?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Qimbaba, a prioritized forest area in the northwest of Ethiopia. However, it is experiencing deforestation that limits restoration possibilities. Hence, a detailed assessment of soil seed bank composition and regeneration potential could permit to improve the management of the forests. The study aimed at comparing native with plantation relative to the soil seed bank, as well as their effect on soil properties. A total of 40 circular sample plots (314 m2) were established, half in the natural and half in the plantation forests. Aboveground vegetation was recorded in each plot. Samples were collected for the soil seed bank analysis in the litter layer and the top 9 cm of the soil (3 cm layers) and for soil analysis down to 30 cm (15 cm depths). Vegetation and soil seed bank composition were compared and the effect of forest type (natural vs plantation) on soil seed bank and soil physical and chemical parameters was evaluated. A total of 14 plant species (11 families) were recovered from the soil seed bank (12 species in the natural and 7 in plantation forest). The soil seed bank was not similar according to the Sorenson’s similarity values. There were 11,022 and 10,667 seeds/m2 in the soil seed bank of the natural and plantation forest, respectively. There was no significant difference between the two forests in bulk density, CEC and P but SOC, N, and K were significantly higher in natural. Plantation forest pH was significantly lower than the natural forest.
PUBLIC INTEREST STATEMENT
Soil seed bank, natural storage of seeds in the leaf litter, on the soil surface, or in the soil of many ecosystems, which serves as a repository for the production of subsequent generations of plants to enable their survival. The term soil seed bank can be used to describe the storage of seeds from a single species or from all the species in a particular area. In this study, therefore, soil seed banks in the plantation and natural forest were assessed and their respective soil physicochemical properties were evaluated. And, recommendations which will assist Agricultural office, forestry commission, and NGOs were raised, in designing future programs and strategies for enhancing biodiversity conservation and forest plantation via promoting succession rate by soil seed banks.
Competing interests
The authors declares no competing interests.
1. Introduction
Ethiopia was in a diverse agro-ecological zones (Bekele-Tesemma & Tengnäs, Citation2007), ranging from afroalpine to desert vegetation (Zegeye, Teketay, & Kelbessa, Citation2005). Hence, it is one of the six plant biodiversity rich countries in Africa (Nune, Kassie, & Mungatana, Citation2013). About 35–40% of Ethiopia’s lands mass was covered by climax vegetation, which was either coniferous or broad-leaved (FAO, Citation1982). However, over the last 3,000 years, there has been progressive deforestation, loss of biodiversity and impoverishment of ecosystems (Yirdaw, Citation1996) which drastically reduced the forest area to 16% in 1950s and to 3.1% by 1982 (FAO, Citation2001). Due to severe forest clearing and deforestation of the forest resource, Eucalyptus tree was introduced by Emperor Menelik (1894 − 1895) to relieve the shortage of construction and fuelwood (Pohjonen & Pukkala, Citation1990).
Plantation establishments using exotic species have both advantages and disadvantages (Lugo & Waide, Citation1993) for the purpose of timber production and firewood. Nowadays, tree plantations function as an alternative site for the regeneration of native plant species (Senbeta, Teketay, & Näslund, Citation2002) and contribute to the conservation of biodiversity by providing an environment not only for native woody plants but also for the forest herb species (Boothroyd-Roberts, Gagnon, & Truax, Citation2013). Eucalyptus plantations occasionally promote the recruitment, establishment and succession of native woody species by functioning as foster ecosystems (Lugo & Waide, Citation1993; Parrotta, Turnbull, & Jones, Citation1997) on sites where soil seed banks of native forest species are lacking. They enhance the process of native forest succession over time by attracting seed dispersal agents. As a result, tree plantations can enhance plant diversity of indigenous species (Senbeta & Teketay, Citation2001). Moreover, it can also improve degraded lands by stabilizing soils, improving soil nutrient status and increasing soil organic matter through enhancement of aboveground litter production (Jordan & Farnworth, Citation1982; Lugo & Waide, Citation1993; Parrotta, Citation1995). Some regeneration studies on plantations of different species also proved a surprising catalytic role of these monocultures with regard to habitat recolonization by native woody plants (Alem & Pavlis, Citation2012). Qimbaba forest, marginalized forest resource in Ethiopia with human pressure and free animal range that reduced the forest diversity. But there will be seed bank diversity in the soil and the current paper is intends to evaluate the effect of eucalyptus plantation forest on the regeneration capacity of native forest, soil seed bank and selected soil physical and chemical properties.
2. Materials and methods
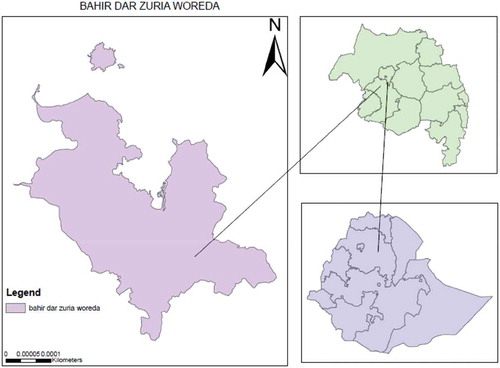
The study was carried out at Qimbaba forest, located south-west of Lake Tana, in Bahir Dar Zuria woreda, Amhara National Regional State (Ethiopia). The forest is geographically located between 12°62ʹ37m” and 12°65ʹ31.6”m N latitude and 32°50ʹ20”m and 32°78ʹ76” m E longitude. Vertisols and lithosols (Schoeneberger, Wysocki, & Benham, Citation2012) was the dominant soil type with a temperature that varies between 19°C and 21°C and receives an annual average of 1,272–1,397-mm rainfall Gebey and Mekuriaw (Citation2013) (Figure ).
