 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The use of botanicals to control phytopathogens of crops is an alternative to the use of synthetic fungicides. The objective of this study was to evaluate the in vitro growth inhibitory effect of Phytolacca dodecandra extracts on Botrytis fabae and their in vivo efficacy to control chocolate spot disease of Vicia faba caused by it. Growth inhibitory effects were evaluated by applying different concentrations of aqueous, methanol or ethanol leaf extracts of P. dodecandra, or extraction solvents (control) by agar diffusion method. Disease incidence and severity were evaluated in vivo by spraying leaf extracts of P. dodecandra on V. fabae plant grown in the field soon after the detection of disease symptoms. All solvents’ extracts significantly reduced B. fabae mycelial growth compared to control, and growth reduction increased with increasing extract concentration. The aqueous extract performed better than methanol and ethanol. Results of field experiment also showed that disease incidence was reduced by at least two and three-fold when extracts and synthetic fungicides were applied, respectively. Similarly, disease severity was reduced by at least two-fold and six-fold when extracts and synthetic fungicides were applied, respectively. Application of extracts under field conditions also improved yield compared to the control. Hence, extracts of P. dodecandra are potential alternatives to synthetic fungicide to control the negative impacts of B. fabae.
PUBLIC INTEREST STATEMENT
Botrytis fabae is a fungal pathogen that causes significant yield loss in faba bean. Though it can be managed by applying synthetic fungicide, smallholder farmers in Ethiopia cannot afford its cost. Moreover, synthetic fungicide may have deleterious effect on human health and other animals. Therefore, the use of plant products that are cheaper and relatively environmentally friendly is better option to manage the severity of the disease caused by this fungus. In this regard, this study came up with the results that help smallholder farmers to increase faba bean yield using water extracts of a plant, which naturally occurs in their surroundings with minimal financial and environmental costs.
Competing Interests
The authors declare no competing interests.
1. Introduction
Crop production is hindered by an array of phytopathogens, of which fungal pathogens are some of the most prominent (Addisu, Egigu, & Bekele, Citation2016). In Ethiopia, faba bean (Vicia faba L., Fabaceae) is one of the main legume crops used as source of protein (Sahile, Ahmed, Fininsa, Abang, & Sakhuja, Citation2008). Its production, however, is severely reduced due to fungal pathogens (Agegnehu, Ghizaw, & Sinebo, Citation2006). The major fungal diseases that threaten faba bean production are chocolate spot (caused by Botrytis fabae Sard.), leaf rust (caused by Uromyces viciae-fabae), ascochyta blight (caused by Ascochyta fabae) and black root rot (caused by Fusarium solani). However, chocolate spot is the most widespread and highly destructive disease causing a yield loss of up to 34–61% on susceptible cultivars in Ethiopia (Addisu et al., Citation2016; Dereje & Beniwal, Citation1988; IAR, Citation1989; International Center for Agricultural Research in the Dry Areas [ICARDA], Citation2006).
Chocolate spot is most commonly controlled by the application of synthetic fungicides (Addisu et al., Citation2016). However, frequent use of synthetic fungicides may present several problems. For example, excessive use of those chemicals may have hazardous effects on humans and the ecosystems. In fact, toxicity to non-target organisms and contribution to environmental pollution are some of the major problems related to the use of synthetic chemicals (Isman, Citation2006). Moreover, though synthetic fungicides have been proven for their effectiveness in controlling fungal plant pathogens, it has been detected recently that Botrytis cinerea and B. fabae are exhibiting resistance to chemical fungicides (Hassan, Abd El-Rahman, El-Abbasi, & Mikhail, Citation2006; Parry, Citation1990).
