Abstract
Glabridin is a prenylated isoflavonoid isolated from the roots of Glycyrrhiza glabra. We investigated the metabolic effects of glabridin, using healthy volunteers and patients with type 2 diabetes. Seven healthy volunteers and 10 patients with type 2 diabetes were recruited, and assigned to receive an oral dose of glabridin (300 mg) once daily for 12 weeks. Total cholesterol, low-density lipoprotein cholesterol, glycated hemoglobin, immunoreactive glucagon, and body fat composition significantly decreased in the healthy individuals, after 12 weeks of glabridin administration, while there were subtle effects in the patients with type 2 diabetes. Glabridin significantly improved flow-mediated vasodilatation, and pulse wavelength in the healthy volunteers. The results of our pilot study indicate that glabridin exerted several favorable metabolic effects and improved vascular functions, especially in healthy volunteers. Therefore, glabridin might be better to use in healthy subjects for preventing metabolic disorder rather than the patients with type 2 diabetes.
PUBLIC INTEREST STATEMENT
Glabridin, which is presently used as a dietary supplement, is a prenylated isoflavonoid first isolated from the roots of Glycyrrhiza glabra (Latin name: Glycyrrhiza glabra Linn. (Fabaceae)). Our study indicate that glabridin improved vascular functions in healthy subjects.
1. Introduction
The current global increase in the incidence of metabolic conditions such as diabetes, dyslipidemia, and hypertension (Guariguata et al., Citation2014; Toth, Potter, & Ming, Citation2012), is expected to translate into an increase in the incidence of cardiovascular and cerebrovascular diseases over time. Furthermore, the increase in healthcare costs, specifically costs for medication to treat diabetes and diabetes-associated vascular diseases is becoming a cause for concern. Therefore, preventive medicine which is opposed to disease treatment has become more and more important. Dietary supplements are functional foods and widely used not only for healthier lifestyle but also for prevention of metabolic disorders.
Glabridin, which is presently used as a dietary supplement, is a prenylated isoflavonoid first isolated from the roots of Glycyrrhiza glabra (Latin name: Glycyrrhiza glabra Linn. (Fabaceae)) by Saito et al. in 1976 (Saito et al., Citation1976). Previous reports indicate that glabridin prevents vascular injury and exerts anti-inflammatory (Kang et al., Citation2005; Yokota, Nishio, Kubota, & Mizoguchi, Citation1998), anti-atherogenic (Kang et al., Citation2015), anti-obesity (Ahn, Lee, Jang, Kim, & Ha, Citation2013; Aoki et al., Citation2007a), anti-osteopenic (Klasik-Ciszewska, Kaczmarczyk-Sedlak, & Wojnar, Citation2016), neuroprotective (Hasanein, Citation2011; Yu et al., Citation2008), nephroprotective (Riccioni et al., Citation2012), and anti-cancer effects (Hsieh et al., Citation2016). However, most of these studies have been performed in animal models or in vitro. Therefore, in this pilot study, we examined the its metabolic effects on healthy volunteers and patients with type 2 diabetes and in vitro effects of glabridin on endothelial cells were also investigated. We hope that data generated from this study will support future large-scale clinical studies.
2. Methods
2.1. Ethical considerations
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and approved by the ethical committee of Chiba University (approval number: 1208).
2.2. Study participants
Seven healthy male volunteers aged 30–45 years who worked at Chiba university, and 10 patients with type 2 diabetes aged 40–65 years (5 males, 5 females) who were outpatients in Chiba university hospital were recruited. The aims and methods of the study were explained and written informed consents were obtained from all study participants.
2.3. Drug administration
All participants were administered an oral dose of glabridin (Glavonoid™ softgels containing 300 mg of licorice glabra polyphenols, Kaneka Corporation, Osaka, Japan) once daily in the morning after a 12 h fast for 12 weeks.
2.4. Sample collection
Venous blood samples were collected before (baseline) and after the 12 week intervention. The samples were obtained in the morning, after a 12 h fast. The serum and plasma samples not used immediately after collection were frozen at—80 °C and only thawed prior to analyses. Clinical parameters were measured by SRL Diagnostics Inc. (Tokyo, Japan)
2.5. Measurement of lean body mass and fat body mass
The assessment of body composition such as lean body mass and fat body mass were evaluated by bioelectrical impedance analysis (BIA) methods (Inbody S10, Wellup, Yokohama, Japan). On the day of BIA examination, participants were requested to fast, abstain from caffeinated beverages, only consume water, and take no medication.