Plantation forests have been increased in the study area since the introduction of eucalyptus by Adiss-Bah project in 1974. Currently, 61% of the 967.50 ha covered by forest is plantation forest and the remaining 39% natural forest. The natural forest is dominated by shrub species of Acokanthera schimperi, Calpurnia aurea, Capparis tomentosa and Maytenus arbutifolia in almost all parts of the forest and there is a high diversity of Solanum giganteum whereas, big trees like Ficus ovate, Sapium ellipticum and Syzygium guineense can be found along small streams inside the forest. The assessment compares Eucalyptus plantation and natural forest using a line transect survey. A circular plot with a size of 314 m2 was established at 200-m intervals along line transect (in the east-west direction) which was 200 m apart. A total of 40 sampling plots (20 in the natural and 20 in the plantation forest) were taken across the study area within 8 transects.
2.1. Soil seed bank sampling
At the centre of each plot, a small plot of 100 cm2 was marked and four separate soil layers consisting of the litter layer, and three successively deeper mineral soil layers, each 3 cm thick (to investigate depth distribution of seeds in the soil), were removed using a sharp knife. The soil seed bank samples were put into plastic bags separately and transported to the lab for analysis. In cases of dissimilarity between soil seed bank and aboveground flora, soil depth was used to speculate on the origin of the seed sources (whether recently dispersed seed or from the soil seed bank) (Teketay, Citation2001). All soil samples were sieved using a mesh size of 0.50 mm to recover seeds of woody species before incubation to stimulate the germination of seeds. The seeds recovered by sieving were collected into paper bags and identified using local reference material. The soil samples were incubated in a glasshouse for 4 months (Figure ). The emerging seedlings were identified, recorded and discarded once every 2 weeks. Those seedlings difficult to identify were transplanted and grown to a larger stage to make identification easier and accurate (Senbeta & Teketay, Citation2001). Nomenclature follows honeybee flora of Ethiopia Fichtl and Adi (Citation1994) and flora of Ethiopia and Eritrea (Edwards, Tadesse, Demissew, & Hedberg, Citation2000; Edwards, Tadesse, & Hedberg, Citation1995; Hedberg & Edwards, Citation1995; Hedberg, Edwards, & Nemomssa, Citation2006).
2.2. Soil sampling for physicochemical properties study
Five representative soil sampling sites were selected randomly for each forest (plantation and natural forests) by using available field methods such as similarity in soil color, structure, and cohesiveness when wet. 20 m × 20 m plots were laid and then one composite was made for each soil depth (constituting five composites per layer for each forest type). The soil samples were collected from the 0–15 and 15–30 cms in five replicates. The samples were collected at the four corners and at the center of each 20 m × 20 m plot and depths to form a single composite sample. So that a total of 10 soil samples (five per each forest type) were collected for the analysis of soil physicochemical properties. Additionally, separate soil core samples from the 0–15 and 15–30 cm depths were taken with a sharp-edged steel cylinder forced manually into the soil for bulk density determination. The soil samples collected from representative fields’ in five replications were air-dried, mixed well and passed through a 2 mm sieve for the analysis of selected soil physical and chemical properties.
2.3. Soil data analysis
The major part of the soil physical and chemical analysis was carried out at the Adet Agricultural center soil testing Laboratory. Standard laboratory procedures were followed in the analysis of the selected physicochemical properties considered in the study. The soil bulk density was analyzed using core method prescribed for undisturbed soils (Kolay, Citation2000). The core sample was oven-dried to a constant weight using an oven at 105°C for 24 h. The soil pH was measured using a pH meter in 1:2.5 soils: water ratio suspension (Anderson & Ingram, Citation1993). The soil organic content was determined following the wet digestion method as outlined by Walkley and Black (Citation1934) and percent OM (organic matter) was obtained by multiplying percent OC by 1.72. Available Phosphorous was determined using the standard Olsen extraction method (Olsen, Citation1954). Total Nitrogen contents in the soil samples were determined titrimetrically following the Kjeldahl method as described by Lim and Jackson (Citation1982). Cation exchange capacity (CEC) was determined after extracting the soil samples by ammonium acetate (1N NH4OAc) at pH 7.0. While exchangeable Ca and Mg in the extracts were analyzed using atomic absorption spectrophotometer, while K was analyzed by a flame photometer (Rowell, Citation1994). Cation exchange capacity was thereafter estimated titrimetrically by distillation of ammonium that was displaced by sodium from NaCl solution (Chapman, Citation1965).
2.4. Statistical analysis
The Shannon-Wiener Diversity Index (H′) was used to determine the diversity of species in both the natural forest and plantation.
where k is the number of species, pi proportion of individual species to the total.
