 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nowadays, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) is becoming a serious insect pest of chickpea in Ethiopia and its management has faced considerable challenges, to improve yields in many crops, in most parts of the world. This experiment was initiated with the objective to evaluate the effectiveness of Azadirachta indica and Milletia ferruginea extracts for the management of H. armigera in chickpea from July to December during 2017 and 2018 cropping season under field conditions. The results revealed that aqueous extracts of M. ferruginea and A. indica at 5% concentration either individually or in combinations at 2.5% concentration of each were more effective in reducing per plant H. armigera larval populations, pod damage with increased the subsequent yields during both cropping seasons as compared to control plot. Comparing the net cost–benefit return of each treatment, during 2017 maximum CBR was from the M. ferruginea 5% treated plot and A. indica 5% treated plots during the 2018 year. Thus, locally available botanical extracts would greatly benefit the resource-poor farmers in chickpea production. Future research attentions and considerations as a part of IPM tools, in pest management, are crucial.
PUBLIC INTEREST STATEMENT
Helicoverpa armigera is becoming a serious insect pest of chickpea in the world. It can cause 21 to 36% and almost 30% chickpea yield losses in Ethiopia and India, respectively. Looking for control methods, most farmers rely on synthetic insecticides which could have an irreversible side-effect to this planet. The demand for plant derived insecticides is increasing to replace the adverse effects. Therefore, the effectiveness of seed extracts of Azadirachta indica and Milletia ferruginea for the management of H. armigera in chickpea under field conditions was evaluated for 2 years. Application of an aqueous extract of M. ferruginea and A. indica at 5% concentration individually or in combinations at 2.5% concentration of each was more effective in reducing H. armigera larval populations and pod damage with increased the yields during both cropping seasons as compared to control plots. During 2017, the maximum cost-benefit return was from the M. ferruginea 5% and A. indica 5% treated plots during the 2018 year.
Competing Interests
The authors declare no competing interests.
1. Introduction
Nowadays, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) management facing considerable challenges in many crops of the world (Li et al., Citation2018; Patil et al., Citation2017; Singh, Bisht, & Dutta, Citation2014). H. armigera, a highly polyphagous and cosmopolitan pest infesting many host plants (Attique, Arif, Ahmed, & Mohyuddin, Citation2000; Pande, Sharma, & Ramkrishna, Citation2000; Pawar, Citation1998; Sarwar, Citation2012), is a serious pest of chickpea (Cicer arietinum L.) (Fite, Tefera, Negeri, Damte, & Sori, Citation2018; Patil et al., Citation2017; Sarwar, Ahmad, & Tofique, Citation2011). It can cause 21 to 36% crop yield losses in Ethiopia (Geletu, Million, & Anbessa, Citation1996) and almost 30% chickpea yield losses in India (Dinesh, Anusha, Bharu, & Dangi, Citation2017). The demand for crop production is high for an increasing world population (Chichaybelu et al., Citation2018; Singh et al., Citation2014). Although several non‐chemical approaches, involving transgenic crops (Das et al., Citation2017; Singh et al., Citation2014, Citation2018), cultural (Jallow, Cunningham, & Zalucki, Citation2004) and biological methods (Reddy & Manjunatha, Citation2003; Revathi, Ravikumar, Kalaiselvi, Gomathi, & Uma, Citation2011), have been available as a part of integrated pest management (IPM) approach but its management is largely based on synthetic insecticides. The adaptability of insect pests to most of conventional insecticides is leading to economic yield losses with increased negative side-effects to environment is a big challenge to the modern agriculture (Ahmad, Bilal, Munir, & Russell, Citation2019; Bird, Citation2017; Li et al., Citation2018; Mironidis et al., Citation2013). Due to the increasing side-effects of synthetic insecticides, there is increasing demand and interest for botanical insecticides worldwide (Ali et al., Citation2017). H. armigera is recognized as a potential insect pest for resistance development to a wide range of synthetic insecticides including Bt. in various crops worldwide (Ahmad et al., Citation2019; Alvi, Sayyed, Naeem, & Ali, Citation2012; Hussain, Muhammad, Ghulam, & Muneer, Citation2015; Li et al., Citation2018). In Ethiopia, farmers are facing serious challenges to manage this resistant insect pest as most of the synthetic insecticides in chickpea have lost their effectiveness under field conditions. Hence, in order to minimize the side effects of synthetic chemicals, the use of naturally occurring botanical extracts as a part of IPM would be an alternative approach for successful management of H. armigera. Numerous studies have been conducted on the use of botanical extracts, essential oils and isolated compounds (Isman, Citation2006; Koul, Citation2016; Ali et al., Citation2018; Younas et al., Citation2016; Junhirun et al., Citation2018) as promising insect pest management tools. Many botanical products have been found to act as oviposition and feeding deterrents, ovicidal, larvicidal agents against a diverse range of insect pests (Schmutter, Citation1990; Gebre-Amlak, Citation1999; Silva et al., Citation2015; Ahmad et al., Citation2015). Additionally, they have no negative effects on beneficial organisms under field conditions (Begg et al., Citation2017).
