Abstract
Zambia has three primary agro-ecological regions, with each region having specific ecological characteristics. Region II agro-ecological zone of Zambia has low nutrient reserves and poor water holding capacity due to moderately leached clayey to loamy soil; this has led to low soybean productivity. Soil fertility management (ISFM) strategies such as the use of inorganic fertilizers and the use of inoculants of rhizobia have been introduced and promoted among small-scale farmers in Eastern Province. Two soybean varieties (Lukanga and Kafue) were used for this study and 96 samples from on-farm soil fertility management trials in Chipata, and Petauke districts were collected for the determinations of nutritional and anti-nutritional properties. The proximate analysis of Chipata samples showed that the ash content 5.10‒6.23%, fat content 17.71‒25.57%, protein content 27.73‒37.11%, amylose content 1.26‒4.56 %, sugar content 6.75‒9.62%, and starch content 4.00‒18.57%, while anti-nutritional properties ranged between 3.07 and 8.21% for phytate and 1.42‒3.35% for tannin. With Petauke, the ash content 3.32‒6.8 %, fat content 19.16‒26.85%, protein content 27.68‒35.62%, amylose 2.00‒4.37%, sugar content 6.23‒9.76%, and starch content 5.70‒18.63%. Phytate and tannin contents were 3.37‒7.90% and 0.14‒3.32%, respectively. The highest protein content was found at the level of 37.11% in Kafue with 40kg P/ha and inoculant, and the least was 27.73% for Lukanga without inputs in Chipata while in Petauke, Lukanga with 40 P/ha and inoculant had the highest protein content of 35.62% and the least was 27.68% for Lukanga with inoculant. The co-application of rhizobia inoculant and P nutrient increased phytate, and tannin content significantly (P < 0.05).
PUBLIC INTEREST STATEMENT
Soya bean is a beneficial legume crop indigenous to Zambia. It has been useful in the fortification of foods to combat malnutrition, especially in infants and pregnant women. Also, it has helped drastically in reducing protein-energy malnutrition among the general populace in Zambia. Thus, the need to encourage small scale farmers to embrace its cultivation to close the malnutrition gap likely to remain by integrating farmers into safe soil fertility management techniques to improve the quality and quantity of soya bean.
Competing Interests
The authors declares no competing interests.
1. Introduction
In Zambia, the soybean is used as an industrial crop for products such as soya oil, soya chunks, and soya meal. Also, the by-product (cake) is processed into animal feed (Hichaambwa, Chileshe, Chimai-Mulenga, Chomba, & Mwiinga-Ngcobo, Citation2014). Soybean has been used in human and animal nutrition because of its abundant protein and oil content (LIU, Citation2000) as well as its essential functional properties for the development of different types of food for humans (TRAINA & BREENE, Citation1994). Soybean has highly valuable proteins and oils (on average ranging from 39 to 41% crude protein and 18‒21% oil), which makes it a good feed alternative to animal proteins and oils. According to its digestibility and amino acid composition, soybean has very similar proteins with those of animal origin, except that the sulphur amino acid (cysteine and methionine) content is limited (Anderson & Wolf, Citation1995). Soybean is the leading protein source used in poultry and livestock diets around the world as its high-quality protein is equivalent to milk protein when supplemented with methionine (Liener, Citation2000). It also has many of the essential amino acids that are deficient in most cereal, grain-based diets fed to poultry and swine (Bruce et al., Citation2006). According to (Stein et al., Citation2008), this unique composition of amino acids (AA) complements the AA composition of many cereal grains in complete animal diets. In Zambia, soybean has extreme market potential in the animal feed and food processing industry. As soybean is an excellent source of protein and other nutrients, it has been used to fortify fritters for improving the nutritional status of rural communities and the Zambian population in general (Alamu, Popoola, & Maziya‐Dixon, Citation2018). However, production is dominated by commercial farmers and is not very attractive to smallholder farmers due to several challenges such as limited access to improved soybean varieties and knowledge on how to cultivate the crop especially about the use of inoculum and phosphate fertilizers for boosting yields (Hichaambwa et al., Citation2014). Based on these challenges, for the smallholder farmers that grow soybean, yields have remained poor, with an average of less than one ton per hectare (Lubungu, Burke, & Sitko, Citation2013). This currently reported yields of 0.5–0.9 t ha−1 are estimated to be about 30% of potential yield. This is notwithstanding the availability of high yielding varieties on the market, improved production technologies, and ready markets for the crop (Hichaambwa et al., Citation2014; Lubungu et al., Citation2013).
