Abstract
Banana is a key crop in the livelihoods of many people in the region of East and Central Africa. For so many years, the fruit has been threatened by Banana Xanthomonas Wilt (BXW) which has spread throughout the region but at different rates. The disease attacks all banana cultivars and can cause up to 100% yield losses at the farm level if effective control measures are not put in place. Despite, the impact of BXW on banana production in eastern and central Africa is not yet fully determined. Although the economic loss of banana bacterial wilt on the welfare of the farmers and the economy of the countries is not well documented, the impact of banana bacterial wilt on food security is very significant. Since banana is a key component of these farming communities, the banana production losses resulted in a significant reduction in household food security and incomes. There is, therefore, need for overviewing the impact, New research directions for sustainable management of BXW in east and central are suggested, including development of resistant banana cultivars through genetic engineering; exploring the potential use of endophytes as biological control agents; fine tune existing cultural control practices; farmer field schools; and current knowledge related to the deadly bacterial wilt disease in the region.
PUBLIC INTEREST STATEMENT
Banana is a key crop in the livelihoods of many people in the region of East and Central Africa. For so many years, the fruit has been threatened by Banana Wilt disease which has spread throughout the region but at different levels. The disease attacks all banana cultivars and can cause up to 100% yield losses at the farm level if effective control measures are not taken. The economic loss of banana bacterial wilt on the welfare of the farmers and the economy of the countries is not well documented. Since banana is a key component of these farming communities, the banana production losses resulted in a significant reduction in household food security and incomes. Therefore, need for overviewing the impact, new research directions for sustainable management of BXW in east and central Africa are suggested, including development of resistant banana cultivars through genetic engineering; exploring the potential use of endophytes as biological control agents; existing cultural control practices; and farmer field schools.
Competing Interests
The author declares no competing interests.
1. Introduction
Approximately a third of global banana production is grown in sub-Saharan Africa where the crop provides more than 25% of food energy requirements for an estimated 70 million people (Price, Citation1995). The sub-region alone produces nearly 20 million tons of bananas annually. The crop is important as a staple as well as a relatively cheap and easily produced source of minerals and vitamins, particularly vitamin A, C and B6. Although a major staple in Africa, Asia and Latin America, only 13% of bananas produced are internationally traded, indicating the fruit’s importance for domestic markets and food security. In East and Central Africa, it is a significant dietary component, ranging from about 20% of daily total food intake in Uganda up to 80% in parts of Rwanda (Lescot, Citation2013).
Furthermore, banana by-products (peels and pseudostems) provide valuable livestock fodder, especially during the prolonged droughts that affect the regions periodically. The plant has also been used for medicinal purposes (Karamura, Citation1992), for celebrating marriage and for other rituals. Virtually all components of the plant have found a variety of uses in the homesteads and domestic industries, including basket-making, carpets, shoes and a host of indoor decorations (Karamura, Citation1992).
Bananas also play an important environmental role within farming systems by combating soil erosion on steep hills and preserving long-term fertility of the soil. The crop’s canopy also provides protection for other crops that are often inter-cropped with it, such as beans, groundnuts, cucurbits and coffee. Once established as a sole crop, bananas enter a phase of continuous growth, creating a “tropical forest” with beneficial microclimatic effects to the soil. Gardens as old as 150 years are common in Uganda (Tothill, Citation1940).
However, the banana crop is threatened by Xanthomonas wilt (BXW), caused by the bacterium Xanthomonas campestris pv. musacearum (Xcm) in East and Central Africa (ECA). BXW was formally only reported in Ethiopia on both banana (Musa spp.) and Enset (Ensete ventricosum) (Dagnachew & Bradbury, Citation1968). Since its first report in the region in 2001, BXW is now recorded from Uganda, eastern Democratic Republic of Congo, western Kenya, northwest and western Tanzania, the whole of Rwanda and Burundi, with yield losses of up to 80–100% in many sites .
Movement of infected banana material by farmers leads to rapid spread to previously uninfected areas. Sudden outbreaks in old or isolated plantations suggest the bacterium is also air or insect-borne (Ndungo, Bakelana, Eden-Green, & Blomme, Citation2004). Once these pathogens have become established, disease control is very difficult. In east and central Africa, there are cases where the disease has been eradicated using cultural control methods while the disease has reached endemic proportions in other cases (Tinzaara, 2009). There are also areas where the disease has been brought under control only for it to resurge some months later. The adoption of control technologies (debudding, use of clean tools, uprooting diseased plants and use of clean planting material) by farmers in ECA has generally been low, thus limiting the potential for sustainable control of the disease. Inadequate knowledge and sensitization of stakeholders with respect to disease diagnosis, spread and control has been identified as one of the major challenges.
Despite the developing strategies of disease management, the impact of BXW on banana production in eastern and central Africa is not yet fully organized. Although the economic loss of BXW on the welfare of the farmers and the economy of the countries is not well documented. In addition to this paper discussed efforts aimed at generation of information on fine tuning cultural control options and development of resistant cultivars. With this, the objective of this paper is to review banana bacterial wilt distribution, importance, and management techniques in east and central Africa.
2. Literature review
2.1. Causal agents of bacterial wilt diseases
Ralstonia solanacearum, the causal agent of bacterial wilt, is currently found on all continents and numerous islands located between the tropics of Cancer and Capricorn, causing disease on more than 200 plant species in over 50 families (Buddenhagen, Citation2009). According to (Mansfield et al., Citation2012) R. solanacearum is considered as one of the world’s most important/damaging phytopathogenic bacteria due to its lethality, broad geographic distribution and wide host range. In reference to the high geographic and pathogenic diversity of the species, there are many bacterial wilts and there are many “Pseudomonas solanacearums” (syn. R. solanacearum). They have originated and evolved in widely different places, and they have different capabilities with both native flora and introduced hosts and presumably with different soils and environmental conditions. This diversity results in variable disease expression and disease potentials for each host/parasite genotype interaction (Buddenhagen, Citation2009).