Mainly, Azadirachta indica A. Juss has, substantially, been used in pest management worldwide since years (Hussain et al., Citation2015). Also, the seed extract of Milletia ferruginea has been observed to be effective in controlling many insect pests such as Callasobruchus chinensis (Mulatu, Citation2007), Sitophilus zeamais (Jembere, Citation2002), Busseola fusca (Fuller) (Tilahun, Azerefegn, & Reddy, Citation2009), Zebrotes subfaciatus (Habeeb, Citation2010) and mosquito larvae (Asegid, Abede, Mudi, Melaku, & Taye, Citation2007). Tremendous research is undergoing to evaluate the efficacy of various plant extracts against agricultural insect pests (Zanuncio et al., Citation2016). However, most of these researches have investigated the effectiveness of these plant-derived insecticides under laboratory conditions. Botanicals are inexpensive and readily available tools for insect pest management and are eco-friendly. Furthermore, poor farmers in developing countries cannot afford to use synthetic insecticides due to higher financial costs. Moreover, lack of updated information on botanical extracts for proper development of IPM strategies in chickpea is crucial to improve the livelihoods of Ethiopian farmers. It is obvious that there is a need to develop biological alternatives for eco-friendly and sustainable H. armigera management strategies. Therefore, the objective of this study was to evaluate the effectiveness of seed extracts of A. indica and M. ferruginea for the management of H. armigera in chickpea under field conditions.
2. Materials and methods
2.1. Site description
A field experiment was conducted on chickpea under field conditions at Minjar-Shenkora district of Amhara Regional State for two consecutive years, from July to December during 2017 and 2018 cropping season. The district was among the major chickpea producing area and H. armigera is regular pest of this crop at that area. Moreover, this criterion allows a good infestation level to test the management options. The district is part of the temperate, cool sub-humid highlands of midland areas at 08°54.703ʹ N latitude and 039°26.940ʹE longitude with 10 years annual rainfall between 162 (the lowest) to 1028 mm (the highest) and 20°C (maximum) and 7.3°C (minimum) annual temperatures (Getu, Zemede, & Ensermu, Citation2015).
2.2. Field experiments
The field was thoroughly ploughed to remove all the previous crop residuals and weeds for better crop growth in July. Habru (Kabuli chickpea type) was selected for preference by farmers. Seeds were sown at 5 cm depth of soil with spacing between rows and plants were 30 cm and 10 cm, respectively, on a plot area of 3*2 m. The spacing between plots and blocks was 1 m and 1.5 m, respectively. All agricultural practices were done as practiced by farmers in that particular location. Fertilizers have not been used for this experiment. Plot weeding was done twice after 30 and 40 days of sowing.
2.3. Preparations of the plant extracts
Mature and healthy A. indica seeds were collected from Dire Dhawa area, Ethiopia in 2017. The pulp of the seeds was removed, cleaned and shade dried after removing seed coat from the dried seeds. Then, the kernel was powdered using micro plant grinding machines (Jianjin Taisite Instrument Co., Ltd, AU6114-03). The seeds of M. ferruginea were collected from Dilla town (Southern Nations, Nationalities and Peoples (SNNP) region), Ethiopia, in the same year. The seed powder was obtained as explained above.
An aqueous extract from each of the prepared powders was prepared as using Jain and Bhargava (Citation2007); briefly, the aqueous extracts of both A. indica and M. ferruginea seeds were extracted as per the formula illustrated below, and 5% concentration of aqueous was prepared by mixing 5 kg of either A. indica or M. ferruginea powder in 100 l of water to spray 1 ha, left for 24 h and stirred periodically to mix the contents well, then filtered through doubled muslin cloth. Then, the amounts of both botanicals required for the experimental plot were estimated based upon this procedure. Then, filtrate was used for field spray.