To improve the yield and quality of soybean grain, previous studies have reported that integrated soil fertility management (ISFM) practices such as co-application of organic manure and P fertilizers are very useful (Chiezey & Odunze, Citation2009; Manral & Saxena, Citation2003; Rurangwa, Vanlauwe, & Giller, Citation2018). Also, there is evidence that seed inoculation with Rhizobium strains enhance nitrogen (N) fixation in soybean whether the cultivar is promiscuous or not. For example, there is evidence in the literature that significant yield increase was obtained by inoculation of soybean with the right bacteria before sowing (Ibrahim & Mahmoud, Citation1989; Joshi, Nkumbula, & Javaheri, Citation1986; Kim et al., Citation1988). The P nutrient can play an essential role in seed yield as it is one of the limiting plant nutrients to produce soybean (Rao, Krishna, Prasad, & Raghavan, Citation1995). Its uptake and utilization are essential for ensuring proper growth and improving yield and quality of the crop. It influences the growth of roots, helps the uptake of more nutrients and nodule formation, balances the N deficiency in the soil, and aids in seed maturation. Thus, it is essential to find out the optimum amount of N and P nutrients needed for achieving better soybean yield. This study aimed to investigate the impact of the soil fertility management practices on the nutritional and anti-nutritional properties of Soybean varieties grown in Eastern Zambia. Consequently, a chemical analysis of two soybean varieties with different treatments was carried out to show the effects of ISFM practices on their nutritional and anti-nutritional properties.
2. Materials and methods
2.1. Description of experiment area
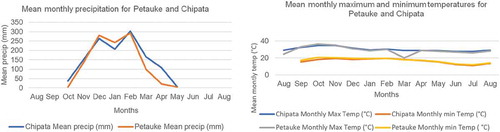
The experiments were conducted in the cropping seasons of 2015/2016 and 2016/2017. The sites were Chipata (13.64598°S, 032.55849°E) and Petauke (14.49810°S, 0.31.32428°E). All sites are in the Eastern Province of Zambia situated in agro-ecological zone II with an average annual rainfall of between 800 and 1000 mm. All sites have a single rainy season which extends from October to April. The trends of monthly mean minimum and maximum temperatures and rainfall of the 2016/2017 cropping season are shown in Figure . From Figure , in both Chipata and Petauke, the rainfall increases sharply from October to December and then declines in January before increasing sharply again up to February. Maximum rainfall was recorded in February, which indicates that the crop was free of water deficit stress. However, the rainfall declined in March to May, and by then the crop had reached physiological maturity. Therefore, the development or full growth period of the crop was not subjected to any water deficit.
2.2. Experimental treatments, design, and procedures
The treatments were arranged in a randomized complete block design (RCBD) in factorial combinations with three replications. The treatments were two varieties (Kafue and Lukanga), two levels of P application (0 and 40 kg P/ha) with single super phosphate (SSP) and with and without rhizobia inoculant (500g/ha) (Table ). The P fertilizer was broadcast in the rip lines. A total of 8 treatments were used in the experiments. The size of each of the 16 plots was 5 m × 10 m (50 m2). The land was manually ridged. The space between plots was 1 m. Each plot had 8 planting rows, and the space between rows was 50 cm. At planting, soybean seeds were planted 5 cm apart within a row at a depth of 4 cm. Harvesting was done at physiological maturity, and 48 samples collected per location.
2.3. Chemical and physical analysis of soil properties
The results of the chemical and physical properties of soils from the two study sites are presented in Table . Soil pH was measured in 0.01 M CaCl2. The Walkley and Black (Citation1934) was used to determining the organic carbon content (Org. C) of the soil. The total N content of the soil was determined using the Kjeldahl digestion, distillation, and titration procedure (Jackson, Citation1958). Available P was analysed based on the Bray II method (Bray & Kurtz, Citation1945). The ammonium acetate method was employed to determine the cation exchange capacity (CEC) of the soil. CEC was determined by leaching the 10 g of sampled soil with neutral 1M ammonium acetate (FAO, Citation2008). The excess ammonium is removed, the amount of exchangeable ammonium is determined. The pH of the soils is below 5, and this indicates that both soils were slightly acidic. The % organic carbon, % Nitrogen, and minerals (ppm) of soil from Chipata are far higher than that of Petauke except for the pH and %CEC that are lower. The amount of K, Na, Ca and Mg were determined using the Atomic Absorption Spectroscopy (AAS) technique.
Table 1. Codes for the different treatments
Table 2. Chemical and physical properties of soils from the two locations
2.4 Proximate Composition Analyses
The harvested, dried soybean samples were milled using a Laboratory mill 310 (PERTEN) using a sieve size of 0.5 mm, packed in a well-labelled polyethylene whirl-pack bag, and stored at 4 °C until analysed for proximate composition and anti-nutritional properties. The samples were analysed in duplicate, for moisture, ash, protein, fat, total sugars, starch, amylose, crude fibre, phytate, and tannin content using methods described by (Alamu et al., Citation2015) according to Association of Official Analytical Chemists (AOAC) (Citation2005).
2.5 Anti-nutritional property determination
(a) Phytate determination: Phytate (Phytic acid) was analysed using two methods, where the extraction and precipitation of phytic acid were done following the method of (Wheeler & Ferrel, Citation1971) as described by (Maziya‐Dixon, Alamu, Popoola, & Yomeni, Citation2017). This was followed by Iron (Fe) precipitation that was measured according to the method of (Makower, Citation1970). A 4:6 Fe/P atomic ratio was used to calculate the phytic acid content. (b) Tannin determination: Tannins were determined following the method described by (Maziya‐Dixon et al., Citation2017). The measurement was based on the reduction reaction of phosphotungstomolybdic acid by tannin-like compounds in an alkaline solution, producing a brightly coloured blue solution, measuring 760 nm.