The BIA were examined between 8:30 and 9:30 am.
2.6. Plasma glabridin measurements
Plasma levels of glabridin were determined only in healthy volunteer using solid-phase extraction and liquid chromatography–mass spectrometry (LC–MS/MS), according to a slightly modified protocol by Aoki et al., by Kaneka Techno Research Corporation (Aoki et al., Citation2005) before (baseline) and after the 12 week intervention. Blood samples were obtained in the morning, after a 12 h fast.
2.7. Brachial–ankle pulse wave velocity
Brachial–ankle pulse wave velocity (baPWV) were measured only in healthy volunteer at the baseline and the end of the intervention, using a Colin Waveform Analyzer (model BP203RPE II) (Colin, Komaki, Japan).
2.8. Flow-mediated vasodilatation
The flow-mediated vasodilation (FMD) was measured only in healthy volunteer using a novel semi-automatic vessel chasing system (UNEX-EF18G) (UNEX Corporation, Aichi, Japan), according to the standard protocol at the baseline and the end of the intervention. On the day of FMD examination, participants were requested to fast, abstain from caffeinated beverages, only consume water, and take no medication.
The FMD measurements were obtained between 8:30 and 9:30 am.
2.9. Statistical analyses
All data are expressed as mean ± standard deviation (SD). We analyzed changes in clinical parameters from baseline to 12 weeks, using the paired t-test and Spearman’s correlation coefficient. Comparisons between more than two groups were made using one-way analysis of variance (ANOVA) and Dunnett’s or Tukey’s multiple comparison tests. P < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software Inc. San Diego, CA).
2.10. Reagents
The following antibodies were used (catalog numbers shown in brackets): phospho-eNOS (ser1177) antibody (#9571), phospho-Akt (ser473) rabbit monoclonal antibody (#4060), Akt antibody (#9272), and ICD54/ICAM1 antibody (#4915). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) rabbit monoclonal antibodies (#5174) were purchased from Cell signaling technology, NOS3 antibodies (#sc-654) from Santa Cruz Biotechnology, and anti-VCAM1 antibodies (#ab134047) from Abcam.
LPS from Escherichia coli O55: B5 (#L4524), 2-metcaptoryhanol (#M3148), and bovine serum albumin (BSA; #A3294) were purchased from Sigma-Aldrich. PI3K inhibitor, LY294002 (#129-04861), Akt inhibitor, Triciribine (#206-19371), and dimethyl sulfoxide (DMSO; #043-07216) were purchased from WAKO.A licorice flavonoid oil concentrate solution for human study containing Glycyrrhiza glabra L. and medium-chain triglycerides was provided by Kaneka Corporation. The concentration of glabridin, the major component of the solution, was adjusted to 3% (w/w).
2.11. Cell culture
Human Umbilical Vein Endothelial Cells (HUVECs) purchased from Takara-bio and were cultured in EGM2 Bullet kit (#CC-3162, Lonza) at 37°C in 5% CO2. In all experiments, HUVECs were seeded in 6-well plates (1 x 105/well, #4810-010, IWAKI) with a growth arrest period of 24 h in Endothelial Cell Basal Medium (#CC-3121, Lonza) containing 0.1% BSA.
2.12. Immunoblotting
Cells were lysed in boiled Laemmli sample buffer. The samples were boiled for 10 min and centrifuged at 15,000 rotations per minute (rpm) for 5 min at room temperature. An aliquot of 30μl from the protein samples was separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) on 7.5%, 10%, or 12% (w/v) polyacrylamide gels. Signals were detected using ECL Western Blot Substrate (Thermo Fisher Scientific, Waltham, MA) and Hyper film ECL (GE Healthcare, Little Chalfont, UK). Films were scanned and signals in the linear range were quantified using Image J and normalized to the control levels.