Shannon evenness index (Peet, Citation1974; Krebs, Citation1989).
where J = evenness, H’ = Shannon-wiener diversity index and H’max = maximum Shannon-wiener diversity indexes,
The Sorensen similarity coefficient was used to compare the similarity between the community types in their species richness. Kent (Citation2011) realized that the index were widely used because it gives more weight to the species that are common to the samples rather than to those that only occur in either sample. Accordingly, Sorenson coefficient of similarity (Ss) was calculated using the formula of,
where, Ss = Sorenson similarity coefficient
a = number of species common to both samples, b = number of species in sample 1 and c = number of species in sample 2.
Data obtained were subjected to statistical analysis to determine the mean values, standard deviation and the standard error of the means using Microsoft Excel 2010. Independent T-tests were used to compare the mean differences for all the tested parameters between soil samples of the Eucalyptus plantation and natural forest. Tables and bar charts were used.
3. Results and discussion
3.1. Species composition of soil seed banks
A total of 14 plant species (11 families) were recorded from the soil seed bank, of which 12 species (10 families) occurred in the natural forest and 7 of them (5 families) in the Eucalyptus plantation forest. Trees were the dominant group represented by 8 species (57.2%), while shrubs and climbers were represented by 5 species (35.7%) and 1 species (7.1%), respectively. This result is in agreement with the studies of Assefa (Citation2011) that reported the emergence of five woody plant species in the seed bank study, Bezawit forest at Abay Millennium Park, Northwest Ethiopia. The low number of seeds in the soil seed bank for most species could also be the result of short residence time of most woody species Teketay (Citation2005). The only Exception Acokanthera schimperi, which was the dominant species, accounting to more than 80% of the seed bank flora. This high number of seeds of Acokanthera schimperi in Qimbaba natural and plantation forest contradicts with other result of Senbeta et al. (Citation2002); Tenkir (Citation2006) and Sileshi and Abraha (Citation2014). They reported Juniperus procera as one of the dominant species that were found in the soil seed banks of Ethiopian afromontane forests. The Qimbaba forests is situated in a heavily populated area, where forest disturbance and the expansion of quarries inside the forest aggravate the sustainability of a diverse forest cover. (Lemenih & Teketay, Citation2006) also noted that as long as seeds of woody species remain on the surface they are either attacked by predators or immediately germinate. As previous studies (Lemenih &, Teketay Citation2006; Senbeta & Teketay, Citation2001; Senbeta et al., Citation2002) have indicated, most woody species in the dry Afromontane forests of Ethiopia depend on seed rain and formation of seedling banks under the shades of mature forest canopy as strategies for regeneration.
3.2. Species richness, diversity and evenness of the soil seed bank
The Shannon diversity index for the diversity of the soil seed bank in Qimbaba natural and Eucalyptus plantation forests were (H’ = 0.94 and H’ = 0.706), respectively (Table ). These index values are lower than Hgumbirda National Forest Priority Area (H’ = 1.76) (Sileshi & Abraha, Citation2014) and Harenna forest (Tesfaye, Teketay, Assefa, & Fetene, Citation2004). In our study, the highest diversity occurred in the litter layer followed by 0–3 cm, 3–6 cm and 6–9 cm, respectively. Generally, species richness decreased with greater soil depth.
Table 1. Soil seed bank species richness, diversity and evenness of Qimbaba forest
The Shannon evenness index had no consistent trend for the soil layers of each forest site, but overall the value ranked as litter > 0–3 cm > 6–9 cm > 3–6 cm. The overall soil seed bank evenness of the eucalyptus plantation forest was 0.36, which is less than the adjacent natural forest evenness (0.38). This may be due to variation in seed rain and seed deterioration due to differences in current vegetation between the two forest sites (Perera, Citation2005; Sileshi & Abraha, Citation2014). The highest Shannon diversity and evenness index were recorded from the natural forest (Table ), which shows the better status of this forest compared to the plantation forest. Likewise, differences in magnitude and intensity of disturbances could add up to the variation in diversity and evenness of the soil seed bank (Kellerman, Citation2006; Sileshi & Abraha, Citation2014).
3.3. Similarity between soil seed bank and above ground flora
The similarity in species composition of the soil seed bank between the two forest sites was generally low with the value of 0.208 (Table ). These findings were low which is similar to other studies, Tenkir (Citation2006) Dodola forest; (Sileshi & Abraha, Citation2014) in Hgumbirda National Forest Priority area and Lemenih and Teketay (Citation2006) they reported low Sorenson similarity index in tropical dry afromontane forests of Ethiopia. (Table )
Table 2. Sorenson’s coefficient of similarity in species composition of soil seed banks
The similarity between the different soil layers was very low (SCS = 0.07–0.25) in the natural forest, where the similarity between the litter layer and 0–3 cm soil depth (SCS = 0.076) was the minimum and between 0 and 3 cm and 3–6 cm soil depth (SCS = 0.25) was relatively higher (Table ).