Many plants and plant products have been reported to possess antimicrobial properties (Egamberdieva, Wirth, Behrendt, Ahmad, & Berg, Citation2017), and their properties are attributed to secondary metabolites, mainly terpenoids, phenolics and alkaloids. Literatures suggest that plant extracts are among the most prospective biological plant protection aids. Their impact is effective, extraction is not so complicated and time-consuming. They are also relatively harmless to environment and people as they are less persistent in an environment, compared to synthetic ones (Isman, Citation2006; Koul, Citation2008; Nerio, Olivero-Verbel, & Stashenko, Citation2010). Also, pathogens develop less resistance toward them. As a result, phytochemicals are potential alternatives to synthetic chemicals in the control of phytopathogens. In this regard, there are certain successes so far in many countries in the development and practical establishment of new, ecologically safe plant protection aids. In Ethiopia, for instance, extracts from specific plants are used traditionally as natural fungicides in small-scale farming systems where synthetic chemicals are out of the reach of the average subsistence farmer, but no scientific basis exists for this practice.
Although Ethiopia is rich in plant resources, scientific studies of natural products’ effectiveness against fungal plant diseases are limited. Previously, however, Roman (Citation2010) used crude extracts of seven plant species (Croton macrostachyus, Solanum incanum, Datura stramonium, Solanum marginatum, Calpurnia aurea, Clematis simensis, and C. hirsute) to evaluate their efficacy against B. fabae in the laboratory, and under greenhouse conditions, and found satisfactory results. Similar work has also been done recently by Addisu et al. (Citation2016). The objective of this study was to investigate the efficacy of crude extracts of Phytolacca dodecandra (Phtolaccaceae) against a fungus (Botrytis fabae), a causative agent of chocolate spot disease of faba bean (V. faba, Fabaceae). The African soapberry plant, P. dodecandra, locally called “Endod”, produces a series of triterpenoid saponins that possess very potent and useful biological properties including antifungal, anti-protozoan, spermicidal and insecticidal properties (Esser, Semagn, & Wolde-Yohannes, Citation2003).
2. Materials and methods
2.1. Test plant material and pathogen
Fresh healthy-looking mature leaves of P. dodecandra were collected from natural habitats from areas in the vicinity of Sinana Agricultural Research Center (SARC) located at 07° N and 40° 10ʹ E in Bale Zone, Ethiopia. This was done when P. dodecandra was bearing flowers. Pure culture of Botrytis fabae was developed in the laboratory at SARC.
2.2. Preparation of plant extracts
Leaf samples were thoroughly washed with running tap water and distilled water, and allowed to dry in the laboratory. The dried leaves were pulverized using a sterilized blender and the powder was extracted using three different solvents viz distilled water, methanol and ethanol, separately. Extraction was done by soaking leaf powder in distilled water, 99.8% methanol or ethanol in 1:4 (w:v) ratio of leaf powder to the respective solvents and left for 24 h in the laboratory with intermittent stirring using a sterile glass rod to ensure uniform soaking (Egigu, Ibrahim, Yahya, & Holopainen, Citation2010; Simeon, Materechera, & Hae, Citation2008). After 24 h of soaking, the extracts were filtered in two steps; first by using fourfold cheese cloth (Wokocha & Okereke, Citation2005) and then using sterilized Whatman No. 1 filter paper. The filtrates were centrifuged for 15 min at 6000 rpm and then dried using rotary evaporator under reduced pressure. The dried extracts were then reconstituted by dissolving 5, 10, 20 and 40 g of them in 100 ml of the respective solvents to have 5%, 10%, 20% and 40% (w/v) concentrations (Shovan, Bhuiyan, Begum, & Pervez, Citation2008; Yeni, Citation2011). The extracts were then stored in airtight bottles in a refrigerator (4°C), until used in bioassay (Naduagu, Ekefan, & Nwankiti, Citation2008; Prince & Prabakaran, Citation2011).