Bacterial diseases of banana and enset can be classified into three distinct groups: i) Ralstonia-associated diseases (Moko/Bugtok disease caused by Ralstonia solanacearum and banana blood disease caused by R. syzygii subsp. celebesensis); ii) Xanthomonas wilt of banana and enset, caused by Xanthomonas campestris pv. musacearum and iii) Erwinia-associated diseases (bacterial head rot or tip-over disease (Erwinia carotovora ssp. carotovora and E. chrysanthemi), bacterial rhizome and pseudostem wet rot (Dickeya paradisiaca formerly E. chrysanthemi pv. paradisiaca).
2.2. Distribution of banana bacterial wilt in east and central Africa
In 2001, a banana wilting disease was discovered for the first time in central Uganda, eastern Democratic Republic of Congo (DR Congo) (Ndungo et al., Citation2004), Rwanda, Tanzania (Carter et al., Citation2010), Kenya and Burundi (Carter et al., Citation2010). In the same year, east Congolese farmers observed the same wilting disease on a few plants at Bwere Hill, Bashali Mokoto village in the Masisi district, in North Kivu Province.
The disease was described as destroying banana fruit bunches and known to result in total loss of production, threatening the livelihoods of millions of people who depend on banana as a food and income source. Subsequently, the disease was identified as Xanthomonas campestris pathovar musacearum. Previously known only from its attack on Ensete and banana in Ethiopia, in just four years since its discovery in central Uganda, the disease developed into a full-blown epidemic, spreading throughout the eastern, central and north-western districts of the country. Although the disease was first observed in 2001, in the Democratic Republic of Congo, it was only confirmed in 2004 (Ndungo et al., Citation2004; Ndungo, Eden-Green, Blomme, Crozier, & Smith, Citation2006).
In Tanzania Xanthomonas wilt was first identified in Muleba District Kagera region in January 2006; subsequently, the disease has been reported in Bukoba, Karagwe, Biharamulo, in Kagera region and Tarime District in Mara region. The other banana growing regions- Meru-Kilimanjaro axis, Kigoma region, the southern highlands bordering Malawi and Mozambique and the islands of Pemba and Zanzibar are free from the disease. In Kenya, Xanthomonas wilt was first discovered in 2006 in Teso, Bungoma and Busia in Western province. In the year 2007, the disease has also been reported in Kakamega district in Western province and Siaya in Nyanza province. The highland agroecologies of Kisii, the rift valley and central Kenya remain disease free and so does the coastal regions.
2.3. Economic impact of banana bacterial wilt and implications for management strategies
Compared to pre-infection levels, the total banana yield loss due to BXW infection is estimated at 30–52% between 2001 and 2004 (Karamura et al., Citation2008). Although an economic analysis of BXW has to be based on findings from Central Uganda, where the disease has occurred first and is presently most common, it is possible to forecast the economic impact of a BXW pandemic in Uganda by extrapolating the observations made in this region. BXW has now been reported in 34 districts in Uganda, apparently spreading from Central Uganda, where banana production is less intensive and mainly subsistence oriented to the high-production areas in Western Uganda. However, whereas in Central Uganda infestation rates reach levels of 18–27%, the major banana producing areas in the South-West of Uganda still show little or no infection (Tushemereirwe & Opolot, Citation2005).
If uncontrolled BXW spreads at an infection rate of 8% per annum in cooking bananas, the total production loss of bananas is expected to be about 56% over a ten-year period, translating into a reduction from 4.5 million tons to eventually 2.1 million tons per year. spread over the whole of Uganda would induce economic losses of 2 billion dollars over a decade, arising from price increases and significant reductions in production. However, producers would benefit either in the first few years of the pandemic, or during a whole decade if the infestation rates are lower than 8% (Abele and Pillay, Citation2007).
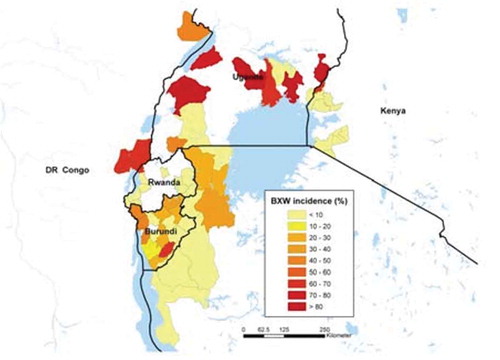
Bananas are important in the Great Lakes region, not only in Uganda, but also in adjacent countries, where they are both a food security and a cash crop (Figure ). Food security studies reveal that in Uganda, Rwanda, and Burundi, bananas constitute more than 30% of the daily per capita caloric intake, rising to 60% in some regions. Bananas also provide a major source of cash income for the farmers in the Great Lakes region (Okech et al., Citation2004). Between 2001 and 2007, BXW spread into more than 35 districts in Uganda, apparently from central Uganda, where banana production is less intensive and mainly subsistence-oriented, to the highly intensive production areas in western Uganda. In central Uganda, BXW incidence, measured as the proportion of affected sites within a district, reached up to 60%, but the major banana production areas in southwestern Uganda showed little or no infestation by 2006 (Tushemereirwe et al., Citation2006).