3. Statistical analysis
The laboratory analyses of the samples were carried out in duplicate, and the mean ± standard deviation of the results was calculated and using the Statistical Analysis System (SAS) software package (version 9.3). Also, Duncan’s multiple range test was used to separate the differences in the mean scores at a significant level of P = 0.05.
4. Results
4.1. Nutritional properties of various soybean using different ISFM practices in Petauke
Data on the proximate composition of the two soybean varieties in Petauke are shown in Table . Significant (p < 0.05) differences existed in moisture content, ash, protein, amylose, sugar, and starch. The moisture content of Kafue ranged from 4.36 % to 6.20 %. T16 led to increased moisture content compared to T7 that had no inputs. That of Lukanga ranged from 4.59 % to 6.32 % with the application of 40 kg P ha−1 and T10 having higher moisture content compared to T5. The ash content for Kafue ranged from 5.86 % to 6.80 %, with T1 having a higher increase compared to T7. That of Lukanga ranged from 6.25 % to 6.57 % with T4 being higher compared to T9. The protein content of the variety Kafue ranged from 29.89 % to 35.18 % with T2 having the highest increase in the value of the protein content, while that of Lukanga ranged from 27.68 % (T4) to 35.62% (T10). The amylose content of Kafue was in the range of 2.0 % to 4.37 % for T2 and T14, respectively. That of no input plots was 2.74 % and 2.43 % for T9 and T15, respectively. That of Lukanga increased from 2.21 % to 3.61 %. The lowest values of 2.21 % were T12 and T4. T5 and T9 had an amylose content of 3.61 % and 2.74 % respectively. The sugar content ranged from 6.23 % to 9.76 %, with T14 having the highest increase in the sugar content (9.76%) compared to T7 while that of Lukanga ranged from 9.05 % to 9.62 %; T12 having the highest increase and T5 having the lowest sugar content. The amount of starch in Kafue ranged from 5.75 % to 18.39 %, with T2 having the highest increase in the starch content and T16 having the lowest . That of Lukanga ranged from 5.70 % to 18.18 %; with T4 having the highest starch content, and T9 had the lowest starch content.
Table 3. Nutritional properties of various soybean using different ISFM practices in Petauke
4.2. Nutritional properties of various soybean using different ISFM practices in Chipata
Table shows the results of the nutritional properties of two soybean varieties (Kafue and Lukanga) using different ISFM practices in Chipata. A significant difference existed in moisture content, ash, fat, protein, amylose, sugar, and starch. The moisture content ranged from 4.49% to 5.95%, and the ash content for Kafue increased from 5.10% to 6.03%. T16 and T3 had the highest ash content as compared to T7 and T15 while that of Lukanga ranged from 5.22% to 6.23%. The highest increase was due to T10 while the lowest was due to T6. The fat content increased from 17.71% to 20.57%, with the lowest T14 and the highest T1, respectively. That of Lukanga ranged from 18.84% (T8) to 25.57% (T10). The protein content of Kafue ranged from 32.02% (T16)to 37.11% (T14), while that of Lukanga ranged from 27.73% to 34.98% with the highest being T9.
Table 4. Nutritional properties of soybean using different ISFM practices in Chipata
The amylose content for Kafue ranged from 1.37% to 4.56%, and the highest amylose content was as a result of T1and T2. T15 showed the lowest amylose content. That of Lukanga ranged from 1.26% to 4.26% with the T8 and T4 having the lowest and the highest amylose content, respectively. As for the sugar content, it ranged from 7.38% to 9.10 % with T2 and T1 having the lowest and highest sugar content, respectively, for Kafue. Also, the sugar content of Lukanga ranged from 6.75 to 9.01%, with T10 and T13 having the lowest and the highest sugar content, respectively. The amount of starch in Kafue ranged from 4.00 % to 18.57% in T16 and T2 respectively, while that of Lukanga ranged from 6.65% to 16.93% with T12 and T6 having the lowest and the highest starch content, respectively.
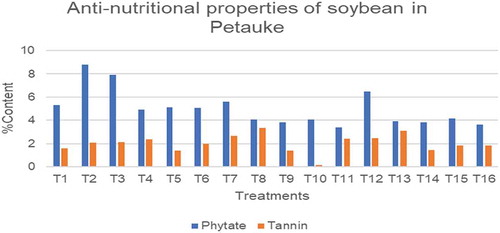
4.3. Anti-nutritional properties of various soybean using different ISFM practices in Petauke
In Figure , the results showed significant differences in the phytate and tannin content of soybean. The phytate content of Kafue ranged from 3.37% to 8.8%, the lowest and the highest phytate content were T11 and T2, respectively. The phytate content of Lukanga ranged from 3.82% to 6.46%. The highest amount was T12 while T9 recorded the lowest phytate content. Tannins in Kafue ranged from 1.44% to 2.65%, T14 and T7 respectively, while that of Lukanga ranged from 0.14% to 3.32 %, T10 and T8 respectively.