Alcoholic seed extracts of A. indicawere prepared as follows; the seed kernel powder (100 g) of A. indica was mixed with 100 ml of absolute ethanol (97%). Then, the solution was stirred repeatedly with a magnetic stirrer for 2 h to facilitate thorough mixing and filtered through a doubled muslin cloth, after which the alcohol was evaporated using a rotary evaporator at 20°C for 1 h (Hashemi & Hossain, Citation2016). The obtained crude extract was stored under dark area until required for field application. Then, all of the solutions were sprayed on to the respective experimental plots by using 1 l capacity concentrations used which were prepared as the following equation:
where C = percent concentration of botanicals, W = weight of solute (Botanicals), V = Volume of solution (volume of botanical + volume of solvent (water)).
2.4. Treatments and experimental design
Aqueous extracts of A. indica and M. ferruginea seeds at two concentration levels (5% and 2.5%) and combined, alcoholic extract of A. indica, standard check (deltamethrin 25% EC at 250 mL/ha) and untreated control (water) were used (Table ) under Randomized complete block design (RCBD) with three replications during both 2017 and 2018 cropping seasons.
Table 1. Treatment details used for the experiments
2.5. Treatment application
The treatments were applied at 15-day intervals two times (at 5:00 pm) with the designed concentrations of each treatment at the economic threshold level of larvae (one larva/meter row). The economic threshold level of larvae was based on the assessments made on larval counts prior to treatment applications. The first treatment was applied after the first larval infestation and damage symptom was noted after 60 days of sowing based on assessment result. Two sprays were applied from 61 days after sowing. Pre- and post-spray larval count was noted.
2.6. Sampling
2.6.1. Larval population (number of larvae per plant)
Larval population was counted (at 8:00 pm) starting from the time of half flowering, 60 days after sowing from 20 randomly selected plants from the central four rows per plot, excluding two border rows, one from each side. Sampling of larvae was counted 1 day before spray and at one, three and 7 days’ after spray (DAS) for the two application rounds. The number of damaged pods was counted at the seventh day of the last spray before drying and harvesting. Then, the collected data were converted to percentage data as follows:
The crop was harvested from each plot separately for the 2 years. At the end of the experiment, after harvesting, the yield from each experimental plot was weighed using a digital balance. The mean yield obtained from each plot was converted into kg ha−1 to check the effectiveness of the applied treatments. The cost of the different treatments and gross income was estimated on the basis of the inputs and products of that time market price. Benefit–cost ratio was calculated for all treatments (Wakil, Ashfaq, Ghazanfar, Afzal, & Riasat, Citation2009) (from cost of cultivation and plant protections) applied in field experiment as follows:
where CB is cost benefit from the treatments.
3. Data analysis
Prior to subjecting the data to ANOVA, all the data were checked for normality using Shapiro-Wilk’s test (Shapiro & Wilk, Citation1965). All data analyses were performed using R (version 3.5.2) statistical software packages (R Development Core Team, Citation2018). Statistical significance differences among the means were analyzed using LSD test at p < 0 .05 for mean separations.
4. Results
4.1. Population of H. armigera
In 2017 cropping season, larval population was significantly differed after the treatment application on 1st day (df = 7, 16; f = 12.83; p < 0.05), 3rd days (df = 7, 16; f = 5.90; p < 0.05) and 7th days (df = 7, 16; f = 2.88; p < 0.05) after the first treatment application. Similarly, it was significantly differed during the second treatment applications on 1st day (df = 7, 16; f = 37.76; p < 0.05), 3rd days (df = 7, 16; f = 17.38; p < 0.05) and 7th days (df = 7, 16; f = 8.67; p < 0.05) of post-treatment applications (Table ). The average larval population per plant in A. indica 2.5% + M. ferruginea 2.5% treated plot was 1 for the 1st treatment and 2 for the 2nd round treatment applications; implying that reduction of larval infestation by 23.09% and 50% at 7 ATA, respectively. Likewise, A. indica 5% treated plots were 1 and 2.33 larval infestations per plant which corresponds to 25% and 63.48% infestation reduction recorded at 7 ATA after the first and second treatment applications, respectively. Relative to treated plots and pre-treatment larval population, the infestation was increased over time in the control plot, in which the infestation was increased by 156% during the first treatment application and 113.48% for the second treatment application in 2017 cropping season.