2.3. Preparation of growth medium
Faba bean dextrose agar (FDA) was prepared and used as a growth medium (Haggag, Kansoh, & Aly, Citation2006; Hanounik & Maliha, Citation1986). For this, coarsely chopped fresh faba bean leaf (400 g) was mixed with 1 L of tap water in a 1.5 L conical flask and autoclaved at 121°C for 20 min. The sterilized leaf-water mixture was then filtered and the filtrate was mixed with agar medium (18 g) and dextrose (20 g). Thereafter, the mixture was heated along with uniform mixing and the volume was made up to 1 L with tap-water. This mixture was again autoclaved (121°C for 20 min), cooled down to about 40°C and poured into Petri-dishes (9 cm diameter) to serve as growth medium.
2.4. In vitro growth test
Prior to pouring the growth medium into Petri dishes, two perpendicular lines that divide the Petri-dishes into four equal sections were drawn at their bottom (Amadioha & Obi, Citation1999). The point of intersection of the lines represented the centre of the Petri-dishes. Thereafter, growth medium was poured into the Petri-dishes with immediate addition of 2 ml of the different concentrations of the extracts (Joseph, Dar, & Kumar, Citation2008; Shovan et al., Citation2008). Petri-dishes that received distilled water or organic solvents represented negative control. Subsequently, a 4 mm diameter disk of mycelial pure culture was cut out using sterile cork borer and placed into the hole cut out from the solidified medium-extract mixture just at the point of intersection of the two lines drawn at the bottom of the plate (Yeni, Citation2011). Mycelial radial growth measurement commenced as soon as the growth in the control Petri dishes reached maximum (90 mm) using an ordinary ruler, with a centimeter scale. Fungi-toxicity of test extracts was then calculated in terms of percentage mycelial growth inhibition using the formula indicated in Sundar, Das, and Krishnaveni (Citation1995) and Ahmed et al. (Citation2002). The experiment was arranged in completely random design with three replications.
2.5. In vivo experiment
A faba bean variety, Shallo, which was released by Sinana Agricultural Research Centre, was used for the field experiment. The study was arranged in RCB design with three replications. Each plot (2 m x 1.2 m) comprised three rows of 0.4 m spacing and had 2 m length. Plots were spaced 1 m apart within a block and blocks were 2 m apart. Plants were subjected to natural infection and disease development (El-Sayed, El-Shennawy, & Ismail, Citation2011; Ogbebor & Adenkunle, Citation2008). Thereafter, extracts were applied to the treatment plots soon after disease infection was noticed. Plots that received extracts were sprayed with 40% (w/v) concentration of crude extracts (i.e. 40 g of the three solvents’ extracts dissolved in 100 ml of distilled water). Extracts were applied so as to sufficiently cause wetting of the leaves. The extract concentration was not diluted as in the case of in vitro experiment, deliberately to compensate for the probable loss of active principles under field conditions. Application of a standard synthetic fungicide, Mancozeb 80 WP (Dethane M-45) at the recommended rate (2.5 kg ha−1) and distilled water were sprayed on control plots. In the case of field experiment, we used only distilled water as negative control as there were no significant differences between organic solvents and water when applied as negative controls. Prior to extract application, potential phytotoxic effect of the extract was also evaluated by applying onto sample leaves of the crop to inspect the development of leaf injury symptoms for a week time, but no visible leaf injury was noticed. For collection of some agronomic data, five plants from middle row were randomly selected and marked with a colored thread for identification. Data related to percent disease incidence and disease severity were then collected. Disease severity was recorded using 1–9 scale: where 1 = no disease symptoms or very small specks; 3 = few small discrete lesions; 5 = some coalesced lesions with some defoliation; 7 = large-coalesced sporulating lesions, 50% defoliation and some dead plant; and 9 = extensive lesions on leaves, stems and pods, severe defoliation, heavy sporulation, stem girdling, blackening and death of more than 80% of the plants (Bernier, Hanounik, Hussein, & Mohamed, Citation1993). Disease severity data were then converted to Percent Severity Index (PSI) using the following formula developed by Wheeler (Citation1969).