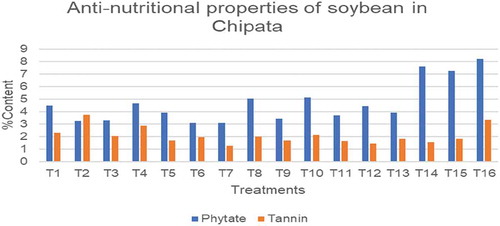
4.4. Anti-nutritional properties of various soybean using different ISFM practices in Chipata
As shown in Figure , there were significant (p < 0.05) differences in the phytate and tannin content of soybean; the phytate content of Kafue ranged from 3.07 % to 8.21%, the lowest phytate content was T7, and the highest was T16. That of Lukanga ranged from 3.44% to 5.12%. T9 had the lowest phytate content, while T10 had the highest amount. The tannin amount ranged from 1.26 % to 3.76% for Kafue. The lowest and highest tannin content was a result of T7 and T2, respectively. That of Lukanga ranged from 1.42 to 2.86%, T12 and T10 respectively.
5. Discussion
The moisture content was found to be different from 1.02 to 1.08% reported by (Eshun, Citation2012). The low moisture content of 4.32% for T8 in Chipata implies that the sample can be stored for a very long time since moisture, which is an essential medium in the multiplication of microorganisms, is very low in the sample. The high moisture content of 6.32% for T10 in Chipata can be attributed to the above-normal rainfall (1242.6 mm) received in the area, and the organic carbon content of 0.9% despite it is lower than the range of 1.6‒3.3% recognized by (Jackson, Citation1958) and (Nelson & Sommers, Citation1982). Soil organic matter content and composition affect both soil structure and adsorption properties; therefore, water retention may be affected by changes in soil organic matter that occur because of both climate change and modifications of management practices. The highest ash content of 6.80% which is an indication that the sample could be an essential source of minerals for T1 in Petauke, was higher than the range of 1.01‒1.67% reported by (Eshun, Citation2012). The increase in the ash content can be attributed to the application of P, which leads to the absorption of P, and other minerals by the developing root. It plays a vital role in the plant’s energy transfer system since its deficiency retards growth (Qasim, Farrukh Saleem, Khan, & Anjum, Citation2009). Symbiotic nitrogen fixation needed high levels of phosphorus as the vast amounts of energy being consumed during the process of photosynthesis, or energy-generating metabolism, depend on the availability of phosphorus (Schulze, Temple, Temple, Beschow, & Vance, Citation2006). The fat content of 26.85% obtained for T9 in Petauke was not consistent with the reported 16.82‒19.30 range (Eshun, Citation2012).
Soybean contributes to 28% of the world’s edible oil (Foster, Williamson, & Lunn, Citation2009; Kim et al., Citation2015) and is second in production of edible oils to palm oil. It should be noted that oils are produced from plant sources for other purposes than food such as detergents, candles, pharmaceuticals, and biofuels; considering these products, soy contributes to half of the world production of oil (Foster et al., Citation2009). Edible oils from soybean are processed to create numerous food products such as salad dressings, margarine, and spreads. Oil comprises of 17‒19% of soybean dry weight, of which most is polyunsaturated fatty acids (Hammond, Citation1991; Krishnan, Natarajan, Mahmoud, & Nelson, Citation2007; Natarajan, Xu, Bae, Caperna, & Garrett, Citation2006; Sharma, Kaur, Goyal, & Gill, Citation2014; Yaklich, Citation2001). The high-fat content suggests that the soybean sample is a viable source of oil, going by its fat content. Most legumes contain 1.5% fat. Soybean fat is very high compared to most legumes because it is an oilseed. The protein content of 37.11% obtained for T14 in Chipata compared favorably with the value of 36% (Edema, Sanni, & Sanni, Citation2005) and 36.94‒40.10% (Eshun, Citation2012). Through its essential functions in plants as an energy source, phosphorus affects nodule development, production of protein, phospholipids, and phytin in grain legumes (Rahman, Bhuiyan, Sutradhar, Rahman, & Paul, Citation2008). The high protein content suggests that it could be used in the management of protein deficiency cases such as kwashiorkor.
Amylose content is one of the critical factors affecting starch pasting and retrogradation. It provides surface and textural regularity, elasticity, and sticky characteristics to starch-based products (De La Guerivier, Citation1976). The high amylose content in Chipata was due to T1 and T2. The sugar content of 9.76% was obtained for T14 in Petauke. Adequate P nutrient application promotes root growth, the process of photosynthesis, translocation of sugars, and other such functions, which directly influence N fixation by legume plants (Abdul-Aziz, Citation2013). The value is lower than the 16.6% reported by (Hymowitz & Collins, Citation1974). The principal sugars present in soybean seed include glucose, fructose, sucrose, raffinose, and stachyose (Eldridge, Black, & Wolf, Citation1979; Yazdi-Samadi, Rinne, & Seif, Citation1977). Sucrose makes up 41.3–67.5%, raffinose 5.2–15.8%, and stachyose 12.1–35.2% of the total soluble sugars in soybean seed (Yazdi-Samadi et al., Citation1977). Trace amounts of other sugars have also been reported, such as pinitol, myo-inositol, verbascose, galactose, arabinose, and mannose (Schweizer, Horman, & Würsch, Citation1978; Yazdi-Samadi et al., Citation1977). Sugars in soybean seed affect soy food quality, digestibility, and nutritional values. Soy food such as soymilk, tofu, and natto are part of a healthy diet, and consumption is highly recommended by nutritionists and medical doctors (Messina, Citation2003).