3. Results
3.1. Glabridin induced metabolic changes in healthy volunteers but not in patients with type 2 diabetes
In the healthy volunteer group, total cholesterol (T-Cho), low-density lipoprotein cholesterol (LDL-C), glycated hemoglobin (HbA1c), immunoreactive glucagon, and body fat composition significantly decreased after 12 weeks of glabridin administration (Table ). Serum soluble intercellular adhesion molecule-1 (ICAM1) levels decreased, though not significantly. While, high sense C-reactive protein and high-density lipoprotein cholesterol (HDL-C) levels significantly decreased in patients with type 2 diabetes (Table ).
Table 1. Clinical parameters of healthy volunteers before and after oral administration of 300 mg glabridin once daily for 12 weeks
Table 2. Clinical parameters of patients with type 2 diabetes before and after oral administration of 300 mg glabridin once daily for 12 weeks
3.2. Glabridin exerts beneficial effects on vascular function in healthy volunteer
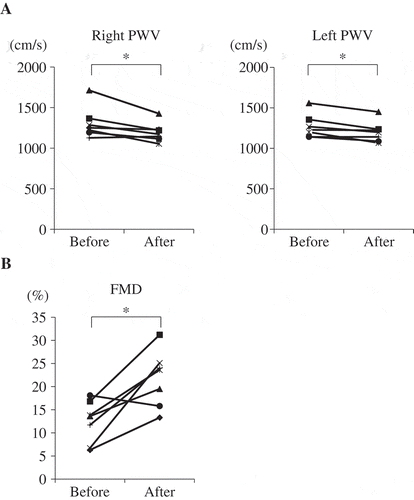
Glabridin decreased total cholesterol and LDL-C levels in healthy volunteer but not in the patients with type 2 diabetes. We focused on the healthy volunteer and examined plasma glabridin concentration, and its effects on vascular function. Plasma glabridin concentration was measured before and after the administration of 300 mg glabridin (Table ) for 12-week intervention in healthy volunteers. Mean glabridin concentration was 1.73 ng/ml, corresponding to approximately 5.3 nM glabridin. The vascular functions FMD and baPWV significantly improved after the 12-week intervention (Figure ). The association between changes in clinical parameters and the observed improvements in FMD and baPWV was examined. Univariate analyses revealed that changes in HbA1c, T-Cho, and LDL-C were significantly associated with changes in FMD (Table ), while uric acid was the only parameter significantly associated with changes in baPWV (data not shown). Due to the small sample size, multivariate analysis was not performed.
Table 3. Plasma glabridin concentration before and after 300 mg glabridin administration in healthy volunteer. N.D.: not determined, SD: standard deviation
Table 4. Univariate analyses of clinical parameters significantly associated with changes in flow-mediated vasodilatation
Figure 1. Glabridin improved vascular function in healthy volunteer.
Seven healthy volunteers were assigned to receive an oral dose of glabridin (300 mg) once daily for 12 weeks. Brachial–ankle pulse wave velocity (baPWV) (a) and flow-mediated vasodilatation (FMD) (b) were examined before and after the treatment period. *p < 0.05.
3.3. Glabridin activates eNOS via the PI3K/Akt pathway and inhibits LPS-induced endothelial cell injury
Endothelial nitric oxide synthase (eNOS) mediates the production of nitric oxide (NO) and reportedly plays an essential role in endothelial cell function under normal and pathogenic conditions (Rudic et al., Citation1998). Because glabridin significantly improved vascular function in healthy volunteers, we examined the effects of glabridin in vitro, by investigating the expression of eNOS in cultured human endothelial cells (Human Umbilical Vein Endothelial Cells: HUVECs purchased from Takara-bio, Shiga, Japan). In the presence of 50 nM glabridin, the phosphorylation of eNOS was significantly upregulated (Figure ). Several pathways (such as the AMP-activated protein kinase (AMPK) and PI3K/Akt pathways) and proteins have been reported to be associated with the regulation of eNOS. In this study, we examined the involvement of the Akt pathway, by using Triciribine (10 μM) and LY-294002 (10 μM) to inhibit Akt and P13K phosphorylation, respectively. Both triciribine and LY-294002 inhibited glabridin-induced eNOS phosphorylation, indicating that glabridin induced its phosphorylation effects via the PI3K/Akt signaling pathway (Figure ).
Figure 2. Glabridin activates eNOS via the PI3K/Akt pathway and protected against LPS-induced endothelial cell injury.