Where: PSI = Percent severity index, Snr = Sum of numerical ratings, Npr = Number of plants scored and Mss = the maximum scale of the disease.
2.6. Statistical analysis
Statistical analyses were conducted with the statistical packages of the computer software SPSS for Windows 16.0 (SPSS; Chicago, IL, USA). Data were first checked for normality of distribution using Shapiro–Wilk test and P-values of all data sets were found to be >0.05 confirming their normal distribution. As a result, general linear model procedure was used to analyze laboratory data while one-way ANOVA was performed for field data and means were separated by the least significant difference (LSD). The differences between means were considered to be statistically significant at P <0.05
3. Results
3.1. Invitro growth of Botrytis fabae
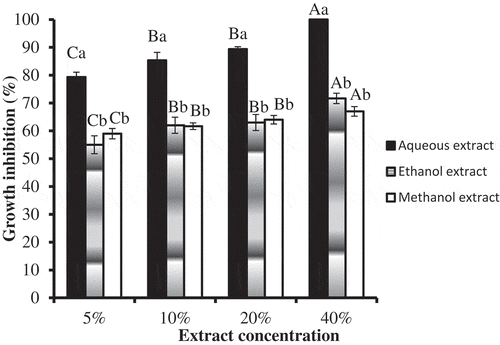
Extract concentrations and solvents used to make extracts were the main effects. The extracts, at all levels of concentration, significantly (df = 4, F = 897.233, P < 0.001) inhibited mycelial growth of B. fabae when compared with control that had no growth inhibitory effect (data for control not presented in the graph as it is zero), and growth inhibitory effect appeared to increase with increasing extract concentration, though no significant differences were evident between 10% and 20% extract concentrations (Figure with mean separation indicated by capital letters over error bars). Mycelial growth was also significantly (df = 2, F = 197.646, P < 0.001) varied with the type of solvent used to make extraction with aqueous extract exhibiting the highest inhibitory effect than ethanolic and methanolic extracts (Figure with mean separation indicated by small letters over error bars). That is, aqueous extracts were found to be superior to ethanolic and methanolic extracts in inhibiting mycelial growth at all concentration levels (Figure ). Particularly, its efficacy was 100% at 40% concentration. However, no significant difference was observed between ethanolic and methanolic extracts in inhibiting mycelial growth (Figure )
Figure 1. Comparison of growth inhibitory effects between: the same extract concentration of the three extraction solvents (mean comparison indicated by small letters over error bars at each concentration level) and different extract concentrations of each extraction solvent (mean comparison indicated by capital letters over error bars for different concentrations of each extraction solvent). Values are Mean ± S.E., n = 3. Error bars with same letter are not significantly different, whereas those with different letters are significantly different at P< 0.05.
3.2. Disease incidence and severity symptoms
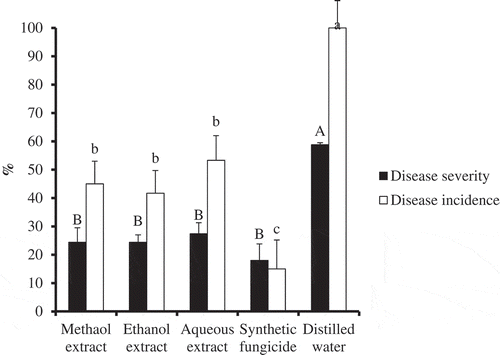
Disease incidence was 100% in plots sprayed with distilled water (control), compared to synthetic fungicide or plant extract sprayed plots (Figure ). Although disease incidence was significantly reduced by plant extracts, their efficacy was lower than that of synthetic fungicide. No significant differences were observed among the three solvents’ extracts of P. dodecandra in terms of disease incidence (Figure ). Compared to the control (58.8%), disease severity was significantly reduced by all extract types of P. dodecandra, with severity scores of 24.4% each for methanol and ethanol extracts, and 27.4% for aqueous extract (Figure ). However, there was no significant difference between the three solvents’ extracts of P. dodecanda and synthetic commercial fungicide in terms of disease severity reduction (Figure ).