T14 had the highest starch content of 18.63%, the amount of starch in this sample can be attributed to the P nutrient content of the soil in Petauke (25 ppm); this value obtained in this study compared reasonably with the range (18 to 45%) reported for other legume starches (GUJSKA, D.‐REINHARD, & KHAN, Citation1994; Yañez‐Farias, Moreno‐Valencia, Falcón‐Villa, & Barrón‐Hoyos, Citation1997). Starch remains a significant source of calories in the human diet and can be found in high concentrations in the primary storage organs of plants, including roots and tubers, stems, seeds, and grains. Starch is the essential carbohydrate consumed on a worldwide basis because of its high uninterrupted abundance and low cost.
Phytic acid chelates calcium, magnesium, potassium, iron, and zin is rendering them unavailable to non-ruminant animals. Many phytates in diets decrease the availability of these minerals, mainly calcium, phosphorus, and zinc. Phytates also decrease the activity of enzymes (pepsin, trypsin, and amylase) as well as the availability of protein, amino acids, starch, and energy (Ravindran, Cabahug, Ravindran, Selle, & Bryden, Citation2000; Sebastian, Touchburn, & Chavez, Citation1998). The high phytate content of 8.81% in Petauke was due to T2. Phytic acid concentration appears to be a function of plant P status during soybean seed development. This agrees with the findings of Earley and DE TURK (Citation1944), who demonstrated a requirement for nutrient P during maize kernel development. Asada, Tanaka, and Kasai (Citation1970) demonstrated the positive relationship between nutrient P and phytic acid accumulation in rice. Recently, in a study with wheat grown in a potting soil medium, Michael, Zink, and Lantzsch (Citation1980) also demonstrated the effectiveness of late P fertilization (at or after flowering) as a means of inducing variation in phytic acid content. Phytates influence the decrease of feed consumption by chickens as well as their growth (Shan & Davis, Citation1994). Liener (Citation2000) estimated that two-thirds of the phosphorus in soybean is bound as phytate and unless freed is mostly unavailable to animals. Phytic acid is present in soybean and most soybean products at a level of 1‒1.5 g/100 g dry matter. The higher tannin content value of 3.76% in Chipata was a result of T2, this value was in the range 4.9‒7.0 mg g−l for the tannin content in faba bean (Vicia faba) seed (Helsper, Hoogendijk, van Norel, & Burger-Meyer, Citation1993) and in seed of six cowpea cultivars ranging from 3.6 to 4.1 mg g-l (Xavier-Filho et al., Citation1989). The presence of tannin in seed has been associated with lower nutritive value and lower biological availability of macromolecules, such as proteins, amino acids, carbohydrates, vitamins, and minerals (Deshpande & Cheryan, Citation1985). Tannin content is affected by many factors such as genotype, time of harvest, and temperature. Rhizobium inoculation significantly increased the tannin content of faba bean and groundnut seed. (Babiker, El Sheikh, Osman, & El Tinay, Citation1995; Elsheikh & Ahmed, Citation2000; Elsheikh & Elzidany, Citation1997; Elsheikh & Mohamedzein, Citation1998).
6. Conclusion
The study investigated the impact of different integrated soil fertility management practices on the nutritional and anti-nutritional properties of Soybean varieties grown in Eastern Zambia. The results show that application of P nutrient significantly increased ash, protein, fat, amylose, and sugar content. The results also show that soybean is rich in protein, fat, and starch and is, therefore, an inexpensive source of macronutrients that can be used in intervention programs aimed at alleviating protein-energy malnutrition.
Acknowledgements
The authors would like to acknowledge the support received from the African Green Revolution Alliance (AGRA), Development Aid for People to People (DAPP), the Zambia Agricultural Research Institute (ZARI), the CGIAR Grain Legumes and Dryland Cereals (GLDC) Research program and all the project staff who contributed to the success of the study.
Data availability statement
The csv and xlsx format of the data used to support the findings of this study were deposited to IITA CKAN- open access repository for research database (http://data.iita.org/dataset) and also available from the corresponding author upon request.
Additional information
Funding
Notes on contributors
Emmanuel Oladeji Alamu
Dr. Emmanuel Oladeji, a Nigerian, is a Food Scientist and Technologist working with the IIITA, Zambia. His research interests are Chemistry of Food formulations, Biofortification and New Food products development.
Therese Gondwe
Dr. Therese Gondwe is a Social Scientist with more than 10 years in formulating, managing and implementing Food and Nutrition Projects in Southern Africa.
Gbenga Akinwale
Dr. Gbenga Akinwale, a Nigerian, is a Plant Breeder and Program Manager at IIITA, Chitedze Research Station, Lilongwe, Malawi.
Kanako Suzuki
Dr. Kanako Suzuki is an Associate Scientist of Agriculture and Development and soil expert at IIITA, Lusaka, Zambia.
Chipo Chisonga
Mr. Chipo Chisonga is a data management assistant at IITA) Southern Africa Hub, Lusaka, Zambia.
Godfree Chigeza
Dr. Godfree Chigeza G is a soybean breeder at IITA, Southern Africa Hub, Lusaka, Zambia.