(a, b) Triciribine (10 µM) was used to inhibit Akt phosphorylation (A), and LY-294002 (10 μM) was used to inhibit phosphoinositide 3-kinase (PI3K) (B). HUVECs were pretreated with each inhibitor or vehicle for 1 h, then incubated in the presence of 50 nM glabridin (a) or 100 nM glabridin (b) for 30 min. The cells were then lysed and analyzed using SDS–PAGE and immunoblotting. Both triciribine and LY-294002 inhibited glabridin-induced eNOS phosphorylation (a, b). The experiments were repeated at least five times. *p < 0.05.(c, d) HUVECs were incubated in the presence or absence of 50 nM glabridin for 1 h. LPS (1 µg/ml) was then added and the cells were further incubated for 6 h. The cells were then lysed and analyzed using SDS–PAGE and immunoblotting. All films were scanned, and signals in the linear range were quantified using Image J software and normalized to the control levels (c, d).The experiments were repeated at least four times. *p < 0.05.
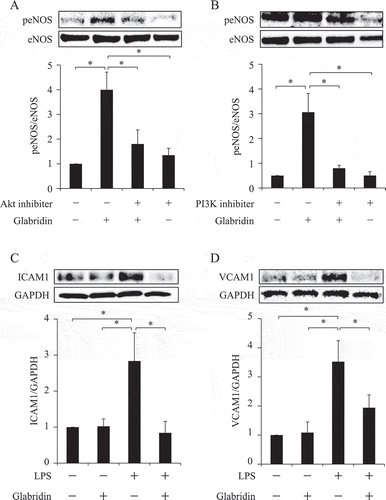
Lipopolysaccharides (LPSs), which are components of gram-negative bacteria, have been reported to induce endothelial cell injury. Serum LPS activity also enhances acute bacterial infection and contributes to lifestyle-related conditions such as diabetes, dyslipidemia, and obesity; collectively referred to as metabolic endotoxemia. Therefore, LPSs trigger acute and chronic inflammation, thereby promoting the development of atherosclerotic disease. In this study, we used LPSs to understand how glabridin protects against vascular injuries. We found that LPSs induced endothelial cell injury in HUVECs, demonstrated by an increase in ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) protein levels. However, glabridin inhibited these effects (Figure ).
4. Discussion
In this study, we demonstrated that T-Cho, LDL-C, HbA1c, immunoreactive glucagon, and body fat composition significantly decreased after 12 weeks of glabridin administration in healthy volunteers. We also showed that treatment exerted subtle or no metabolic effects on patients with type 2 diabetes. Oral ingestion of glabridin significantly improved vascular functions in healthy volunteers, as demonstrated by improvements in FMD and baPWV in healthy volunteers. We also demonstrated that the PI3K/Akt pathway mediated a significant increase in the eNOS levels of HUVECs exposed to glabridin. Additionally, in the HUVECs, glabridin inhibited the LPS-induced increase in ICAM-1 and VCAM-1 levels.
Glabridin has been reported to possess anti-inflammatory, anti-obesity and anti-diabetic (Simmler, Pauli, & Chen, Citation2013). Studies mostly conducted in diabetic animal models reported that glabridin administration increases body weight, glucose tolerance, and superoxide dismutase activities in the liver, kidneys, and pancreas, while decreasing fasting blood glucose levels and malondialdehyde content in these organs (Wu, Jin, & Jin, Citation2013). It has also been reported that glabridin significantly reduces high-fat diet-induced weight gain, in a dose-dependent manner (Aoki et al., Citation2007a). Glabridin has also been reported to ameliorate adiposity and lipid dysregulation in animal models of obesity via AMPK activation (Lee et al., Citation2012). However, these studies were performed either in rodent models or in vitro. Therefore, we examined the effects of glabridin on human subjects. Glabridin exerted more favorable metabolic effects in the healthy volunteers, compared to the subtle effects it exerted in patients with type 2 diabetes. In this study, 300 mg glabridin was used; a dose equivalent to what is generally used as dietary supplements for healthy subjects. It has been reported that glabridin exhibits linear pharmacokinetics in the dose range of 300–1200 mg/individual, and its steady state plasma level (1.42 to 4.4 ng/mL) is attained after two weeks of daily administration in healthy humans (Aoki et al., Citation2007b). The mean glabridin concentration of 1.73 ng/ml observed in this study therefore falls within the expected blood concentration range.