Table 3. Sorenson’s coefficient of similarity in soils depths (the natural forest)
In the Eucalyptus plantation forest, the similarity was lower between the litter layer and 0–3 cm soil depth (SCS = 0.111) and higher in the soil depth of 0–3 cm and 6–9 cm (SCS = 0.29). Acokanthera schimperi, species is abundant and commonly found in the soil seed bank of both the Eucalyptus plantation forest and natural forest (Table ).
Table 4. Sorenson’s coefficient of similarity in different soils depths (plantation forest)
The exotic species, Eucalyptus camndulnsis was abundant in the soil seed bank of Eucalyptus plantation forest compared to Lemenih and Teketay (Citation2006) and (Sileshi & Abraha, Citation2014) who instead reported Eucalyptus globulus and Cupressus lusitanica in the soil seed bank of Ethiopian afromontane forests, respectively. The presence of exotic species in soil banks could potentially become a source of invasion and risk for future regeneration by competing and changing the ecosystem (Ferreira, Elosegi, Gulis, Pozo, & Graça, Citation2006; Matthew et al., Citation2004). However, scanty distribution of the standing canopy of those two exotic species may not affect the regeneration ability of most native flora.
3.4. Species density of soil seed bank (SSB) flora
The total seed bank densities in the litter and the upper 9 cm of the soil were 11,022 seeds/m2 in the natural forest and 10,667 seeds/m2 in the Eucalyptus plantation forest. In the natural forest, 10,844.44 seeds/m2 were viable and 177.56 seeds/m2 were non-viable. Similarly, in the plantation forest 10,311.11 seeds/m2 were viable and 355.89 seeds/m2 were non-viable. These values are considerably lower than those obtained for the Hgumbirda National forest Priority area (12,611.1 seeds/m2) by Sileshi and Abraha (Citation2014), the Dodola Dry Afromontane Forest (30,267 seeds/m2) by Tenkir (Citation2006), the Menagesha-Suba and Munessa-Shashemene forest sites (ranged from 27,200 seeds/m2 to 82,600 seeds/m2) by Senbeta et al. (Citation2002) and the Gera Ades Moist Evergreen Afromontane Forest (10,333–93,293 seeds/m2) by Teklu (Citation2014). The dominant species in the soil seed bank was Acokanthera schimperi in both forest types, accounting for over 80% of all seeds in the seed bank in natural forest and 84% in the plantation forest.
4. Effects of plantation and natural forest on selected soil physicochemical properties
4.1. Bulk density
The measured bulk density did not show significant differences (P > 0.05) between the two forests or between soil depth (Table ). Soil bulk density is used as a measure of soil compaction, health and an indirect measure of the total pore space. The bulk density of fine-textured mineral soils usually ranges from about 1.0 to 1.5 g/cm3 (Kolay, Citation2000), a higher soil bulk density means less water held in the soil at field capacity and at lower soil bulk densities, soils are less compacted and are able to retain water (Kakaire, Makokha, Mwanjalolo, Mensah, & Menya, Citation2015). In a similar study Abiyu et al. (Citation2011) found 0.9 g cm−3 in 0–10 cm and 0.9 g cm−3 in the 10–20 cm soil depths of Eucalyptus plantation forest in Tehuledere district of Northern Ethiopia. However, Ravina (Citation2012) showed a higher soil bulk density of 1.24 g cm3 under the Eucalyptus Plantation forest compared to the native forests (0.66 g cm3) in a Brazilian soil at a soil depth of 0–15 cm. Since the soil bulk densities found in both the plantation forest and natural forest soils were within this range, the findings from this study did not confirm those of Aweto and Moleele (Citation2005), who concluded that Eucalyptus plantations increased soil bulk density more than the native forest in Botswana.
Table 5. Mean ± SE result of bulk density (BD) for depths of 0–15 cm and 15–30 cm for the plantation and natural forest, and corresponding P values from T-table
4.2. Soil pH
The pH of the soils range from 6.28 in the plantation and 6.61 for the natural forest (Table ). The pH of the soils under the plantation forest was significantly lower (P < 0.05) than the natural forest soils. However, there was no significant difference (P > 0.05) with soil depth in the two forests. But, according to Horneck, Sullivan, Owen, and Hart (Citation2011), the two forests within the depth fit on different acidic strength groups, where plantation forest was in moderately to slightly acidic and the natural forest in the group of neutral. Nutrient elements occur in available forms at a soil pH range of 5.5 to 6.5 (Horneck et al., Citation2011); therefore, the natural forest soil is likely to be more fertile with a greater availability of plant nutrients as compared to the plantation forest soil. The result is in agreement with Abiyu et al. (Citation2011) who reported a decrease in soil pH from exclosure to cupresses Lusitania plantation forest and finally to Eucalyptus plantation forest.