Maziya-Dixon Busie
Dr. Maziya-Dixon Busie is an internationally recognized Food Science & Nutrition expert. She is a Principal Scientist at Western Africa hub, Ibadan, Nigeria.
References
- Abdul-Aziz, A. L. (2013). Contribution of Rhizobium and phosphorus fertilizer to biological nitrogen fixation and grain yield of soybean in the tolon district (Doctoral dissertation, MSC Thesis (unpublished)). Faculty of Agriculture, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
- Alamu, E. O., Maziya‐Dixon, B., Menkir, A., & Olaofe, O. (2015). Effects of husk and harvesting time on provitamin A activity and sensory properties of boiled fresh orange maize hybrids. Journal of Food Quality, 38(6), 387–13. doi:10.1111/jfq.12158
- Alamu, E. O., Popoola, I., & Maziya‐Dixon, B. (2018). Effect of soybean (Glycine max (L.) Merr.) flour inclusion on the nutritional properties and consumer preference of fritters for improved household nutrition. Food Science & Nutrition, 6(7), 1811–1816. doi:10.1002/fsn3.751
- Anderson, R. L., & Wolf, W. J. (1995). Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. The Journal of Nutrition, 125(suppl_3), 581S–588S. doi:10.1093/jn/125.3_Suppl.581S
- Asada, K., Tanaka, K., & Kasai, Z. (1970). Formation of phytic acid in cereal grains. Annals of the New York Academy of Sciences, 165(2), 801–814.
- Association of Official Analytical Chemists (AOAC). (2005). Official methods of analysis of the association of analytical chemists international (18th ed.). Gaithersburg, MD: USA.
- Babiker, E. E., El Sheikh, E. A. E., Osman, A. J., & El Tinay, A. H. (1995). Effect of nitrogen fixation, nitrogen fertilization and viral infection on yield, tannin and protein contents and in vitro protein digestibility of faba bean. Plant Foods for Human Nutrition, 47(3), 257–263.
- Bray, R. H., & Kurtz, L. T. (1945). Determination of total, organic, and available forms of phosphorus in soils. Soil Science, 59(1), 39–46. doi:10.1097/00010694-194501000-00006
- Bruce, K. J., Karr-Lilienthal, L. K., Zinn, K. E., Pope, L. L., Mahan, D. C., Fastinger, N. D., … Ellis, M. (2006). Evaluation of the inclusion of soybean oil and soybean processing by-products to soybean meal on nutrient composition and digestibility in swine and poultry. Journal of Animal Science, 84(6), 1403–1414. doi:10.2527/2006.8461403x
- Chiezey, U. F., & Odunze, A. C. (2009). Soybean response to application of poultry manure and phosphorus fertilizer in the Sub-humid Savanna of Nigeria. Journal of Ecology and the Natural Environment, 1(2), 025–031.
- De La Guerivier, J. F. (1976). Principles of the extrusion cooking process application to starch foods. Bul Des Anc Ele De Ecol Fran De Meu, 276, 305.
- Deshpande, S. S., & Cheryan, M. (1985). Evaluation of vanillin assay for tannin analysis of dry beans. Journal of Food Science, 50(4), 905–910. doi:10.1111/jfds.1985.50.issue-4
- Earley, E. B., & DE TURK, E. E. (1944). Time and rate of synthesis of phytin in corn grain during the reproductive period. Journal of the American Society of Agronomy, 36, 803–814. doi:10.2134/agronj1944.00021962003600100002x
- Edema, M. O., Sanni, L. O., & Sanni, A. I. (2005). Evaluation of maize-soybean flour blends for sour maize bread production in Nigeria. African Journal of Biotechnology, 4(9), 911–918.
- Eldridge, A. C., Black, L. T., & Wolf, W. J. (1979). Carbohydrate composition of soybean flours, protein concentrates, and isolates. Journal of Agricultural and Food Chemistry, 27(4), 799–802. doi:10.1021/jf60224a056
- Elsheikh, E. A., & Elzidany, A. A. (1997). Effect of Rhizobium inoculation, organic and chemical fertilizers on proximate composition, in vitro protein digestibility, tannin and sulphur content of faba beans. Food Chemistry, 59(1), 41–45. doi:10.1016/S0308-8146(96)00046-5
- Elsheikh, E. A., & Mohamedzein, E. M. M. (1998). Effect of Bradyrhizobium, VA mycorrhiza and fertilisers on seed composition of groundnut. Annals of Applied Biology, 132(2), 325–330. doi:10.1111/aab.1998.132.issue-2
- Elsheikh, E. A. E., & Ahmed, E. I. A. (2000). A note on the effect of intercropping and Rhizobium inoculation on the seed quality of faba bean (Vicia faba L.). University of Khartoum. Journal of Agricultural Science, 8, 171–172.
- Eshun, G. (2012). Nutrient composition and functional properties of bean flours of three soya bean varieties from Ghana. African Journal of Food Science and Technology, 3(8), 176–181.
- FAO. (2008). Fertilizer and plant nutrition bulletin (pp. 220). Rome, Italy: Author.