Figure 1. Incidence of banana Xanthomonas wilt measured as percentage of farms infected in the Great Lakes region of Africa.
In response to the outbreak of BXW, the Ministry of Agriculture, and Animal Industry and Fisheries (MAAIF) in Uganda formed a task force in December 2001 to develop a strategy to eradicate the disease (Tushemereirwe et al., Citation2006). The strategy emphasized cutting and burying infected banana plants, restricting movement of banana materials, decapitating male buds, sterilizing tools, and raising awareness about the disease. BXW management strategies reduced disease incidence to less than 10% in areas where they were adopted, but implementation was not sustainable due to the high costs.
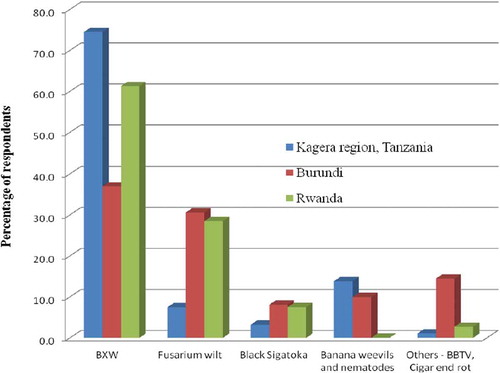
2.4. Ranking of banana bacterial wilt versus other banana production constraints in East and Central Africa
Farmers‟ ranking of banana production constraints in Burundi, Rwanda and Tanzania show that BXW disease was ranked first by most farmers (Figure ). The importance of other banana constraints greatly varied between countries. Banana weevils (Cosmopolites sordidus), nematodes (including Radopholus similis, Helicotylenchus multicinctus and Pratylenchus goodeyi), Fusarium wilt (Fusarium oxysporum cv cubence) and Black Sigatoka (Mycosphaerella fijiensis) followed in that order for Tanzania. In Rwanda, the second banana production constraint was Fusarium wilt followed by Black Sigatoka and Banana Bunchy Top Virus (BBTV) while in Burundi the second constraint was Fusarium wilt, BBTV, banana weevils and nematodes. The high ranking of BXW is probably due to the high yield losses the disease causes (up to 100% loss) if not controlled early enough (Karamura et al., Citation2010). This implies that for any banana intervention or investment, the control of BXW should be given first priority.
2.5. Effect of banana bacterial wilt on household food security and dietary intake
Household food security was heavily affected by the outbreak of BXW in these countries. Usually, over 50% of the household diet comes from bananas in Kagera region. Likewise, banana contributes substantially to household diets in Rwanda with 32% of the households having banana contributing more than 50% to their diets. The outbreak of BXW affected about 34% and 44% of the villages in Kagera in 2009 and 2011, respectively. About 70% of the interviewed farmers reported that there was a significant change in their dietary patterns due to the outbreak of BXW disease (Yadav, Verma, & Tiwari, Citation2006)
3. Management methods
3.1. Understanding of the pathogen
According to Schut, Rodenburg, Klerkx, vanAst, and Bastiaans (Citation2014c), who concluded that there is generally much less attention for other problem dimensions (e.g. socio-cultural (e.g. stakeholder beliefs, or locally preferred practices), economic, and institutional). Capturing the impact of these system dimensions, e.g. on BXW transmission at farm and regional scales, as well as the role of surveillance and control mechanisms, and their impact on combating BXW, becomes gradually more important now that focus shifts from developing knowledge to developing suitable interventions (Tinzaara et al., Citation2016).
This includes investigating diversity among farmers, their production objectives and barriers for adopting (BXW) technologies, effective strategies of information provision and capacity development for farmers, information needs and communication preferences to better understand and address constraints and challenges, and how multi-stakeholder processes can support joint problem identification, analysis and collective action. This diagnostics paper does not offer such an investigation, yet it conveys the importance of each problem dimension by providing a comprehensive assessment of their contribution to the persistence of BXW.
3.2. Current management challenges of banana bacterial wilt
Differential responses of the cultivars to the pathogen have been observed in the field. The mechanisms leading to differential responses by the host plants need to be investigated. Germ-plasm screening trials and field observations in Uganda have shown that some cultivars are able to escape the disease because of their inflorescence morphology.
One of the major challenges is the identification of resistant germplasm and the development of resistant cultivars through conventional breeding. Screening of both local and elite banana germplasm for resistance is a major ongoing activity. Differential responses of the cultivars to the pathogen have been observed in the field. The mechanisms leading to differential responses by the host plants need to be investigated. Germ-plasm screening trials and field observations in Uganda have shown that some cultivars are able to escape the disease because of their inflorescence morphology. Such an observation was also made by Buddenhagen et al. (1987).
Very little is known about the life cycle of Xvm. A clear understanding of the pathogen’s life cycle and its significance to the epidemic development in bananas is critical to the wilt research agenda. Information on pathogen population structure, pathogen diversity and phylogeny is still lacking and yet important in determining the best strategy for deployment of resistance. The duration of survival of the bacteria in the soils is not well documented, and the relative importance of different routes of infection remains a major challenge (Brandt, Spring, & Cliffton, Citation1997)
Even farmers in Uganda are reluctant to destroy “kayinja” and other ABB cultivars such as “Bluggoe” (best performing cultivars) even if the plants are infected. It is difficult to persuade farmers to destroy diseased mats since occasionally a diseased mat may still produce a normal bunch. Many farmers also obtain cash income from selling “kayinja” leaves that are used in the preparation of food. “Kayinja” produces numerous suckers, which are able to proliferate well in large mats, needs very little attention and produces large laminas that are not shredded easily by wind. Therefore, farmers tend to preserve stands of these plants even if they are infected with banana bacterial wilt.