Glabridin also improved vascular function. FMD and baPWV are widely used as non-invasive measures of vascular function among healthy subjects. FMD is an indicator of endothelial function (Benjamin et al., Citation2004; Celermajer et al., Citation1992), whereas baPWV is an indicator of vascular stiffness (O’Rourke, Staessen, Vlachopoulos, Duprez, & Plante, Citation2002). Multiple studies have reported that FMD is an independent predictor of cardiovascular diseases and might be associated with eNOS function and oxidative stress in endothelial cells (Corretti et al., Citation2002). Many factors have been implicated in FMD improvement, including reduced blood glucose (Nakamura et al., Citation2014) and cholesterol levels (Sahebkar et al., Citation2016), and weight loss (Wycherley et al., Citation2016). In this study, glabridin significantly reduced T-Cho, LDL-C, and HbA1c levels in the healthy volunteers. This study also demonstrated that glabridin reduces serum levels of soluble ICAM1, albeit not significantly. These results suggest that the improvements of the FMD of healthy volunteers by glabridin might be dependent on its effects on vascular endothelial cells directly and/or mediated by its beneficial effects on metabolic parameters. Glabridin has been reported to possess anti-inflammatory (Kang et al., Citation2005; Yokota et al., Citation1998) and anti-obesity (Ahn et al., Citation2013; Aoki et al., Citation2007a), which is consistent with this hypothesis.
A defective eNOS function leads to reduced levels of NO, which promote vascular dysfunction and cardiovascular disease. The link between endothelial cell defects and eNOS dysfunction, also referred to as eNOS uncoupling, results in the transition of eNOS from an NO-producing enzyme to an enzyme that generates superoxides such as hydrogen peroxide (Karbach, Wenzel, Waisman, Munzel, & Daiber, Citation2014). Therefore, to enable proper vascular function and prevent cardiovascular disease incidence, eNOS function must be properly maintained. Therefore, this study focused on the direct effects of glabridin on cultured endothelial cells, and observed that it enhanced eNOS activation via the PI3K/Akt signaling pathway. These results indicate that glabridin possesses anti-atherogenic properties, consistent with previous reports. Glabridin also prevented LDL oxidation (Kang et al., Citation2006). Oxidized LDLs have been shown to promote vascular inflammation and atherogenesis. A previous study demonstrated that glabridin inhibited the translocation of NADPH oxidase p47 to the plasma membrane via protein kinase C (Rosenblat et al., Citation1999). In addition, glabridin has been shown to inhibit tumor necrosis factor (TNF)-α-induced ICAM-1 and VCAM-1 expression in HUVECs (Kang et al., Citation2006). Furthermore, glabridin inhibits the expression of multiple inflammatory cytokines, including TNF-α, interleukin (IL)-1β, interferon (INF)-γ, and IL-12, in macrophages (Kim et al., Citation2010).
The major limitation of this study was the small sample size used. The number of participants was below the standard number required for multivariate analyses of the clinical parameters associated with FMD improvements. Vascular functions were not evaluated in the patients with type 2 diabetes. Further, the reason why the glabridin had less favourable effects on type 2 diabetes compared to healthy volunteer are unclear. It might be so that a higher dose of glabridin administered for longer periods may be required for the achievement of similar results in patients with type 2 diabetes. These hypotheses will be examined in the future.
5. Conclusion
Glabridin exerted several favorable metabolic effects and improved vascular functions, especially in healthy volunteers. However, this was a pilot study. A large-scale, long-term study of healthy subjects is required to further evaluate the preventive value of glabridin.
Competing interests
The authors declare that they have no competing interests
Acknowledgements
This study is supported by Grants-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labor and Welfare.
Additional information
Funding
Notes on contributors
M. Takemoto
Prof. Takemoto`s research group has focused on the research about the mechanisms of diabetes complications especially on the diabetic kidney disease, sarcopenia and progeria syndrome.