- Foster, R., Williamson, C. S., & Lunn, J. (2009). BRIEFING PAPER: Culinary oils and their health effects. Nutrition Bulletin, 34(1), 4–47. doi:10.1111/nbu.2009.34.issue-1
- GUJSKA, E., D.‐REINHARD, W. A. N. D. A., & KHAN, K. (1994). Physicochemical properties of field pea, pinto and navy bean starches. Journal of Food Science, 59(3), 634–636. doi:10.1111/jfds.1994.59.issue-3
- Hammond, E. G. (1991). The raw materials of the fats and oils industry. In P. Wan (Ed.), Fat and Oil Technology (pp. 1–15). Champaign, IL: American Oil Chemists’ Society.
- Helsper, J. P., Hoogendijk, J. M., van Norel, A., & Burger-Meyer, K. (1993). Antinutritional factors in faba beans (Vica faba L.) as affected by breeding toward the absence of condensed tannins. Journal of Agricultural and Food Chemistry, 41(7), 1058–1061. doi:10.1021/jf00031a008
- Hichaambwa, M., Chileshe, C., Chimai-Mulenga, B., Chomba, C., & Mwiinga-Ngcobo, M. (2014). Soybean Value Chain and Market Analysis. Indaba Agricultural Policy Research Institute (IAPRI), Final Draft Report. Zambia. pp. 67.
- Hymowitz, T., & Collins, F. I. (1974). Variability of sugar content in seed of Glycine max (L.) Merrill and G. soja Sieb. and Zucc. 1. Agronomy Journal, 66(2), 239–240. doi:10.2134/agronj1974.00021962006600020017x
- Ibrahim, S. A., & Mahmoud, S. A. (1989). Effect of inoculation on growth, yield and nutrients uptake of some soybean varieties. Egyptian Journal of Soil Science (egypt), 29(2), 133–142.
- Jackson, M. L. (1958). Soil chemical analysis prentice Hall. Inc., Englewood Cliffs, NJ, 498. pp. 183–204.
- Joshi, J. M., Nkumbula, S., & Javaheri, F. (1986). Seed inoculation response for promiscuous soybean cultivars. Soybean genetics newsletter United States, Agricultural Research Service.
- Kim, H. J., Ha, B. K., Ha, K. S., Chae, J. H., Park, J. H., Kim, M. S., … Lee, J. D. (2015). Comparison of a high oleic acid soybean line to cultivated cultivars for seed yield, protein and oil concentrations. Euphytica, 201(2), 285–292. doi:10.1007/s10681-014-1210-5
- Kim, S. D., Hong, E. H., Park, R. K., Yoo, I. D., Shin, M. K., Choe, J. H., … Song, I. M. (1988). Effect of rhizobium inoculant application on nodulation and nitrogen fixation at different soil types in soybeans. The Research Reports of the Rural Development Administration-Upland and Industrial Crops (Korea R.). doi:10.3168/jds.S0022-0302(88)79586-7
- Krishnan, H. B., Natarajan, S. S., Mahmoud, A. A., & Nelson, R. L. (2007). Identification of glycinin and β-conglycinin subunits that contribute to the increased protein content of high-protein soybean lines. Journal of Agricultural and Food Chemistry, 55(5), 1839–1845. doi:10.1021/jf062497n
- Liener, I. E. (2000). Non-nutritive factors and bioactive compounds in soy. Soy in animal nutrition (Drackley, JK ed.). pp.1–12.
- LIU, K. (2000). Expanding soybean food utilization. Food Technology, 54, 46–58.
- Lubungu, M., Burke, W. J., & Sitko, N. J. (2013). Analysis of the soya bean value chain in Zambia’s Eastern Province ( No. 1093-2016-88052).
- Makower, R. U. (1970). Extraction and determination of phytic acid in beans (Phaseolus vulgaris). Cereal Chemistry, 47, 288–295.
- Manral, H. S., & Saxena, S. C. (2003). Plant growth, yield attributes and grain yield of soybean as affected by the application of inorganic and organic sources of nutrients. Bioresource Technology, 92, 110–118.
- Maziya‐Dixon, B., Alamu, E. O., Popoola, I. O., & Yomeni, M. (2017). Nutritional and sensory properties: Snack food made from high‐quality cassava flour and legume blend. Food Science & Nutrition, 5(3), 805–811. doi:10.1002/fsn3.464
- Messina, M. J. (2003). Emerging evidence on the role of soy in reducing prostate cancer risk. Nutrition Reviews, 61(4), 117–131. doi:10.1301/nr.2003.apr.117-131
- Michael, B., Zink, F., & Lantzsch, H. J. (1980). Effect of phosphate application on phytin‐phosphorus and other phosphate fractions in developing wheat grains. Zeitschrift Für Pflanzenernährung Und Bodenkunde, 143(4), 369–376. doi:10.1002/(ISSN)1522-2624
- Natarajan, S. S., Xu, C., Bae, H., Caperna, T. J., & Garrett, W. M. (2006). Characterization of storage proteins in wild (Glycine soja) and cultivated (Glycine max) soybean seeds using proteomic analysis. Journal of Agricultural and Food Chemistry, 54(8), 3114–3120. doi:10.1021/jf052954k
- Nelson, D. W., & Sommers, L. (1982). Total carbon, organic carbon, and organic matter 1. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties (methodsofsoilan2), 9(2), 539–579. Madison: Publisher Madison.