Figure 2. Percent disease severity (black bar) and percent disease incidence (open bar) caused by B. fabae on V. faba treated with P. dodecandra extracts under field conditions. Values are Mean ± S.E., n = 3. Error bars with same letter are not significantly different, whereas those with different letters are significantly different at P< 0.05. Capital letters compare disease severity between treatments, whereas small letters compare disease incidence between treatments.
3.3. Yield and yield components
All solvents’ extracts of P. dodecandra significantly reduced flower abortion, except the aqueous extract. There was no significant difference among the three solvents’ extracts of P. dodecandra in protecting flower abortion. Likewise, the number of tillers counted from plots treated with all solvents’ extracts of P. dodecandra was significantly higher than that of the control plot. The number of pods per plant in plots treated with all solvents’ extracts of P. dodecandra was significantly higher than the control. There was no significant difference among the three solvents’ extracts of P. dodecandra in yielding pods per plant. All solvents’ extracts of P. dodecandra did not significantly vary from the control and synthetic fungicide with respect to number of seeds per pod. Values of hundred kernel weight obtained from plots treated with methanol extract showed no significant difference from that of control and synthetic fungicide treatments except ethanol and aqueous extracts of P. dodecandra. Grain yield obtained from plots treated with all solvents’ extracts and synthetic fungicide significantly increased when compared to the control, and ethanolic and methanolic extracts treated plots gave better yield than synthetic fungicide (Table ).
Table 1. Effects of P. dodecandra extracts on the agronomic traits of faba bean
4. Discussion
4.1. In vitro growth inhibitory effects
The three solvents used had no fungicidal effects, suggesting any fungicidal effect observed is attributed to the extract contents. As there was no significant difference among the three solvents in their fungicidal effect, the negative control taken for comparison with extracts’ effects was that of distilled water throughout. The in vitro bioassay results showed that all solvents extracts of P. dodecandra had negative effects on the growth of B. fabae, and growth inhibitory effect increased with extract concentration. Previously, Lamma, Heyneman, and Silangwa (Citation1984) and Esser et al. (Citation2003) reported that P. dodecandra possesses secondary compounds (e.g. triterpenoid saponin), which has been proven to have fungicidal property.
Yadava and Chakravarti (Citation2009) tested the in vitro antifungal activity of triterpenoid saponin obtained from Launaea pinnatifida on some other fungal species and found growth inhibitory effect, which increased with its concentration. Previously, Tadeg, Mohammed, Asres, and Gebre-Mariam (Citation2005) used P. dodecandra extracts to evaluate their efficacy against some human bacterial and fungal strains causing skin infections, and showed that this plant had antimicrobial property. Solvents of different polarities have different potential of extracting compounds (Egigu et al., Citation2010). In the present experiment, comparison among the different solvents’ extracts of P. dodecandra showed that aqueous extract had greater growth inhibitory effect on B. fabae than ethanol and methanol extracts. Its growth inhibitory effect was greater than 90% only at 20% concentration, suggesting the more extractability of potent active principles in water than ethanol and methanol solvents. Contrary to this result, Abera, Lemessa, and Muleta (Citation2011) found that aqueous extract of P. dodecandra performed less than its ethanolic extract in inhibiting in vitro mycelial growth of other fungal species, Colletotrichum kahawae, which causes coffee berry disease. This most probably is attributed to natural sensitivity difference that exists between B. fabae and C. kahawae towards active compounds extracted by the same solvent. It is also possible that strength of extract concentration obtained with the same solvent may elicit different responses as has been witnessed in this experiment.