3.3. Management of bacterial wilt of banana
Once bacterial diseases of plants are established, it is difficult to control owing to the lack of an effective chemical or other curative treatment. Early detection and destruction of the diseased plants is a key step in preventing disease spread (Karamura et al., 2005). Lack of adequate information on the biology and epidemiology of the pathogen and the perennial nature of the plant have affected the development of effective control measures as yet. Consequently, management options have focused on methods that reduce the initial inoculum and subsequent spread of the pathogen (Handoro, Hunduma, & Hailu, Citation2012). The following are some of effective methods for management of BWE. Integration of two or more of them could be the effective option for the control of the disease.
3.4. Cultural control measures
Bacterial wilt constitutes a potential threat to the cultivation of enset in Ethiopia. Because of its severe damage to enset, farmers undertake a variety of traditional practices (smoking bones, tires, burning porcupine body, including local spiritual beliefs, such as “Dua” prayer ceremony and slaughtering black goat, etc.) for the control of the disease. Here the table shows some cultural management practices conducted by farmers in Uganda and Kenya (Table ).
Table 1. Farmer awareness and application of BXW control measures in Uganda and Kenya
Once BWE occurs in a field, there is no remedy other than to cut down all infected plants, completely dig out the corm, and place the field under fallow or a prolonged crop rotation regime (Tripathi et al., Citation2009). Some farmers fallow the enset field and practice rotation with annual crops. In contrast, in the area that is relatively free of bacterial wilt, farmers practice control measures such as uprooting the infected bacterial wilt enset plants and keeping them away from the household, other enset plants, and cattle. Farmers also try to keep healthy plants away from contaminated farm and processing tools (Brandt et al., Citation1997). A six months fallow period is adequate to avoid re-infection from soil-borne inoculum if all infected plant materials are removed . However, some farmers also uproot and throw away infected plants on the road or near the enset farm, which further spread the disease. Farmers in peasant associations, where some training on sanitary measure was advocated do not practice the measure correctly.
As described earlier, the pathogen can survive in the soil and plant debris, and hence sanitation could be important control option. Sanitation has been recommended for enset bacterial wilt by different authors (Ashagari, 1985; Brandt et al., Citation1997; Dagnachew & Bradbury, Citation1974). This measure includes the use of disease-free suckers as planting material, uprooting and burying of diseased plants far from the field, cleaning and flaming of equipment that has come in contact with diseased plants and rotation of crops if the damage is severe (Brandt et al., Citation1997). Such measures should be taken in a manner of campaign and as a regular practice in all enset growing areas.
3.5. Host plant resistance
Like all other bacterial diseases, developing and use of resistant or tolerant enset clones could be one of the best approaches in the management of enset bacterial wilt. This method is cheaper to the farmer and safe to the environment. Continuous and intense evaluation of enset clones for disease resistance is one of the basic requirements for effective and sustained implementation of integrated disease management programs.
It is estimated that there are over 200 different enset clones described by vernacular names in Ethiopia and more than 66 clones in the Gurage zone. Variable levels of clonal response to the Xcm disease have been observed under farmers’ field conditions and while using artificial inoculation. Enset farmers commonly grow combinations of clones in their enset field, and each clone is basically grown for its specific use. One of the selection criteria of the clone is resistance/tolerance to BWE disease. Ashagari (1985) identified five enset clones, namely Ado, Kembate, Hedesso, Soskella, Genticha and Ade as tolerant clones from 60 test clones by artificial inoculation. Similarly, Welde-Michael et al. (Citation2008) identified some enset clones, which showed a relative tolerance to Xcm. These are Anikefye, Eminiye, Lemat and Nechwe from Gurage zone and Abate, Arkya, Heila, Mezya and Sorpie from Hadiya zone and some clones like Buacho, Wonigoro, Bazeriet and Dere recovered from an initial infection from Xcm. Fikre and Welde-Michael et al. (Citation2008) also reported that Meziya enset clone is resistant/tolerant to bacterial wilt of enset. However, they may found to be susceptible in other experiment, due to variation in pathogen isolate or the clone type
3.6. Biological control
Biocontrol organisms offer environmentally friendly alternatives to chemical control methods to manage plant diseases or pests. Biological control agents could be used where chemical pesticides are banned (organ chlorines) or being phased out (methyl bromide) or where pests or pathogens have developed resistance to conventional pesticides or to grow organic food to satisfy consumer perception (Butt, Jackson, & Magan, Citation2001). It has been reported that some bacterial species can serve as biological control agents against soil-borne pathogens.
Biological control depends upon the establishment and maintenance of a threshold population and viability below that level may eliminate the possibility of biological control. P. putida B56, P. fluorescens A506 and P. syringae Cit7 provided significant reductions in disease severity of bacterial spot of tomato (Xanthomonas campestris pv. vesicatoria) under greenhouse conditions. Similarly, foliar application Pseudomonas syringae consistently provided significant disease suppression in control of bacterial spot of tomato caused by Xanthomonas campestris pv. vesicatoria. In addition, seed and root application of Pseudomonas fluorescens and Bacillus pumilus provided significant suppression of bacterial spot in the field trials conducted in Alabama.
3.7. Limitation of biological control agents
Although biological control agents have shown to protect crop plants from disease under experimental conditions, inconsistent performance between under experimental conditions and field locations has been reported. Biocontrol is less consistent than chemical control in field condition. Variation in consistence and performance of biological control agents has been attributed to many factors like biotic and ecological factors. Moreover, the survival and efficacy of biocontrol agents affected by host plant genotype, agricultural practices, mutation of biocontrol organism and resistance of pathogen to biocontrol mechanisms (Ownley & Windham, Citation2006). Biological control may also competitively displace non-target organisms.