Table 2. Infestation of chickpea plants by H. armigera larvae (larvae/plant) before and after the first and second treatment application during the cropping season of 2017
In the same way, in the 2018 cropping season, reduction in the larval population after the treatment (both aqueous and alcoholic extracts) spray application was more evident relative to both cropping seasons. Larval population was significantly differed after the treatment application on 1st day (df = 7, 16; f = 12.49; p < 0.05), 3rd days (df = 7, 16; f = 9.13; p < 0.05) and 7th days (df = 7, 16; f = 3.18; p < 0.05) after the first treatment application. Also, it significantly differed during the second treatment applications on 1 day before treatment application (df = 7, 16; f = 1.99; p < 0.05) and 1st day (df = 7, 16; f = 17.29; p < 0.05), 3rd days (df = 7, 16; f = 7.29; p < 0.05) and 7 days (df = 7, 16; f = 23.1; p < 0.05) of post-treatment applications (Table ).
Table 3. Infestation of chickpea plants by H. armigera larvae (larvae/plant) before and after the first and second treatment application during the cropping season of 2018
4.2. Pod damage
During both cropping seasons, the pod damage caused by H. armigera larvae was significantly differed (df = 7, 16; f = 14.44; p < 0.05) and (df = 7, 16; f = 16.33; p < 0.05), respectively (Figure ).
Figure 1. Percent chickpea pod damage due to H. armigera in the experiment. Bars denote means ± SE. Those values indicated by the same letter do not differ significantly (LSD test) (p < 0.05)
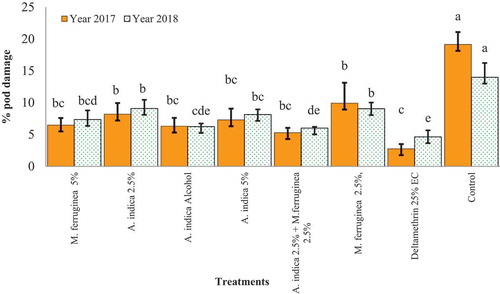
In comparison to the control plot, the lowest pod damage was observed in the treatment applied plots with botanical extracts (Figure ). Implying that, the maximum pod damage was recorded from the control plot and minimum was from a plot treated with A. indica 2.5% + M. ferruginea 2.5%, this value is comparable with the most of botanically treated plots. Interestingly, in both cropping years, the maximum pod damage was recorded from control plots (Figure ).
4.3. Yield and cost–benefit ratio
The yield of chickpea in treated plots differed significantly (df = 7, 16; F = 22.48; p < 0.05) in 2017 cropping season (Table ). From the botanical extract treated plots, the maximum average yield (2224.08 kg ha−1) was recorded from aqueous extract of M. ferruginea 5% treated plot with CBR = 22.47, followed by A. indica alcoholic extraction (1896.41 kg ha−1) and A. indica 2.5% + M. ferruginea 2.5% (1805 kg ha−1) with CBR = 15.30 and minimum (793.37 kg ha−1) yield was achieved from control treated plot (Table ). Comparing the cost–benefit ratio (CBR) of each treatment, M. ferruginea 5% treated plot was more profitable than the other treatments (Table ). Similarly, in 2018 cropping year the yield of chickpea differed significantly (df = 7, 16; F = 7.75; p < 0.05). A. indica alcoholic extracted and/or A. indica 5% treated plots showed the maximum yield (1283.0–1233.6 kg/ha) with CBR = 5.86 and 5.90, respectively, in comparison to the control plot generated the lowest yield (667.0 kg/ha) (Table ). Interestingly, in both copping seasons, maximum CBR was achieved by M. ferruginea and A. indica at 5% treated plots.