4.2. In vivo experiment
All solvents’ extracts of P. dodecandra significantly reduced disease incidence and severity when compared to control treatment. Similarly, most of the measured yield related agronomic traits and yield were found to increase in extract and synthetic fungicide applied plots when compared to control treatment. Results of this experiment accord with that of Tegegne and Pretoriu (Citation2007) who found significantly lower level of disease incidence and higher yield of sorghum when powder of berries of P. dodecandra was applied to artificially inoculated seeds of sorghum with smut spores under field condition. Contrary to in vitro experiment, no significant difference was observed between the different extract types in reducing disease incidence and severity under field condition. For example, aqueous extract of P. dodecandra was superior to ethanol and methanol extracts in inhibiting growth of B. fabae under laboratory condition, but not under field condition. This may be due to the susceptibility of active compound(s) extracted by water to degradation by uncontrolled light and temperature under field conditions so that their activity in sole or synergistically can be reduced. Previously, Schmutterer (Citation1990) explained that some secondary metabolites are easily degradable under field conditions due to ultraviolet light and/or extreme temperatures.
5. Conclusions
This study showed that extracts of P. dodecandra are effective in suppressing growth of B. fabae in in vitro experiment. Moreover, they significantly reduce disease incidence and severity, and improve yield and yield-related traits under field conditions compared to the control. The results also reveal that the efficacy of extract varies with solvent used, where aqueous extract appears to perform better than ethanol and methanol solvents’ extracts in vitro. However, this situation is not observed under field condition where all solvents’ extracts perform more or less equally probably due to the susceptibility of active principles extracted by aqueous solvent to degradation under field condition. The study also showed that organic solvents used have no negative impact on test plant as there is no uniquely visible leaf injury compared to distilled water. The two organic solvents used also showed no antifungal effect compared to distilled water, when used as negative control, suggesting that the antifungal property observed by the three solvents’ extracts is generally attributed to the plant compound(s).
Acknowledgements
We thank members of the Sinana Agricultural Research Centre for providing necessary support during the experiments at the center. We also thank the School of Biological Sciences and Biotechnology of Haramaya University, Ethiopia for technical support received.
Additional information
Funding
Notes on contributors
Addisu Tegegn
Addisu Tegegn (M.Sc.) and Bekele Hundie (PhD)are plant pathologists at Sinana and Kulumsa agricultural research centers Ethiopia, respectively. They have long years of experience in the areas of crop-microbial pathogen interactions and published many articles. M.C.E. (Corresponding author, photo attached) is M.Sc. in Botany and Ph.D. in Plant Chemical Ecology. Presently, he is working as an Associate professor in the School of Biological Sciences and Biotechnology, Haramaya University, Ethiopia. He has many years of teaching (at undergraduate and postgraduate levels) and research experience in the area of plant chemical ecology. He has advised several M.Sc. students and published many research articles on reputable journals.
References
- Abera, A., Lemessa, F., & Muleta, D. (2011). The antifungal activity of some medicinal plants against Coffee berry disease caused by Colletotrichum kahwae. International Journal of Agricultural Research, 6, 268–9.
- Addisu, T., Egigu, M. C., & Bekele, H. (2016). Efficacy of pepper tree (Schinus molle) extracts to suppress growth of botrytis fabae and manage chocolate spot severity on faba bean (Vicia faba) at Sinana, Bale Zone, Southeastern Ethiopia. East African Journal of Sciences, 10, 111–118.
- Agegnehu, G., Ghizaw, A., & Sinebo, W. (2006). Yield performance and land-use efficiency of barley and faba bean mixed cropping in Ethiopian highlands. European Journal of Agronomy, 25, 202–207. doi:10.1016/j.eja.2006.05.002
- Ahmed, M. D., Khalequzzaman, F., Islam, K. M., Md, N., Anam, M. K., & Islam, M. T. (2002). Effect of plant extracts against Bipolaris oryzae of rice under in vitro conditions. Pakistan Journal of Biological Sciences, 5, 442–445. doi:10.3923/pjbs.2002.442.445
- Amadioha, A. C., & Obi, V. I. (1999). Control of anthracnose disease of cowpea by Cymbopogon citratus and Ocimum gratissimum. Acta Phytopathologicaet Entomologica Hungarica, 34, 85–89.