Unintended effects would occur if this competitive displacement were to seriously affect a nontarget organism; perhaps even leading to its extinction or in some other way detrimentally affecting a component of the ecosystem. Furthermore, some fungi that intended for biological control could infect a wide variety of hosts, which sometimes include mammals. Thus, evaluation of potential microbial control agents must include an evaluation of their virulence towards non-target organisms (Goettel, Hajek, Siegel, & Evans, Citation2001).
3.8. Chemical control measures
So far no bactericide has been recommended against enset bacterial wilt. Likewise, chemical control of bacterial wilt of banana (caused by a similar pathogen with BWE) is not effective. However, various in-vitro trials were done on antibiotics and plant extracts against X. campestris pathovars that cause diseases in different crops. For example, streptomycin, oxytetracycline, chloroamphenicol and rifampicin were tested for the control of black rot of cauliflower caused by X. campestris pv. campestris and streptomycin was the most effective, giving 100% control, followed by oxytetracycline .
In-vitro test was also done on X. campestris pv. mangiferaeindicae and all the chemicals and antibiotics used, aureofungin, bavistin, erythromycin, streptocycline, streptomycin and tetracycline inhibited the bacterium. Test of some antibiotics for Xanthomonas axonopodis pv. punicae also shows an inhibitory effect against the pathogen. Consequently, streptocycline + copper oxychloride had showed highest inhibition (2.8 cm), followed by copper oxychloride (2.63 cm) and streptocycline (2.59 cm), which are on par with each other.
Several studies have also indicated the potential of plant extracts in the control of diseases caused by X. campestris in several important crop plants. About 208 diffusates from various plants such as forest trees, shrubs, herbs, fruit seeds, etc., against X. campestris pv. citri. and diffusates from various parts of Phyllanthus emblica, Acacia nilotica, Sapindus mukorossis and Terminalia chebula exhibited an inhibition zone 4.83–6 mm at 50 g/liter appeared to be the most effective. Extracts from Acacia arabica, Achras zapota, and from other six higher plants were also found inhibitory to various pathovars of X. campestris. Chamomilla recutita and Chamaemelum nobile extracts also inhibited the growth of X .campestris pv. citri strains causing citrus bacterial canker disease .
A researchs also reported that in vitro evaluation of plant-extracts (such as Sapin-dus laurifolia, Asclepias curassavica, Helicteres isora, Piper betel, Tamarindus indica, Tridax procumbens and Azadirachta indica) inhibits bacterial growth of X. campestris pv. mangiferaeindicae causing bacterial black spot of mango. However, the in vitro and in vivo effectiveness of these chemicals for Xcm is not documented for both enset and banana.
Even though biological control of bacterial diseases using microbial antagonists are known to be effective (Priou, Marquez, & Gutarra, Citation2006), this option has not yet been tried so far in the management of bacterial wilt of enset (Handoro et al., Citation2012). But control of insect vectors may prove possible once these have been identified, especially for banana. Plants sprayed with streptomycin sulphate, Agrimycin—100, Vitavax, Dithane M-45 and Benlate each at 0.2% concentration and then inoculated with exhibited infection index values of 3.00, 2.33, 2.40, 1.90 and 1.83, respectively, as compared with 2.93 in case of control, 10 days after inoculation.
3.9. Disadvantage of chemical control
Although chemical compounds have been applied to control plant diseases, they result in a negative impact on a wide range of organisms. The increasing use of agrochemicals is negatively perceived by consumers and supermarket chains. Chemical pesticides contaminate groundwater, enter foodchains, and pose hazards to animal health and to the spraying personnel of the chemical pesticides. Several members of the European Union (EU), such as Sweden, Denmark, and the Netherlands have decided in the mid-late 1980s to decrease the chemical input in agriculture by 50% within a 10-year period. Frequent applications of copper-based bactericides amended with an ethylenebisdithiocarbamate fungicide (maneb or mancozeb, class B2 carcinogens) provide some disease suppression of Xanthomonas leaf blight and other bacterial diseases of onion in Colorado, but they must be applied regularly eight or more times to be effective and suppress disease per season (Schwartz & Otto, Citation1998). This approach to the management of disease onion is expensive for onion growers. In addition, Copper resistance has been reported in Barbados (Butt et al., Citation2001).
4. Future research
The Production of transgenic resistant cultivars is very advanced with some lines under field evaluation in Uganda . Improved understanding of the molecular basis of interaction between the bacterium and the host plant, and an analysis of the intermediate products produced by both the pathogen and plant following infection has been attributed to this advancement in transgenic breeding. Reactions of these transgenic banana cultivars to BXW under field conditions are promising but what remains an issue is the acceptability of transgenic material by farmers as a replacement to their indigenous cultivars. Exclusion of farmers in transgenic research due to its stringent and bio-safety protocols is likely to hamper the adoption of transgenic bananas. Use of transgenic approaches in the development of resistant banana cultivars is however relatively faster than conventional breeding. Continuous donor support for the development of transgenic cultivars is an important strategy (Tripathi et al., Citation2014).
5. Major achievement through biological method
5.1. Promising biological control
Among micro-organisms, there are forms that inhibit the growth of other microbes. They are usually called antagonists. Bacterial antagonists have been used in many studies to suppress bacteria of the same or similar genus. Establishment of bacterial antagonists on plant surfaces is a critical phase in disease control. Populations of antagonists established on plant surfaces are necessary for competition with pathogens for sites and nutrients (Priou et al., Citation2006).