Table 4. Yield and cost–benefit ratio of the evaluated botanical extracts against H. armigera in chickpea, Ethiopia, 2017
Table 5. Yield and cost–benefit ratio of the evaluated botanical extracts against H. armigera in chickpea, Ethiopia, 2018
5. Discussion
The present research work revealed that the tested A. indica and M. ferruginea seeds were effective against H. armigera by reducing larval infestations in chickpea under field conditions during both years. Lower larval infestations were noticed when both botanical extracts were used at 5% concentrations (aqueous) and also alcoholic extraction of A. indica. Previous study indicated that seed extracts of A. indica and M. ferruginea treated plots exhibited a lower level of H. armigera larval infestations (Lulie & Raja, Citation2012). Similarly, Rahman, Haque, Alam, Mahmudunnabi, and Dutta (Citation2014) also reported reduced H. armigera infestation over control with neem seed kernel extracts in tomato crop under field conditions. In addition to larvicidal properties, most botanicals also have various modes of action such as; repellent, deterrent to oviposition and/or feeding with unpleasant odors or irritants and having adverse toxicity effects to insect pests (Chermenskaya, Stepanycheva, Shchenikova, & Chakaeva, Citation2010; Zanuncio et al., Citation2016), making the hosts unpalatable. Moreover, the efficacy of aqueous seed extracts of A. indica has been also reported against larvae of Spodoptera frugiperda (Birhanu, Tefera, Wakgari, Ayalew, & Mendesil, Citation2019; Zuleta-Castro et al., Citation2017).
We noted that lower chickpea pod damage was recorded in plots treated with M. ferruginea and A. indica at 5% concentration, A. indica alcoholic extraction and a combination of aqueous extract of A. indica (2.5%) and M. ferruginea (2.5%) compared to control plot during both cropping seasons. The lower pod damage in treated plots could be due to the lower larval infestations; therefore, these botanicals were effective in reducing chickpea pod damage under field conditions. Additionally, lower pod damage was noticed when A. indica (2.5%) and M. ferruginea (2.5%) were combined together for applications. Combining botanicals with different modes of actions have been reported to be more effective than using them singly which is in correspondence with the previous reports of (Sharma et al., Citation2007; Younas, Waqas, Zaeema, Muhammad, & Sean, Citation2016). Mode of actions and the mechanism of botanicals vary especially when they are combined depending on the type of compound and ingredient contents (Esmaeili & Asgari, Citation2015) so that maximum plant protection will be achieved by having synergic effect due to the multiple modes of actions. For instance, rotenone is one of the dominant, among the three rotenoid compounds (rotenone, deguelin and tephrosin) (Clark, Citation1943) found in Millettia seeds (Bekele, Citation1988; Dagne & Bekele, Citation1990; Jembere, Namukobe, Benard, & Dagne, Citation2007) which might possess insecticidal properties against the H. armigera in the present field experiments. In line with our results, applying Neem Seed Kernel Extract (NSKE 5%) greatly reduced the pod borer population in chickpea (Hussain et al., Citation2016).
The seed extract of M. ferruginea has also been observed to be effective in controlling various insect pests such as; Callasobruchus chinensis (Mulatu & Gabremedhin, Citation2000), Zebrotes subfaciatus (Habeeb, Citation2010), adult termites (Jembere, Getahun, Negash, & Seyoum, Citation2006), H. armigera larvae (Fite et al., Citation2018; Lulie & Raja, Citation2012) and Busseola fusca (Tilahun & Azerefegne, Citation2013), mosquito larvae (Asegid et al., Citation2007), ticks (Choudhury, Shiferaw, & Hussen, Citation2015) and other insect pests (Damte & Chichaybelu, Citation2002; Eyob, Azerefegne, Tameru, Temesgen, & Blomme, Citation2010).
In response to lower larval population and pod damage, maximum yields were recorded from plots treated with M. ferruginea 5%, alcoholic extraction of A. indica and combination treated plots during both 2017 and 2018. Similar results were reported by Lulie and Raja (Citation2012); testing M. ferruginea and A. indica against H. armigera under field condition. Tilahun and Azerefegne (Citation2013) also reported that higher maize yields were obtained from maize plots treated with aqueous crude seed extracts M. ferruginea 5% against B. fusca; thus, M. ferruginea applied two to three times is recommended for the control of B. fusca in maize, as effective dose under field conditions. A similar report also approves our result, in that Millettia extract significantly reduced the number of surviving larvae and damage to maize compared with the untreated plots even at the lower concentration (1 and 3%) tested (Tilahun & Azerefegne, Citation2013). Likewise, a seed extract of M. ferruginea was more effective than synthetic insecticides by causing 45-60% mortality on the Pachnoda interrupt (Oliver) within 24–48 h (Jembere et al., Citation2006). The effectiveness of water extracts of M. ferruginea seed has been reported against many insect pests including storage and field insect pests.