- Bernier, C. C., Hanounik, S. B., Hussein, M. M., & Mohamed, H. A. (1993). Field manual of common faba bean diseases in the Nile Valley. International Center for Agricultural Research in the Dry Areas (ICARDA) Information Bulletin No. 3. Aleppo, Syria.
- Dereje, G., & Beniwal, S. P. (1988). Yield losses caused by chocolate spot in faba bean. EPC Newsletter, 13, 16–20.
- Egamberdieva, D., Wirth, S., Behrendt, U., Ahmad, P., & Berg, G. (2017). Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Frontiers in Microbiology, 8, 1–11. doi:10.3389/fmicb.2017.00199
- Egigu, M. C., Ibrahim, M. A., Yahya, A., & Holopainen, J. K. (2010). Yeheb (Cordeauxia edulis) extract deters feeding and oviposition of Plutella xylostella and attracts its natural enemy. Biocontrol, 55, 613–624. doi:10.1007/s10526-010-9287-9
- El-Sayed, A. S., El-Shennawy, R. Z., & Ismail, A. I. (2011). Fungicidal management of chocolate spot of faba bean and assessment of yield losses due to the disease. Annals of Agricultural Sciences, 56, 27–35. doi:10.1016/j.aoas.2011.05.004
- Esser, K. B., Semagn, K., & Wolde-Yohannes, L. (2003). Medicinal use and social status of the soap berry endod (Phytolaccadodecandra) in Ethiopia. Journal of Ethnopharmacology, 85, 269–277. doi:10.1016/S0378-8741(03)00007-2
- Haggag, W. M., Kansoh, A. L., & Aly, A. M. (2006). Proteases from Talaromyces flavus and Trichoderma harzianum: Purification, characterization and antifungal activity against brown spot disease on faba bean. Plant Pathology Bulletin, 15, 231–239.
- Hanounik, S. B., & Maliha, N. (1986). Horizontal and vertical resistance in Vicia faba to chocolate spot caused by Botrytis fabae. Plant Disease, 70, 770–773. doi:10.1094/PD-70-770
- Hassan, M. E. M., Abd El-Rahman, S. S., El-Abbasi, I. H., & Mikhail, M. S. (2006). Inducing resistance against faba bean chocolate spot disease. Egyptian Journal of Phytopathology, 34, 69–79.
- IAR. (1989). Crop protection department progress report for the period 1985/86 (pp. 140–147). Addis Ababa: Author.
- ICARDA. (2006). Technology generations and dissemination for sustainable production of cereals and cool season legumes (pp. 256). International Center for Agricultural Research in the Dry Areas, Aleppo, Syria.
- Isman, M. B. (2006). Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology, 51, 45–66. doi:10.1146/annurev.ento.51.110104.151146
- Joseph, B., Dar, M. A., & Kumar, V. (2008). Bioefficacy of plant extracts to control Fusarium solani F. Sp. melongenae incitant of Brinjal Wilt. Global Journal of Biotechnology and Biochemistry, 3, 56–59.
- Koul, O. (2008). Phytochemicals and insect control: An antifeedant approach. Critical Review of Plant Science, 27, 1–24. doi:10.1080/07352680802053908
- Lamma, A., Heyneman, D., & Silangwa, S. M. (Editors). (1984). Phytolacca dodecandra (endod). Towards controlling transmission of schistosomiasis with the use of a natural product. Final report the international scientific workshop (pp. 318), March 1983, Lusaka, Zambia. Dublin, Ireland: Tycooly International Publications Ltd.
- Nduagu, C., Ekefan, E. J., & Nwankiti, A. O. (2008). Effect of some crude plant extracts on growth of Colletotrichum capsici (Synd) Butler and Bis by causal agent of Pepper anthracnose. Journal of Applied Biosciences, 6, 184–190.