Table 6. Soil chemical property in the two adjacent forests plantation Forest and natural forest
4.3. Soil organic carbon (SOC)
The concentrations of soil organic carbon (SOC) recorded in the plantation and natural forest were 1.83% and 3.45%, respectively (Table ). The SOC in the plantation forest was significantly lower (t (5) = 2.14, P = 0.00013) than in the natural forest soil. The comparison of the SOC across depths showed significant differences when assessed by using T-test (T (5) = 2.57, P = 0.0089) in the depth of 0–15 cm, and also there is a significant difference at (T (5) = 2.44, P = 0.0095) with the depth of 15–30 cm. It is also clearly visible that as the soil depth increases, the SOC content decreased continuously in the Eucalyptus plantation forest and adjacent natural forest (Table ). According to the ratings, SOC in the natural forest is low, but numerically higher than the eucalyptus plantation forest which is in the range of very low. This result is in line with Abiyu et al. (Citation2011) in Tehuledere district, Mensah (Citation2016) in Kenya, and Mengist, Georg, and Sieghardt (Citation2011) in Jufi plantation forest, Ethiopia.
4.4. Soil available phosphorus
The mean concentration of soil available P recorded was 0.92 and 2.00 ppm for PF and NF, respectively (Table ). The concentration of available P in the plantation forest soil was not significant (t (5) = 2.2, P = 0.18) compared to that in the natural forest soil. In the two depths also the concentration of available P was not significantly different. In the plantation forest P concentration was increased due to depth but not in the natural forest. The available P in the plantation and natural forest soil falls below the medium sufficiency range. The relatively lower available P registered for the plantation forest soil may be due to the low pH value which might have resulted in a higher amount of soil available P fixed/adsorbed Mensah (Citation2016). Aweto and Moleele (Citation2005) reported that Eucalyptus species have a higher capacity of immobilizing phosphorus, and make them inaccessible for plant use. Berendse (Citation1998) adds that at soil pH of below 5.5, soil trace nutrients like Aluminum (Al) availability increases to levels that are unsuitable for most plant growth and soil soluble phosphorus (P) tends to form insoluble compounds with Al and Fe in acidic soils, which are inaccessible to native plants. Even if there is a change in the level of range (plantation forest in low range and natural forest in the medium range) there is no significant difference between the two forests.
These findings are in agreement with those of Polglase, Attiwill, and Adams (Citation1992) who found lower available phosphorous in the 0–5 cm depth of soil under the Eucalyptus Plantation forest. Aweto and Moleele (Citation2005) also found low available phosphorus (below 5μg g − 1) in soils of Eucalyptus plantation forest in both the 0–10 and 10–20 cm depths of soil in Botswana. Furthermore, Alemie et al. (Citation2009) reported that the concentration of available phosphorus in Eucalyptus plantation within 2 cm depth of soil depth was in the very low range (< 5mg kg-1) in Ethiopia. But Abiyu et al. (Citation2011) reported that there is a relatively higher amount of available phosphorus concentration in Eucalyptus glubules plantation than in the enclosure area and cupresses Lusitanica plantation forest in the depth of 0–10 cm.
4.5. Soil nitrogen
The mean concentrations of soil N were 0.18% and 0.31% for plantation and natural forest, respectively (Table ). The total nitrogen concentration found in the soil under plantation forest was significantly lower (t (5) = −2.14, P = 0.02) than under natural forest. However, there was no significant difference between the two forests with soil depth (Table ). According to the ratings of nitrogen, the concentrations in the plantation forest with soil depth were in low range, whereas, it was in the average range in the natural forest. The low level of total nitrogen in the plantation forest is in line with the studies of Abiyu et al. (Citation2011) in the Tehuledere district plantation forest and Mengist et al. (Citation2011) in Jufi plantation, Ethiopia. Alemie et al. (Citation2009) also reported lower soil total nitrogen under plantation forests of Eucalyptus species in koga, Ethiopia. The concentration of nitrogen in a soil is partly reflected by the rate of decomposition of the plant material (Cao et al., Citation2010). Eucalyptus produce a litter with low nutrient concentrations, which decomposes slowly to release low concentrations of nutrients (Aweto & Moleele, Citation2005; Cao et al., Citation2010; Demessie, Singh, Lal, & Strand, Citation2012), coupled with low soil pH and N mineralization. These could be factors in Qimbaba Eucalyptus plantation forest to have low levels of total nitrogen than the adjacent natural forest.
4.6. Potassium
The Eucalyptus stand contained low exchangeable K+ compared with the natural forest (Table ). The K+ content that was observed in the plantation forest was significantly lower than the natural forest at (t (5) = 2.11, P < 0.05). This result was in line with Abiyu et al. (Citation2011). There is a high K+ concentration in the natural forest at the depth of 0–15 cm (261.6) while in the plantation forest the amount is much lower than the natural forest in the two depths, but no significant difference was detected between the two forests types. The Change in K+ content under plantation and natural forest across the soil depth mostly showed a regular reduction rate (Table ). Abiyu et al. (Citation2011) and Mengist et al. (Citation2011) in Jufi plantation also reported there is a reduction in K+ concentration with an increase in soil depth.
4.7. Cation exchange capacity (CEC)
The Cation exchange capacity (CEC) recorded in the plantation forest (50.01) soil was lower than the natural forest soil (55.45) (Table ), the figures were found to be statistically similar. Across depths, the data were not significantly different. Although the CEC tended to increase with depth. This result is in line with Mengist et al. (Citation2011) in Jufi plantation. Under plantation forest there are CEC of 50.3 for the 0–15 cm depth and 49.7 for 15–30 cm. In the natural forest, there is a similar trend of decreasing from 56.7 in the upper soil and 54.1 in the depth of 15–30 cm.