According to Abayneh (2010) reported that using microbial agents against BXW has been evaluated in pot experiments and under greenhouse conditions four bacterial antagonistic (inhibit growth of other microbes) isolates tested against Ensete Xanthomonas wilt pathogen demonstrated a reduction in disease severity ranging from 56.4% to 74.8%. Bacterial antagonistic isolates showed significant disease reduction relative to the positive control, indicating that Xanthomonas wilt could be potentially managed with bacterial antagonists. Biological control of Xcm using antagonistic bacteria has not been discussed in detail and remains an option for controlling the disease.
5.2. Disease diagnostics
Ocimati et al. (Citation2013) reported that range from 30 days to 16 months, making its early detection of the pathogen and control very difficult. In addition, latent Xcm infection levels of 15–43% have also been reported in Uganda in symptomless suckers of mother plants that have succumbed to the disease (Ocimati et al., Citation2013). A semi-selective medium developed has improved the robustness of disease diagnostics, but it is time-consuming and labor-intensive, especially when testing a large number of samples. Furthermore, advanced PCR-based diagnostic methods with high specificity to Xcm have been developed.
ELISA based Xcm-specific techniques that are suitable for handling large plant samples have also been developed for laboratory assays. More recent polyclonal diagnostic studies conducted through collaboration between Bioversity International, the National Agricultural Research Organization (NARO, Uganda), IITA and the Food and Environment Research Agency (FERA, UK) successfully developed a lateral flow device (LFD) which is suitable for Xcm detection in banana. These LFDs are currently being tested under field conditions, and the technology will enhance diagnosis of BXW in material suspected to be infected as opposed to solely relying on visual symptoms and ease BXW surveillance work.
5.3. Refinement cultural practices for BXW
At the beginning of the disease epidemic in ECA (East and Central Africa), the complete uprooting of diseased mats, use of clean garden tools and early removal of male buds to prevent insect vector transmission were recommended (Tushemereirwe et al., 2004). Uprooting a complete mat is time-consuming and labor intensive and becomes logistically difficult when large numbers of diseased mats need to be removed.
According to Ssekiwoko, Turyagyenda, Mukasa, Eden-Green, and Blomme (Citation2010) it is possible to remove the infected stems only from the infected mat and that non-diseased suckers remain healthy. This is because most infection begins from the upper parts of the plant and takes over 30 days to reach the corm. This method is less intensive compared to the removal of a complete mat and involves continued removal of single diseased plants in a field to reduce the inoculum level and bring the disease incidence down to an acceptable level (Kubiriba et al., Citation2014). Single stem removal has been evaluated in Kenya and DR Congo and has been found to be effective (Kubiriba et al., Citation2014). This method, however, needs to be closely coordinated with other strategies to prevent new infections, such as the use of decontaminated garden tools or reducing insect vector transmission.
Recent research findings also show that the incubation period for the bacterium is between 2 weeks and 16 months (Ocimati et al., Citation2013). During this period, infection can be spread from the asymptomatic plant to healthy plants contaminated tools. Such activity is responsible for perpetuating field infection for years, even for farmers who are actively using other recommended practices. Therefore, it follows that those farmers who suspend the use of cutting tools in the same field for at least three months and practice removal of all infected plants will gradually reduce the number of infected plants to zero. It, therefore, reduces the cost of BXW control and improves farmer confidence in BXW control measures.
5.4. Farmer field schools (FFS)
Farmers Field Schools are traditionally an adult education approach that assists farmers to learn in an informal setting within their own environment. FFS are schools without walls where groups of farmers meet weekly with facilitators. The FFS is a community-based approach that empowers farmers to make logical crop management decisions, exposes farmers to new ways of thinking and problem solving, and encourages them to implement and discuss their own solutions (Nankinga & Okasaai, Citation2006).
The FFS approach that was coordinated by NARO and the Food and Agricultural Organization (FAO) worked in Uganda to effectively disseminate information and equip farmers with knowledge to control BXW. In Uganda, it has been reported that the proportion of fields that had low BXW infection (<10 infected plants) was higher on sites that hosted FFS (68%) than in sites with farmers that employed community action (51%) or accessed information and technologies for BXW control traditionally (38%) in the East Africa highland banana cropping system. A similar trend was reported for the Kayinja cropping system where farms with low BXW infection were observed in communities hosting FFS (56.3%) compared to communities mobilized traditionally (28.3%) or using community action (36.6%) (Kubiriba et al., 2012). In western Kenya, the FFS approach was recently initiated by the Kenya Agricultural Research Institute (KARI) and the Rural Electricity and Food Security Organization (REFSO). The approach demonstrated an increased adoption of BXW management practices and a reduction in the disease incidence from 80% to less than 10% in 12 months (M. Onyango, pers. commun.). This led to increased banana production and incomes of smallholder banana farmers in western Kenya.
6. Conclusion
Banana (Musa sp.) is the fourth most edible global food crop plant after rice, wheat and maize in contrast to its vast production. Nowadays the production and yield of banana are mostly affected by banana bacterial wilt pathogen (Xanthomonas campestris pv. musacearum). Since banana is a key component of east and central Africa farming communities, the banana production losses resulted in a significant reduction in household food security and incomes. The management of the pathogen is very important in order to increase its production and reduce its yield loss. The use of different management practices separately is not sufficient for controlling this deadly bacterial disease, therefore creating awareness on using of integrated pest management tactics is best option to eradicate the bacteria. In addition to this isolation and characterization of endophyte bacterial antagonistic agents against bacterial wilt of banana (Xanthomonas campestris pv. musacearum) from different ensete and banana growing regions should be made to find effective isolates that can widely suppress bacterial wilt of banana.