- Nerio, L. S., Olivero-Verbel, J., & Stashenko, E. (2010). Repellent activity of essential oils: A review. Bioresource Technology, 101, 372–378. doi:10.1016/j.biortech.2009.07.048
- Ogbebor, O. N., & Adenkunle, A. T. (2008). Inhibition of Drechslera heveae (Petch) M. B. Ellis, causal organism of Bird’s eye spot disease of rubber (Hevea brasiliensis Muell Arg.) using plant extracts. African Journal of General Agriculture, 4, 19–26.
- Parry, D. W. (1990). Plant pathology in agriculture (pp. 181–189). Cambridge: Cambridge University Press.
- Prince, L., & Prabakaran, P. (2011). Antifungal activity of medicinal plants against plant pathogenic fungus Colletotrichum falcatum. Asian Journal of Plant Science and Research, 1, 84–87.
- Roman, M. (2010). Evaluation of antifungal activity of plant extracts against chocolate spot disease (Botrytis fabae) on Faba bean (M.Sc thesis), Addis Ababa University.
- Sahile, S., Ahmed, S., Fininsa, C., Abang, M. M., & Sakhuja, P. K. (2008). Survey of chocolate spot (Botrytis fabae) disease of faba bean (Vicia faba L.) and assessment of factors influencing disease epidemics in northern Ethiopia. Crop Protection, 27, 1457–1463. doi:10.1016/j.cropro.2008.07.011
- Schmutterer, H. (1990). Properties and Potential of natural Pesticides from the neem tree. Azadirachta Inidica. Annual Review of Entomology, 35, 271–297. doi:10.1146/annurev.en.35.010190.001415
- Shovan, L. R., Bhuiyan, M. K. A., Begum, J. A., & Pervez, Z. (2008). In vitro control of Colletotrichum dematium causing anthracnose of soybean by fungicides, plant extracts and Trichoderma harzianum. International Journal of Sustainable Crop Production, 3, 10–17.
- Simeon, A., Materechera, M., & Hae, E. (2008). Potential of aqueous extracts from parts of the pepper tree (Schinus molle L.) to affect emergence and seedling development of wheat (Triticum sativa L.) and weeds in a manure amended soil. The Open Agriculture Journal, 2, 99–104. doi:10.2174/1874331500802010099
- Sundar, A. R., Das, N. D., & Krishnaveni, D. (1995). In-vitro Antagonism of Trichoderma spp. against two fungal pathogens of castor. Indian Journal of Plant Protection, 23, 152–155.
- Tadeg, H., Mohammed, E., Asres, K., & Gebre-Mariam, T. (2005). Antimicrobial activities of some selected traditional Ethiopian medicine al plants used in the treatment of skin disorders. Journal of Ethnopharmacology, 100, 168–175. doi:10.1016/j.jep.2005.02.031
- Tegegne, G., & Pretoriu, J. C. (2007). In vitro and in vivo antifungal activity of crude extracts and powdered dry material from Ethiopian wild plants against economically important plant pathogens. Biocontrol, 52, 877–888. doi:10.1007/s10526-007-9088-y
- Wheeler, J. B. (1969). An introduction to plant diseases (pp. 347). London: Wiley and Sons.
- Wokocha, R. C., & Okereke, V. C. (2005). Fungitoxic activity of extracts of some medicinal plants on Sclerotium rolfsii, causal organism of the Basal stem rot diseases of Tomato. Nigerian Journal of Plant Protection, 22, 106–110.
- Yadava, R. N., & Chakravarti, N. (2009). New antifungal triterpenoid saponin from Launae pinnatifida Cass. Indian Journal of Chemistry, 48B, 83–87.
- Yeni, I. J. (2011). Evaluation of antifungal effects of extracts of Allium sativum and Nicotiana tobacum against soft rot of yam (Dioscorea alata). Researcher, 3, 1–5.
