doi:10.1016/S0044-8486(00)00382-3
- Refstie, S., Landsverk, T., Bakke-McKellep, A. M., Ringø, E., Sundby, A., Shearer, K. D., & Krogdahl, Å. (2006). Digestive capacity, intestinal morphology, and microflora of 1-year and 2-year old Atlantic cod (Gadus morhua) fed standard or bioprocessed soybean meal. Aquaculture, 261, 269–284. doi:10.1016/j.aquaculture.2006.07.001
- Refstie, S., Sahlstrom, S., Brathen, E., Baeverfjord, G., & Krogedal, P. (2005). Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture, 246, 331–345. doi:10.1016/j.aquaculture.2005.01.001
- Ringø, E., Sperstad, S., Myklebust, R., Refstie, S., & Krogdahl, Å. (2006). Characterisation of the microbiota associated with intestine of Atlantic cod (Gadus morhus L.) the effect of fish meal, standard soybean meal and a bioprocessed soybean meal. Aquaculture, 261, 829–841. doi:10.1016/j.aquaculture.2006.06.030
- Romarheim, O. H., Skrede, A., Gao, Y., Krogdahl, Å., Denstadli, V., Lilleeng, E., & Storebakken, T. (2008). Comparison of white flakes and toasted soybean meal partly replacing fish meal as protein source in extruded feed for rainbow trout (Oncorhynchus mykiss). Aquaculture, 256, 354–364. doi:10.1016/j.aquaculture.2006.02.006
- Salunkhe, D. K., Adsule, R. N., Chavan, J. K., & Kadam, S. S. (1992). World oil seeds: Chemistry, technology, and utilization. New York: Van Nostrand Reinhold.
- Sealey, W. M., Barrows, F. T., Smith, C. E., Overturf, K., & LaPatra, S. E. (2009). Soybean meal level and probiotics in first feeding fry diets alter the ability of rainbow trout Oncorhynchus mykiss to utilize high levels of soybean meal during grow-out. Aquaculture, 293, 195–203. doi:10.1016/j.aquaculture.2009.04.013
- Seong, M., Lee, S., Lee, S., Song, Y., Bae, J., Chang, K., & Bai, S. C. (2018). The effects of different levels of dietary fermented plant-based protein concentrate on growth, hematology, and non-specific immune responses in juvenile olive flounder, Paralichthys olivaceus. Aquaculture, 483, 196–202. doi:10.1016/j.aquaculture.2017.10.023
- Shiu, Y.-L., Hsieh, S.-L., Guei, W.-C., Tsai, Y.-T., Chiu, C.-H., & Liu, C.-H. (2015). Using Bacillus subtilis E20-fermented soybean meal as replacement for fish meal in the diet of orange-spotted grouper (Epinephelus coioides, Hamilton). Aquaculture Research, 46, 1403–1416. doi:10.1111/are.12294
- Smith, L. S. (1991). Introduction to fish physiology. Redmond Washington: Argent Laboratories.
- Sotoudeh, E., Moghaddam, J. A., Shahhosseini, G., & Aramli, M. S. (2016). Effect of dietary gamma-irradiated and fermented soybean meal on the growth performance, body composition, and digestive enzymes activity of Caspian brown trout, Salmo trutta caspius, juvenile. Journal of the World Aquaculture Society, 47, 830–842. doi:10.1111/jwas.12297
- Stickney, R. R. (1994). Principles of aquaculture. New York: Wiley & Sons.
- Strange, R. J. (1996). Field examination of fishes. In B. R. Murphy & D. W. Willis (Eds.), Fisheries Techniques (2nd ed., pp. 433–466). Bethesda Maryland: American Fisheries Society.