Additional information
Funding
Notes on contributors
Ambachew Zerfu Geberewold
The author, Ambachew Zerfu Geberewold, was born on 15 January 1995 in Negale Borena Guji Zone, Oromiya Regional State, Ethiopia. He attended his elementary education at Ogoba primary school from 2000–2005 and his secondary and preparatory education at Addis Ketema Senior secondary and preparatory School from 2006–2011. Then he joined Mizan – Tepi University in 2012 and graduated with the degree of Bachelor of Science in Plant Science in July 2015. After his graduation, he got sponsor from Ethiopian Ministry of Education and joined the School of Graduate studies of Jimma University in September 2015 to pursue a study leading to the degree of Master of Science in Horticulture. Now he is working as a lecturer at Raya University, Ethiopia under Horticulture department and he actively involved in many research activites.
References
- Abele, S., & Pillay, M. (2007). Bacterial wilt and drought stresses in banana production and their impact on economic welfare in Uganda: Implications for banana research in East African highlands. Journal of Crop Improvement, 19, 173–14. doi:10.1300/J411v19n01_09
- Brandt, S. A., Spring, A., & Cliffton, H. (1997). The tree against hunger: Enset based agricultural systems in Ethiopia. Washington DC: American Association for the Advancement of Science.
- Buddenhagen, I. W. (2009). Blood bacterial wilt of banana: History, field biology andsolution. Acta horticulturae, 828, 57–68. doi:10.17660/ActaHortic.2009.828.4
- Butt, T. M., Jackson, C., & Magan, N. (2001). Introduction – Fungal biological control agents
- Carter, B. A., Reeder, R., Mgenzi, S. R., Kinyua, Z. M., Mbaka, J. N., & Doyle, K. (2010). Identification of Xanthomonas vasicola (formerlyX.campestrispv. musacearum), causative organism of banana Xanthomonas wilt, in Tanzania, Kenya and Burundi. Plant Pathology, 59, 403. doi:10.1111/j.1365-3059.2009.02124.x
- Central Statistics Authority (CSA). (2005). Sample/survey for 1996/97 seasons. Addis Ababa, Ethiopia: CSA.
- Dagnachew, Y., & Bradbury, J. F. (1968). Bacterial wilt of enset (Enset ventricosum) incited by Xanthomonas campestris sp. Phytopathology, 59, 111–112.
- Dagnachew, Y., & Bradbury, J. F. (1974). A note on wilt of banana caused by enset wilt organism Xanthomonas campestris. East African Agricultural and Forestry Journal, 40, 111–114. doi:10.1080/00128325.1974.11662720
- Goettel, M. S., Hajek, A. E., Siegel, J. P., & Evans, H. C. (2001). Safety of fungal biocontrol agents. In T. M. Butt, C. Jackson, & N. Magan (Eds.), Fungi as biological pest control agents: Progress, Problems and Potential (pp. 347–369). New York, NY: CAB International, 198 Madison Avenue.
- Handoro, F., Hunduma, T., & Hailu, E. (2012). Research achievements, experiences and future directions on bacterial wilt of enset. In Y. Mohammed & H. Tariku (Eds.), Enset research and development experiences in Ethiopia. Proceedings of enset national workshop, 19–20 August 2010 (pp. 64–96). Ethiopia: Wolkite.
- Karamura, E., Kayobyo, G., Tushemereirwe, W., Benin, S., Blomme, G., Eden, G. S., & Markham, R. (2010). Assessing the impacts of banana bacterial wilt disease on banana (Musa spp.) Productivity and livelihoods of Ugandan farm households. Bioversity International. Kampala. Uganda.
- Karamura, E. B. (1992). Banana and plantain production constraints as basis for selecting research priorities. In: Proceedings of the Regional Advisory Committee (RAC) meeting (pp. 23–25). September 1991. Kampala, Uganda: INIBAP.
- Karamura, E. B., Turyagyenta, F. L., Tinzaara, W., Blomme, G., Molina, A., & Markham, R. (2008). Xanthomonas wilt of banana in East and Central Africa. Diagnostic and management guide. In Biodiversity International. Uganda.
- Kubiriba, J., Muthomi, J., Ndungo, V., Kwach, J., Erima, R., Rwomushana, I., … Opio, F. (2014). Strategies for rehabilitation of banana fields infested with Xanthomonas campestris pv.musacearum. Journal of Crop Protection, 3, 21–29.
- Lescot, T. (2013, September 9–13). World plantain and banana production systems. In Proceedings XX International Meeting ACORBAT (pp 26–34). Fortaleza.
- Mansfield, J., Genin, S., Magori, S., Citovsky, V., Sriariyanum, M., & Ronald, P. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular Plant Pathology, 13, 614–629. doi:10.1111/j.1364-3703.2012.00804.x
- Mesfin, T., & Gebremedhin, W. G. (2008). Enset: Introduction in root and tuber crops: The untapped resources. In W. G. Gebremedhin, G. Endale, & L. Berga (Eds.), (pp. 155–156). Addis Ababa: EAR.
- MOA (Ministry of Agriculture). (2006). General agricultural survey, unpublished data.
- Nankinga, C., & Okasaai, O. (2006). Community approaches used in managing BXW in Uganda. In E. B. Karamura, M. Osiru, G. Blomme, C. Lusty, & C. Picq (Eds.), Developing a regional strategy to address the outbreak of banana Xanthomonas Wilt in East and Central Africa: Proceedings of the banana Xanthomonas wilt regional preparedness and strategy development workshop held in Kampala, Uganda, 14–18 February 2005 (pp. 56–60). Montpellier: INIBAP.