5. Conclusion
Our findings indicated that soils in the forest were with low bulk density and slightly moderate pH, where most nutrient elements occur in available forms. Soil seed bank was the ideal mechanism for restoration of the natural environment by itself through a long time, by the processes of nature when the ideal environment was available. The study area were primarily dominated by shrub species of Acokanthera schimperi, Calpurnia aurea, Capparis tomentosa and Maytenus arbutifolia in almost all parts of the forest and there is a high diversity of Solanum gigantum. Tree/shrub was also regenerated in the gaps that were created by the expansion of stone crashers inside the forest. Whereas, big trees like Ficus ovate, Sapium ellipticum and Syzygium guineense were found along small streams inside the forest. Generally, the forests were heavily disturbed by human-induced factors. Trees were dominant plant form than shrubs and climbers. In the forest there a huge amount of seeds in the soil seed bank, but in dormant stage because of human-induced factors. High dependency of the people on wood from the forest for the generation of income, stone crushers, high population density and shortage of land are the major problems that could pose a serious threat to the forest resources. Therefore, in order to mitigate the existing crises and promote the sustainable management of the forest resources, the following recommendations are forwarded:
The capacity of exotic plantations to facilitate forest restoration was confirmed by the present study. Therefore, despite the perceived negative effects associated with the introduction of exotic species, there is a potential for exotic tree plantations to be used as a tool to restore indigenous forest ecosystems. However, while this potential for restoration or rehabilitation should be acknowledged, much of it depends on the intensity and length of the period of manmade influences which the forests have been exposed to.
Regarding the composition, diversity, vegetation structure and soil seed bank of the two forests, there should be a need of detailed study on the ethnobotany of Qimbaba natural and Eucalyptus plantation forest for the conservation of endangered multipurpose plant species.
Carry out further studies on the socioeconomic importance of the forest for the community, carbon emission amount of the forest for better conservation and sustainable management of the forest with the help of the local people.
Additional information
Funding
Notes on contributors
Getachew Kassa
Getachew Kassa is a forest researcher in Adet Agricultural Research center, Amhara Agricultural Research Institute, Ethiopia. His overall research interest falls within the domain of forestry and natural resource problems as plant diversity in natural and plantation forest, climate change mitigation measures, introduction and adaptation of valuable forest resources and others problems related to enhancement of overall forestry problems. Getachew kassa has designed and conducted many experimental studies on natural resource, forestry and appreciates biodiversity conservation, climate changes and encourages farmers to exploit the integrated potential of cultural practices to manage forest biodiversity and sustain productivity. He is involved in many national and local projects focusing on natural resource management climate change, along with different teams.
References
- Abiyu, A., Lemenih, M., Georg, G., Raf, A., Teketay, D., & Glatzel, G. (2011). Status of native woody species diversity and soil characteristics in an exclosure and in plantations of eucalyptus globulus and cupressus lusitanica in Northern Ethiopia. Mountain Research and Development, 31(2), 144–13.
- Alem, S., & Pavlis, J. (2012). Native woody plants diversity and density under Eucalyptus camaldulensis plantation, in Gibie Valley, South Western Ethiopia. Open Journal of Forestry, 2(4), 232. doi:10.4236/ojf.2012.24029
- Alemie, T., Buytaert, W., Zulkafli, Z., Grainger, S., Acosta, L., Alemie, T. C., … Foggin, M. (2009). Citizen science in hydrology and water resources: Opportunities for knowledge generation, ecosystem service management, and sustainable development. Frontiers in Earth Science, 2, 26.
- Anderson, J. M., & Ingram, J. S. I. (1993). A handbook of methods. Tropical soil biology and fertility (2nd ed., pp. 221). Wallingford, Oxfordshire: CAB International.
- Assefa, T. (2011). Assessment of soil seed bank composition in Bezawit Abay Millennium Park, Bahir Dar, Ethiopia (Doctoral dissertation, MSc. thesis). Bahir Dar University. (unpubl.)).
- Aweto, A. O., & Moleele, N. M. (2005). Impact of Eucalyptus camaldulensis plantation on an alluvial soil in South Eastern Botswana. International Journal of Environmental Studies, 62(2), 163–170. doi:10.1080/0020723042000275141
- Bekele-Tesemma, A., & Tengnäs, B. (2007). Useful trees and shrubs of Ethiopia: Identification, propagation, and management for 17 agroclimatic zones. RELMA in ICRAF Project, World Agroforestry Centre, Eastern Africa Region. doi:10.1094/PDIS-91-4-0467B
- Berendse, F. (1998). Effects of dominant plant species on soils during succession in nutrient-poor ecosystems. Biogeochemistry, 42(1–2), 73–88. doi:10.1023/A:1005935823525
- Boothroyd-Roberts, K., Gagnon, D., & Truax, B. (2013). Can hybrid poplar plantations accelerate the restoration of forest understory attributes on abandoned fields? Forest Ecology and Management, 287, 77–89. doi:10.1016/j.foreco.2012.09.021
- Cao, Y., Fu, S., Zou, X., Cao, H., Shao, Y., & Zhou, L. (2010). Soil microbial community composition under Eucalyptus plantations of different age in subtropical China. European Journal of Soil Biology, 46(2), 128–135. doi:10.1016/j.ejsobi.2009.12.006
- Chapman, D. W. (1965). Food and space as regulators of salmonid populations in streams. The American Naturalist, 100(913), 345–357.