- Qasim, S. M., Farrukh Saleem, M., Khan, H. Z., & Anjum, S. A. (2009). Performance of soybean (Glycine max L.) under different phosphorus levels and inoculation. Pakistan Journal of Agricultural Sciences, 46(4), 237–241.
- Rahman, M. M., Bhuiyan, M. M. H., Sutradhar, G. N. C., Rahman, M. M., & Paul, A. K. (2008). Effect of phosphorus, molybdenum and rhizobium inoculation on yield and yield attributes of mungbean. International Journal of Sustainable Crop Production, 3(6), 26–33.
- Rao, S. B., Krishna, N., Prasad, J. R., & Raghavan, G. V. (1995). Evaluation of nutritional quality of some fibrous resources by in vitro and nylon bag techniques in goats. Indian Journal of Animal Nutrition, 12(2), 85–90.
- Ravindran, V., Cabahug, S., Ravindran, G., Selle, P. H., & Bryden, W. L. (2000). Response of broiler chickens to microbial phytase supplementation as influenced by dietary phytic acid and non-phytate phosphorous levels. II. Effects on apparent metabolisable energy, nutrient digestibility and nutrient retention. British Poultry Science, 41(2), 193–200. doi:10.1080/00071660050022263
- Rurangwa, E., Vanlauwe, B., & Giller, K. E. (2018). Benefits of inoculation, P fertilizer and manure on yields of common bean and soybean also increase yield of subsequent maize. Agriculture, Ecosystems & Environment, 261, 219–229. doi:10.1016/j.agee.2017.08.015
- Schulze, J., Temple, G., Temple, S. J., Beschow, H., & Vance, C. P. (2006). Nitrogen fixation by white lupin under phosphorus deficiency. Annals of Botany, 98(4), 731–740. doi:10.1093/aob/mcl154
- Schweizer, T. F., Horman, I., & Würsch, P. (1978). Low molecular weight carbohydrates from leguminous seeds; a new disaccharide: Galactopinitol. Journal of the Science of Food and Agriculture, 29(2), 148–154. doi:10.1002/(ISSN)1097-0010
- Sebastian, S., Touchburn, S. P., & Chavez, E. R. (1998). Implications of phytic acid and supplemental microbial phytase in poultry nutrition: A review. World’s Poultry Science Journal, 54(1), 27–47. doi:10.1079/WPS19980003
- Shan, A. S., & Davis, R. H. (1994). Effect of dietary phytate on growth and selenium status of chicks fed selenite or selenomethionine. British Poultry Science, 35(5), 725–741. doi:10.1080/00071669408417738
- Sharma, S., Kaur, M., Goyal, R., & Gill, B. S. (2014). Physical characteristics and nutritional composition of some new soybean (Glycine max (L.) Merrill) genotypes. Journal of Food Science and Technology, 51(3), 551–557. doi:10.1007/s13197-011-0517-7
- Stein, H. H., Berger, L. L., Drackley, J. K., Fahey, G. C., Jr., Hernot, D. C., & Parsons, C. M. (2008). Nutritional properties and feeding values of soybeans and their coproducts. In L. A. Johnson, P. J. White & R. Galloway (Ed.), Soybeans (pp. 613–660). Urbana, IL: AOCS Press.
- TRAINA, M. S., & BREENE, W. M. (1994). Composition, functionality and some chemical and physical properties of eight commercial full‐fat soy flours. Journal of Food Processing and Preservation, 18(3), 229–252. doi:10.1111/jfpp.1994.18.issue-3
- Walkley, A., & Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37(1), 29–38. doi:10.1097/00010694-193401000-00003
- Wheeler, E. L., & Ferrel, R. E. (1971). A method for phytic acid determination in wheat and wheat fractions. Cereal Chemistry, 48(3), 312–320.
- Xavier-Filho, J., Campos, F. A. P., Ary, M. B., Silva, C. P., Carvalho, M. M., Macedo, M. L. R., … Grant, G. (1989). Poor correlation between the levels of proteinase inhibitors found in seeds of different cultivars of cowpea (Vigna unguiculata) and the resistance/susceptibility to predation by Callosobruchus maculatus. Journal of Agricultural and Food Chemistry, 37(4), 1139–1143. doi:10.1021/jf00088a071
- Yaklich, R. W. (2001). β-Conglycinin and glycinin in high-protein soybean seeds. Journal of Agricultural and Food Chemistry, 49(2), 729–735. doi:10.1021/jf001110s
- Yañez‐Farias, G. A., Moreno‐Valencia, J. G., Falcón‐Villa, M. D. R., & Barrón‐Hoyos, J. M. (1997). Isolation and partial characterization of starches from dry beans (Phaseolus vulgaris) and chickpeas (Cicer arietinum), grown in Sonora, Mexico. Starch‐Stärke, 49(9), 341–345. doi:10.1002/star.19970490904
- Yazdi-Samadi, B., Rinne, R. W., & Seif, R. D. (1977). Components of developing soybean seeds: Oil, protein, sugars, starch, organic acids, and amino acids 1. Agronomy Journal, 69(3), 481–486. doi:10.2134/agronj1977.00021962006900030037x