The effectiveness of treatments on the protection of the grain yield of chickpea plants was also evaluated through the cost–benefit ratio. Cost–benefit ratio is an indicator of the relative economic performance of the treatments (Aziz, Hasan, Ali, & Iqbal, Citation2012). The differences in pod damage and yields provide enough clues to rank the effectiveness of the treatments with regard to the economic viability of each treatment. Comparing the net cost–benefit return of each treatment, during 2017 maximum CBR was obtained from M. ferruginea 5% and A. indica 5% treated plots during 2018. Thus, the use of these botanicals under field conditions as an alternative to synthetic insecticide. It is economically profitable as plant-based pesticides reduce input cost being locally available, with knowledge-based preparation, which makes them cheaper products compared to synthetic pesticides. Shabozoi, Abro, Syed, and Awan (Citation2011) also obtained a higher cost: benefit ratio with application of a neem-based botanical extract compared to synthetic insecticides in managing insect pests of pigeon pea. Similarly, higher cost–benefit ratio was observed for plots sprayed with Chromolaena odorata (Asterales: Asteraceae) in comparison to other botanical treated plots and synthetic insecticides against Plutella xylostella L. (Lepidoptera: Plutellidae), a key pest of crucifers in cabbage (Amoabeng, Gurr, Gitau, & Stevenson, Citation2014). It was reported that pesticidal extracts derived from plants and their use in Africa show that they are more cost-beneficial for smallholder farmers than using synthetic pesticides because use of pesticidal plants do have many advantages over other pest management tools such as reduced input costs, improved yield, and effective use of labor (Amoabeng et al., Citation2014; Mkenda et al., Citation2015). Thus, locally available M. ferruginea and A. indica extracts would greatly benefit the resource-poor farmers and need further research attentions and considerations as a part of IPM tools in pest management in chickpea.
6. Conclusion
In conclusion, based on two years' field result, we recommend that sprays of aqueous extractions of M. ferruginea (5%), A. indica (5%) or alcoholic extraction of A. indica two times application at 15 days internal starting from the 61 days after planting were effective in reducing H. armigera infestations and pod damages with increased yield. Moreover, maximum profits were obtained from botanically treated plots, indicating that it is economically viable. M. ferruginea and A. indica seed extracts could be a promising part of IPM program for small-scale chickpea farmers. Therefore, future research should have to focus on mechanisms of their mode of action, ease of product availability and repeat trials under different agro-ecological conditions.
Author Contributions
TF, TT, MN, and TD; conceived and designed the experiment; TF; conducted the field experiment, collected and analyzed data; TF, TT, and TD; interpreted the results; TF, TT, MN, and TD; Wrote and approved the manuscript.
Acknowledgements
This study was supported by the USAID Feed the Future IPM Innovation Lab, Virginia Tech, Cooperative Agreement No. AID-OAA-L-15-00001. Tarekegn Fite is an icipe student supported by the USAID Feed the Future IPM Innovation Lab, Virginia Tech, under the provision of Rice, Maize, and Chickpea IPM Project for East Africa. We also gratefully acknowledge the financial support by the UK’s Department for International Development (DFID); Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); and the Kenyan Government. The views expressed herein do not necessarily reflect the official opinion of the donors. We also wish to forward our thankful appreciation to Plant Science Laboratory Technicians of Ambo University, College of Agriculture and Veterinary Sciences for technical support especially Mr. Fula’a Galana and Mr. Habtamu Haile.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Tarekegn Fite
Tarekegn Fite is an icipe PhD student and admitted at Ambo University specializing on Agricultural Entomology and currently working on a research supported by USAID Feed the Future IPM Innovation Lab, Virginia Tech, under the provision of Rice, Maize, and Chickpea IPM Project for East Africa for his Dissertation thesis.
References
- Ahmad, M., Bilal, R., Munir, A., & Russell, D. A. (2019). Resistance and synergism of novel insecticides in field populations of cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) in Pakistan. Journal of Economic Entomology, 112(2), 859–15. doi:10.1093/jee/toy409
- Ahmad, S., Shafiq, A. M., & Muslim, M. (2015). Toxic effects of neem based insecticides on the fitness of helicoverpa armigera (hübner). Crop Protection, 68, 72–78.