- Demessie, A., Singh, B. R., Lal, R., & Strand, L. T. (2012). Leaf litter fall and litter decomposition under Eucalyptus and coniferous plans. In Gambo District, southern Eth. Acta Agriculturae Scandinavica, Section B-Soil & Plant Science, 62(5), 467–476.
- Edwards, S., Tadesse, M., Demissew, S., & Hedberg, I. (eds.). (2000). Flora of Ethiopia and Eritrea (Vol. 2). Uppsala: The National Herbarium Addis Ababa University, Addis Ababa and Department of Systematic Botany, Uppsala University.
- Edwards, S., Tadesse, M., & Hedberg, S. (eds.). (1995). Flora of Ethiopia and Eritrea (Vol. 2). Uppsala: The National Herbarium Addis Ababa University, Addis Ababa and Department of Systematic Botany, Uppsala University.
- FAO. (1982). Les eucalyptus dans les reboisements. Collection fao forêta. 753.
- FAO. (2001). State of the world’s forests. Rome: FAO (Food Aid Organization).
- Ferreira, V., Elosegi, A., Gulis, V., Pozo, J., & Graça, M. A. (2006). Eucalyptus plantations affect fungal communities associated with leaf-litter decomposition in Iberian streams. Archiv Für Hydrobiologie, 166(4), 467–490. doi:10.1127/0003-9136/2006/0166-0467
- Fichtl, R., & Adi, A. (1994). Honeybee flora of Ethiopia (pp. 519). Weikersheim (Germany): Margraf Verlag. ISBN: 3823612344.
- Gebey, T., & Mekuriaw, Z. (2013). Diagnosis and intervention plans for West Gojjam Zone, Amhara region. Report, Livestock and Irrigation Value Chains for Ethiopian Smallholders (LIVES) project. Addis Ababa, Ethiopia.
- Hedberg, I., & Edwards, S. (Eds.). (1995). Flora of Ethiopia and Eritrea, Vol. 7. Poaceae (Graminae). Uppsala: The National Herbarium, Addis Ababa University, Addis Ababa & Department of Systematic Botany, Uppsala University.
- Hedberg, I., Edwards, S., & Nemomssa, S. (eds.). (2006). Flora of Ethiopia and Eritrea (Vol. 4). Uppsala: The National Herbarium Addis Ababa University, Addis Ababa and Department of Systematic Botany, Uppsala University.
- Horneck, D. A., Sullivan, D. M., Owen, J. S., & Hart, J. M. (2011, July 1–12). Soil test interpretation guide. Oregon State University, Extension Service.
- Jordan, C. F., & Farnworth, E. G. (1982). Natural vs. plantation forests: A case study of land reclamation strategies for the humid tropics. Environmental Management, 6(6), 485–492. doi:10.1007/BF01868377
- Kakaire, J., Makokha, G. L., Mwanjalolo, M., Mensah, A. K., & Menya, E. (2015). Effects of mulching on soil hydro-physical properties in Kibaale Sub-catchment, South Central Uganda. Applied Ecology and Environmental Sciences, 3(5), 127 135.
- Kellerman, M. J. S. (2006). Seed bank dynamics of selected vegetation types in Maputaland, South Africa (Doctoral dissertation). University of Pretoria.
- Kent, M. (2011). Vegetation description and data analysis: A practical approach. Oxford: John Wiley & Sons.
- Kolay, A. K. (2000). Basic concepts of soil science (2nd ed., pp. 78 8, 9 91, 1 2 105,138). New Delhi, India: New Age International Publishers.
- Krebs, C. J. (1989). Ecological Methodology. New York, NY: Harper and Row Corporation. Menlo Park, California. Addison Wesley. 1999. xii, 620 p. 24 cm. Edición; 2nd ed. Incluye bibliografía (p. 581–605) e índice (p. 607–620).
- Lemenih, M., & Teketay, D. (2006). Changes in soil seed bank composition and density following deforestation and subsequent cultivation of a tropical dry Afromontane forest in Ethiopia. Tropical Ecology, 47(1), 1–12.
- Lim, C. H., & Jackson, M. L. (1982). Dissolution for total elemental analysis 1. In Methods of soil analysis Part 2. Chemical and microbiological properties, (methodsofsoilan2) (pp. 101–-150).
- Lugo, A. E., & Waide, R. B. (1993). Catastrophic and background disturbance of tropical ecosystems at the Luquillo experimental forest. Journal of Biosciences, 18(4), 475–481. doi:10.1007/BF02703080
- Matthew, L., Brooks, C. M., d’antonio, D. M., Richardson, J. B., Grace, J. E., Keeley, J. M., … David, P. (2004). Effects of invasive alien plants on fire regimes. Bioscience, 54, 677–688. doi:10.1641/0006-3568(2004)054[0677:EOIAPO]2.0.CO;2
- Mengist, M., Georg, G., & Sieghardt, M., (2011). Eucalyptus plantations in the highlands of Ethiopia revisited: A comparison of soil nutrient status after the first coppicing (Master Thesis) Mountain Forestry program. Boku University, Vienna Austria.