- Ndungo, V., Bakelana, K., Eden-Green, S., & Blomme, G. (2004). An outbreak ofbananaXanthomonaswilt(Xanthomonascampestrispv.musacearum)inthe democraticrepublicofcongo. Info Musa, 13, 43–44. doi:10.1111/mpp.12578
- Ndungo, V., Eden-Green, S., Blomme, G., Crozier, J., & Smith, J. (2006). Presence of banana xanthomonas wilt (Xanthomonas campestris pv. musacearum) in the Democratic Republic of Congo (DRC). Plant Pathology, 55, 294. doi:10.1111/ppa.2006.55.issue-2
- Ocimati, W., Ssekiwoko, F., Karamura, E., Tinzaara, W., Eden-Green, S., & Blomme, G. (2013). Systemicity of Xanthomonas campestris pv. musacearum and time to disease expression after inflorescence infection in East African highland and Pisang Awak bananas in Uganda. Plant Pathology, 62, 777–785. doi:10.1111/j.1365-3059.2012.02697.x
- Okech, H. O., Gold, C. S., Abele, S., Nankinga, C. M., Wetala, P. M., Van Asten, P., … Ragama, P. (2004). Agronomic, pests and economic factors influencing sustainability of banana-coffee systems of Western Uganda and potentials for improvement. Uganda Journal of Agricultural Sciences, 9, 432–444.
- Ownley, B., & Windham, M. T. (2006). Biological control of plant pathology. In R. N. Trigiano, M. T. Windiham, & A. S. Windham (Eds.), Plant pathology: Concept and laboratory exercise (pp. 554–570). Washington, D.C.: Boca Raton London, New York.
- Price, N. S. (1995). Banana morphology part i: Roots and rhizomes. In S. Gowen (Ed.), Bananas and plantains (pp. 179–189). London: Chapman and Hall.
- Priou, S., Marquez, M., & Gutarra, L. (2006, July 17–20). Biological control of bacterial wilt of potato (Ralstonia solanacearum) using an antagonistic endophyte strain of Pseudomonas putida. In: Proceedings of the 4th International Bacterial wilt symposium, (pp. 47). New York UK: Central Science Laboratory.
- Schut, M., Rodenburg, J., Klerkx, L., vanAst, A., & Bastiaans, L. (2014c). Systems approaches to innovation in crop protection. A systematic literature review. Crop Protection, 56, 98–108. doi:10.1016/j.cropro.2013.11.017
- Schwartz, H. F., & Otto, K. J., (1998 December 10–12). Onion bacterial disease management in Colorado. In Proc. of the 1998 National Onion (and other Allium) Research Conference (pp. 214–218). Sacramento, CA.
- Ssekiwoko, F., Turyagyenda, L. F., Mukasa, H., Eden-Green, S., & Blomme, G. (2010). Spread of Xanthomonas campestris pv. musacearum in banana (Musa spp.) plants following infection of the male inflorescence. Acta horticulturae, 879, 349–356. doi:10.17660/ActaHortic.2010.879.36
- Tinzaara, W., Karamura, E. B., Kubiriba, J., Ochola, D., Ocimati, W., Blomme, G., & Ssekiwoko, F. (2016). The banana Xanthomonas wilt epidemic in east and central Africa :Currentresearchanddevelopmentefforts. Acta horticulturae, 1114, 267–274. doi:10.17660/ActaHortic.2016.1114.36
- Tothill, J. D. (1940). Agriculture in Uganda. Oxford: Oxford University Press.
- Tripathi, L., Tripathi, J. N., Kiggundu, A., Korie, S., Shotkoski, F., & Tushemereirwe, W. K. (2014). Field trial of Xanthomonas wilt disease-resistant bananas in East Africa. Correspondence. Nature Biotechnology, 32, 865–870. doi:10.1038/nbt.3007
- Tripathi, V., Mwangi, M., Abele, S., Aritua, V., Tushemereirwe, W. K., & Bandyopadhyay, R. (2009). Xanthomonas wilt. A threat for banana production in East and Central Africa. The American phytopathological society. Plant Disease, 93(5), 440–451. doi:10.1094/PDIS-93-5-0440
- Tushemereirwe, W., & Opolot, O. (2005). BXW history, status and national strategies. Paper presented at the workshop “Assessing the impact of the banana bacterial wilt Xanthomonas campestris pv. musacearum on household livelihoods in East Africa”, held on Dec. 20th 2005 in Kampala, Uganda.
- Tushemereirwe, W. K., Okaasai, O., Kubiriba, J., Nanakinga, C., Muhangi, J., Odoi, N., & Opiuo, F. (2006). Status of banana bacterial wilt in Uganda. African Crop Science Journal, 14, 73–82.
- Welde-Michael, G., Bobosha, K., Blomme, G., Addis, T., Mengesha, T., & Mekonnen, S. (2008). Evaluation of enset clones against enset bacterial wilt. African Crop Science Journal, 16(1), 89–95.
- Yadav, J., Verma, J. P., & Tiwari, K. N. (2006). Plant growth promoting activities of Fungi and their effects on Chickpea plant growth. Asian Journal of Biological Sciences, 5, 1–5.
- Zippel, K. (2002). Enset (Ensete ventricosum (WELW.) CHEESM.) in subsistence farming systems in Ethiopia. Germany: Humboldt University Berlin, Institute for Horticultural Sciences.