References
- Ahn, J., Lee, H., Jang, J., Kim, S., & HA, T. (2013). Anti-obesity effects of glabridin-rich supercritical carbon dioxide extract of licorice in high-fat-fed obese mice. Food and Chemical Toxicology : an International Journal Published for the British Industrial Biological Research Association, 51, 439–11. doi:10.1016/j.fct.2012.08.048
- Aoki, F., Honda, S., Kishida, H., Kitano, M., Arai, N., Tanaka, H., … Mae, T. (2007a). Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Bioscience, Biotechnology, and Biochemistry, 71, 206–214. doi:10.1271/bbb.60463
- Aoki, F., Nakagawa, K., Kitano, M., Ikematsu, H., Nakamura, K., Yokota, S., … Mae, T. (2007b). Clinical safety of licorice flavonoid oil (LFO) and pharmacokinetics of glabridin in healthy humans. Journal of the American College of Nutrition, 26, 209–218. doi:10.1080/07315724.2007.10719603
- Aoki, F., Nakagawa, K., Tanaka, A., Matsuzaki, K., Arai, N., & Mae, T. (2005). Determination of glabridin in human plasma by solid-phase extraction and LC-MS/MS. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 828, 70–74. doi:10.1016/j.jchromb.2005.09.012
- Benjamin, E. J., Larson, M. G., Keyes, M. J., Mitchell, G. F., Vasan, R. S., Keaney, J. F., JR., … Vita, J. A. (2004). Clinical correlates and heritability of flow-mediated dilation in the community: The Framingham heart study. Circulation, 109, 613–619. doi:10.1161/01.CIR.0000112565.60887.1E
- Celermajer, D. S., Sorensen, K. E., Gooch, V. M., Spiegelhalter, D. J., Miller, O. I., Sullivan, I. D., … Deanfield, J. E. (1992). Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet, 340, 1111–1115. doi:10.1016/0140-6736(92)93147-f
- Corretti, M. C., Anderson, T. J., Benjamin, E. J., Celermajer, D., Charbonneau, F., Creager, M. A., … Vogel, R. (2002). Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial artery reactivity task force. Journal of the American College of Cardiology, 39, 257–265. doi:10.1016/s0735-1097(01)01746-6
- Guariguata, L., Whiting, D. R., Hambleton, I., Beagley, J., Linnenkamp, U., & Shaw, J. E. (2014). Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice, 103, 137–149. doi:10.1016/j.diabres.2013.11.002
- Hasanein, P. (2011). Glabridin as a major active isoflavan from Glycyrrhiza glabra (licorice) reverses learning and memory deficits in diabetic rats. Acta Physiologica Hungarica, 98, 221–230. doi:10.1556/APhysiol.98.2011.2.14
- Hsieh, M. J., Chen, M. K., Chen, C. J., Hsieh, M. C., Lo, Y. S., Chuang, Y. C., … Yang, S. F. (2016). Glabridin induces apoptosis and autophagy through JNK1/2 pathway in human hepatoma cells. Phytomedicine, 23, 359–366. doi:10.1016/j.phymed.2016.01.005
- Kang, J. S., Yoon, Y. D., Cho, I. J., Han, M. H., Lee, C. W., Park, S. K., & Kim, H. M. (2005). Glabridin, an isoflavan from licorice root, inhibits inducible nitric-oxide synthase expression and improves survival of mice in experimental model of septic shock. The Journal of Pharmacology and Experimental Therapeutics, 312, 1187–1194. doi:10.1124/jpet.104.077107
- Kang, J. S., Yoon, Y. D., Han, M. H., Han, S. B., Lee, K., Lee, K. H., … Kim, H. M. (2006). Glabridin suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking sphingosine kinase pathway: Implications of Akt, extracellular signal-regulated kinase, and nuclear factor-kappaB/Rel signaling pathways. Molecular Pharmacology, 69, 941–949. doi:10.1124/mol.105.017442
- Kang, M. R., Park, K. H., Oh, S. J., Yun, J., Lee, C. W., Lee, M. Y., … Kang, J. S. (2015). Cardiovascular protective effect of glabridin: Implications in LDL oxidation and inflammation. International Immunopharmacology, 29, 914–918. doi:10.1016/j.intimp.2015.10.020
- Karbach, S., Wenzel, P., Waisman, A., Munzel, T., & Daiber, A. (2014). eNOS uncoupling in cardiovascular diseases–The role of oxidative stress and inflammation. Current Pharmaceutical Design, 20, 3579–3594. doi:10.2174/13816128113196660748
- Kim, J. Y., Kang, J. S., Kim, H. M., Ryu, H. S., Kim, H. S., Lee, H. K., … Han, S. B. (2010). Inhibition of bone marrow-derived dendritic cell maturation by glabridin. International Immunopharmacology, 10, 1185–1193. doi:10.1016/j.intimp.2010.06.025
- Klasik-Ciszewska, S., Kaczmarczyk-Sedlak, I., & Wojnar, W. (2016). Effect of glabridin and glycyrrhizic acid on histomorphometric parameters of bones in ovariectomized rats. Acta Poloniae Pharmaceutica, 73, 517–527.