- Mensah, A. K., (2016). Effects of eucalyptus plantation on soil physico-chemical properties in Thiririka sub-catchment, Kiambu County, Kenya (Doctoral dissertation). Kenyatta University.
- Nune, S., Kassie, M., & Mungatana, E. (2013). Forest resource accounts for Ethiopia. In Implementing environmental accounts (pp. 103–142). Dordrecht: Springer.
- Olsen, S. R. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate (No. 939). Washington: United States Department of Agriculture.
- Parrotta, J. A. (1995). Influence of over story composition on understory colonization by native species in plantations on a degraded tropical site. Journal of Vegetation Science, 6(5), 627–636. doi:10.2307/3236433
- Parrotta, J. A., Turnbull, J. W., & Jones, N. (1997). Catalyzing native forest regeneration on degraded tropical lands. Forest Ecology and Management, 99(1–2), 1–7. doi:10.1016/S0378-1127(97)00190-4
- Peet, R. K. (1974). The measurement of species diversity. Annual Review of Ecology And systematic, 5, 285–307. doi:10.1146/annurev.es.05.110174.001441
- Perera, G. A. D. (2005). Diversity and dynamics of the soil seed bank in tropical semi-deciduous forests of Sri Lanka. Tropical Ecology, 46(1), 65–78.
- Pohjonen, V., & Pukkala, T. (1990). Eucalyptus globulus in Ethiopian forestry. Forest Ecology and Management, 36(1), 19–31. doi:10.1016/0378-1127(90)90061-F
- Polglase, P. J., Attiwill, P. M., & Adams, M. A. (1992). Nitrogen and phosphorus cycling in relation to stand age of Eucalyptus regnans F. Muell. III. Labile inorganic and organic P, phosphatase activity and P availability. Plant and Soil, 142, 177–185. doi:10.1007/BF00010964
- Ravina, M. (2012). Impact of Eucalyptus plantations on pasture land on soil properties and carbon sequestration in Brazil. Sweden: Swedish University of Agricultural Sciences.
- Rowell, D. L. (1994). The preparation of saturation extracts and the analysis of soil salinity and sodicity. In Rowell (Ed.), Soil science methods and applications (pp. 3893–3914). Harlow, UK: Longman Group.
- Schoeneberger, P. J., Wysocki, D. A., & Benham, E. C., Soil Survey Staff. (2012). Field book for describing and sampling soils, Version 3.0. Lincoln, NE: Natural Resources Conservation Service, National Soil Survey Center.
- Senbeta, F., & Teketay, D. (2001). Regeneration of indigenous woody species under the canopies of tree plantations in Central Ethiopia. Tropical Ecology, 42(2), 175–185.
- Senbeta, F., Teketay, D., & Näslund, B. Å. (2002). Native woody species regeneration in exotic tree plantations at Munessa-Shashemene Forest, Ethiopia. New Forests, 24(2), 131–145. doi:10.1023/A:1021201107373
- Sileshi, D., & Abraha, B. (2014). Assessment of soil seed bank composition of woody species in Hgumbirda national forest priority area, Northeastern Ethiopia. Momona Ethiopian Journal of Science, 6(1), 25–44. doi:10.4314/mejs.v6i1.102413
- Teketay, D. (2001). Deforestation, wood famine, and environmental degradation in Ethiopia’s highland ecosystems: Urgent need for action. Northeast African Studies, 53–76. doi:10.1353/nas.2005.0020
- Teketay, D. (2005). Seed and regeneration ecology in dry Afromontane forests of Ethiopia: I. Seed production-population structures. Tropical Ecology, 46(1), 29–44.
- Teklu, Y. (2014). Soil seed bank study at Gera moist evergreen Afromontane Forest, Jimma Zone, Southwest Ethiopia (Doctoral dissertation). Addis Ababa University.
- Tenkir, E. (2006). Soil seed bank study and natural regeneration assessment of woody species in Dodola dry Afromontane forest, Bale Mountains ((Unpublished) M. Sc. thesis). Addis Ababa University, Ethiopia.
- Tesfaye, G., Teketay, D., Assefa, Y., & Fetene, M. (2004). The impact of fire on the soil seed bank and regeneration of Harenna forest, Southeastern Ethiopia. Mountain Research and Development, 24(4), 354–362. doi:10.1659/0276-4741(2004)024[0354:TIOFOT]2.0.CO;2
- Walkley, A., & Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37(1), 29–38. doi:10.1097/00010694-193401000-00003
- Yirdaw, E. (1996). Deforestation and forest plantations in Ethiopia. In Sustainable forestry challenges for developing countries (pp. 327–342). Dordrecht: Springer.
- Zegeye, H., Teketay, D., & Kelbessa, E. (2005). Diversity, regeneration status and socio-economic importance of the vegetation in the islands of Lake Ziway, south-central Ethiopia. Flora-Morphology, Distribution, Functional Ecology of Plants, 201(6), 483–498. doi:10.1016/j.flora.2005.10.006