- Sugiura, S. H., Dong, F. M., Rathbone, C. K., & Hardy, R. W. (1998). Apparent protein digestibility and mineral availabilities in various feed ingredients for salmonid feeds. Aquaculture, 159, 177–202. doi:10.1016/S0044-8486(97)00177-4
- Teng, D., Gao, M., Yang, Y., Liu, B., Tian, Z., & Wang, J. (2012). Bio-modification of soybean meal with Bacillus subtilis or Aspergillus oryzae. Biocatalysis and Agricultural Biotechnology, 1, 32–38. doi:10.1016/j.bcab.2011.08.005
- Trushenski, J. T., Rombenso, A. N., Page, M., Jirsa, D., & Drawbridge, M. (2014). Traditional and fermented soybean meal as ingredients in feed for white seabass and yellowtail jack. North American Journal Of Aquaculture, 76, 312–322. doi:10.1080/15222000.2014.911227
- Van Nguyen, N., Hoang, L., Khanh, V., Duy Hai, P., & Hung, L. T. (2018). Utilization of fermented soybean meal for fishmeal substitution in diets of Pacific white shrimp (Litopenaeus vannamei). Aquac Nutr Early View. doi:10.1111/anu.12648
- Vielma, J., Makinen, T., Ekholm, P., & Koskela, J. (2000). Influence of dietary soy and phytase levels on performance and body composition of large rainbow trout (Oncorhynchus mykiss) and algal availability of phosphorus load. Aquaculture, 183, 349–362. doi:10.1016/S0044-8486(99)00299-9
- Wagner, E. J., Intelmann, S. S., & Routledge, D. (1996). The effect of fry rearing density on hatchery performance, fin condition, and agonistic behavior or rainbow trout (Oncorhynchus mykiss) fry. Journal of the World Aquaculture Society, 27, 264–274. doi:10.1111/j.1749-7345.1996.tb00608.x
- Wagner, E. J., Jeppsen, T., Arndt, R., Routledge, M. D., & Bradwisch, Q. (1997). Effects of rearing density upon cutthroat trout hematology, hatchery performance, fin erosion, and general health condition. The Progressive Fish-Culturist, 59, 173–187. doi:10.1577/1548-8640(1997)059<0173:EORDUC>2.3.CO;2
- Wang, Y., Wang, L., Zhang, C., & Song, K. (2017). Effects of substituting fishmeal with soybean meal on growth performance and intestinal morphology in orange-spotted grouper (Epinephelus coioides). Aquac Rep, 5, 52–57. doi:10.1016/j.aqrep.2016.12.005
- Watanabe, T. (2002). Strategies for further development of aquatic feeds. Fisheries Science: FS, 68, 242–252. doi:10.1046/j.1444-2906.2002.00418.x
- Weathercup, R. N., & McCraken, K. J. (1999). Changes in rainbow trout, Oncorhynchus mykiss (Walbaum), body composition with weight. Aquaculture, 30, 305–307. doi:10.1046/j.1365-2109.1999.00320.x
- Yamamoto, T., Iwashita, Y., Matsunari, H., Sugita, T., Furuita, H., Akimoto, A., … Suzuki, N. (2010). Influence of fermentation conditions for soybean meal in a non-fish meal diet on the growth performance and physiological condition of rainbow trout Oncorhynchus mykiss. Aquaculture, 309, 173–180. doi:10.1016/j.aquaculture.2010.09.021
- Yamamoto, T., Matsunari, H., Sugita, T., Furuita, H., Masumoto, T., Iwashita, Y., … Suzuki, N. (2012). Optimization of the supplemental essential amino acids to a fish meal-free diet based on fermented soybean meal for rainbow trout Oncorhynchus mykiss. Fisheries Science : FS, 78, 359–366. doi:10.1007/s12562-011-0456-2
- Yildiz, M., Sener, E., & Timur, M. (2006). Effect of seasonal change and different commercial feeds on proximate composition of sea bream (Sparus aurata). Turkish Journal of Fisheries and Aquatic Sciences, 6, 99–104.
- Yuan, Y. C., Lin, Y. C., Yang, H. J., Gong, Y., Gong, S. Y., & Yu, D. H. (2012). Evaluation of fermented soybean meal in the practical diets for juvenile Chinese sucker, Myxocyprinus asiaticus. Aquaculture Nutrition, 19, 74–83. doi:10.1111/j.1365-2095.2012.00939.x
- Zhou, F., Song, W., Shao, Q., Peng, X., Xiao, J., Hua, Y., … Ng, W.-K. (2011). Partial replacement of fish meal by fermented soybean meal in diets for black sea bream, Acanthopagrus schlegelii, juveniles. Journal of the World Aquaculture Society, 42, 184–197. doi:10.1111/jwas.2011.42.issue-2