- Lee, J. W., Choe, S. S., Jang, H., Kim, J., Jeong, H. W., Jo, H., … Kim, J. B. (2012). AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. Journal of Lipid Research, 53, 1277–1286. doi:10.1194/jlr.M022897
- Nakamura, K., Oe, H., Kihara, H., Shimada, K., Fukuda, S., Watanabe, K., … Ito, H. (2014). DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovascular Diabetology, 13, 110. doi:10.1186/1475-2840-13-80
- O’Rourke, M. F., Staessen, J. A., Vlachopoulos, C., Duprez, D., & Plante, G. E. (2002). Clinical applications of arterial stiffness; definitions and reference values. American Journal of Hypertension, 15, 426–444. doi:10.1016/s0895-7061(01)02319-6
- Riccioni, G., Speranza, L., Pesce, M., Cusenza, S., D’Orazio, N., & Glade, M. J. (2012). Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition, 28, 605–610. doi:10.1016/j.nut.2011.11.028
- Rosenblat, M., Belinky, P., Vaya, J., Levy, R., Hayek, T., Coleman, R., … Aviram, M. (1999). Macrophage enrichment with the isoflavan glabridin inhibits NADPH oxidase-induced cell-mediated oxidation of low density lipoprotein. A possible role for protein kinase C. The Journal of Biological Chemistry, 274, 13790–13799. doi:10.1074/jbc.274.20.13790
- Rudic, R. D., Shesely, E. G., Maeda, N., Smithies, O., Segal, S. S., & Sessa, W. C. (1998). Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. The Journal of Clinical Investigation, 101, 731–736. doi:10.1172/JCI1699
- Sahebkar, A., Giua, R., Pedone, C., Ray, K. K., Vallejo-Vaz, A. J., & Costanzo, L. (2016). Fibrate therapy and flow-mediated dilation: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacological Research : the Official Journal of the Italian Pharmacological Society, 111, 163–179. doi:10.1016/j.phrs.2016.06.011
- Saitoh, T., Kinoshita, S., & Shibata, S. (1976). E New isofiavanand flavanone from licorice Roet. Chemical and Pharmaceutical Bulletin, 24, 752–754. doi:10.1248/cpb.24.752
- Simmler, C., Pauli, G. F., & Chen, S. N. (2013). Phytochemistry and biological properties of glabridin. Fitoterapia, 90, 160–184. doi:10.1016/j.fitote.2013.07.003
- Toth, P. P., Potter, D., & MING, E. E. (2012). Prevalence of lipid abnormalities in the United States: The national health and nutrition examination survey 2003–2006. Journal of Clinical Lipidology, 6, 325–330. doi:10.1016/j.jacl.2012.05.002
- Wu, F., Jin, Z., & Jin, J. (2013). Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Molecular Medicine Reports, 7, 1278–1282. doi:10.3892/mmr.2013.1330
- Wycherley, T. P., Thompson, C. H., Buckley, J. D., Luscombe-Marsh, N. D., Noakes, M., Wittert, G. A., & Brinkworth, G. D. (2016). Long-term effects of weight loss with a very-low carbohydrate, low saturated fat diet on flow mediated dilatation in patients with type 2 diabetes: A randomised controlled trial. Atherosclerosis, 252, 28–31. doi:10.1016/j.atherosclerosis.2016.07.908
- Yokota, T., Nishio, H., Kubota, Y., & Mizoguchi, M. (1998). The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Research / Sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society, 11, 355–361.
- Yu, X. Q., Xue, C. C., Zhou, Z. W., Li, C. G., Du, Y. M., Liang, J., & Zhou, S. F. (2008). In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice). Life Sciences, 82, 68–78. doi:10.1016/j.lfs.2007.10.019