- Ali, S., Farooqi, M. A., Sajjad, A., Ullah, M. I., Qureshi, A. K., Siddique, B., … Asghar, A. (2018). Compatibility of entomopathogenic fungi and botanical extracts against the wheat aphid, Sitobion avenae (Fab.) (Hemiptera: Aphididae). Egyptian Journal of Biological Pest Control, 28, 97. doi:10.1186/s41938-018-0101-9
- Ali, S., Ullah, M. I., Arshad, M., Iftikhar, Y., Saqib, M., & Afzal, M. (2017). Effect of botanicals and synthetic insecticides on Pieris brassicae (L., 1758) (Lepidoptera: Pieridae). Turkish Journal of Entomology, 41(3), 275–284. doi:10.16970/entoted.308941
- Alvi, A. H. K., Sayyed, A. H., Naeem, M., & Ali, M. (2012). Field evolved resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus thuringiensis toxin Cry1Ac in Pakistan. PloS One, 7(10), e47309. doi:10.1371/journal.pone.0047309
- Amoabeng, B. W., Gurr, G. M., Gitau, C. W., & Stevenson, P. C. (2014). Cost: Benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Protection, 57, 71–76. doi:10.1016/j.cropro.2013.11.019
- Asegid, S., Abede, D., Mudi, K., Melaku, D., & Taye, G. (2007). Screening of some Ethiopian medicinal plants for mosquito larvicidal effects and phytochemical constituents. Pharmacology, 3, 3231–3243.
- Attique, M. R., Arif, M. I., Ahmed, Z., & Mohyuddin, M. I. (2000). Host plants and population dynamics of Helicoverpa armigera (Hubner) in the belt of Punjab. The Pakistan Cotton, 44(3 & 4), 31–40.
- Aziz, M. A., Hasan, M., Ali, A., & Iqbal, J. (2012). Comparative efficacy of different management options in Okra. Pakistan Journal of Zoology, 43, 869–878.
- Begg, G. S., Cook, S. M., Dye, R., Ferrante, M., Franck, P., Lavigne, C., & Lövei, G. L. (2017). A functional overview of conservation biological control. Crop Protection, 97, 145–158. doi:10.1016/j.cropro.2016.11.008
- Bekele, A. (1988) Investigation of flavonoids from ‘birbira’. MSc thesis, Addis Ababa University, 167 pp. doi:10.3168/jds.S0022-0302(88)79586-7
- Bird, L. (2017). Genetics, cross-resistance and synergism of indoxacarb resistance in Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Management Science, 73, 575–581. doi:10.1002/ps.2017.73.issue-3
- Birhanu, S., Tefera, T., Wakgari, M., Ayalew, G., & Mendesil, E. (2019). The efficacy of selected synthetic insecticides and botanicals against Fall armyworm, Spodoptera frugiperda, in Maize. Insects, 10, 2–45.
- Chermenskaya, T. D., Stepanycheva, E. A., Shchenikova, A. V., & Chakaeva, A. S. (2010). Insecto acaricidal and deterrent activities of extracts of Kyrgyzstan plants against three agricultural pests. Indian Journal of Crop Production, 32, 157–163. doi:10.1016/j.indcrop.2010.04.009
- Chichaybelu, M., Tesfaye, G., Nigusie, G., Asnake, F., Million, E., & Chris, O. (2018). Innovative partnership in chickpea seed production and technology dissemination: A decade of lessons in Ethiopia. Ethiopian Journal of Crop Science, 6(2), 1–17.
- Choudhury, M. K., Shiferaw, Y., & Hussen, A. (2015). Toxicity of Millettia ferruginea darasana (family: Fabaceae) against the larvae and adult ticks of Amblyomma variegatum Fabricius a three-host tick in cattle. Journal of Parasitic Diseases, 39(2), 298–302. doi:10.1007/s12639-013-0311-8
- Clark, E. P. (1943). The occurrence of rotenone and related substances in the seeds of Berebera tree. A procedure for the separation of deguelin and tephrosin. Journal of American Chemical Society, 65, 27–29. doi:10.1021/ja01241a008
- Dagne, E., & Bekele, A. (1990). C-Prenylated isoflavones from Millettia ferruginea. Phytochemistry, 29, 2679–2682. doi:10.1016/0031-9422(90)85212-X
- Damte, T., & Chichaybelu, M. (2002). The efficacy of some botanicals in controlling Adzuki bean beetle, Callosobruchus chinensis in stored chickpea. Tropical Science, 42, 192–195.
- Das, A., Datta, S., Sh., T., Shukla, A., Ansari, A., Sujayanand, G. K., … Singh, P. (2017). Expression of a chimeric gene encoding insecticidal crystal protein Cry1Aabc of Bacillus thuringiensis in chickpea (Cicer arietinum L.) confers resistance to gram pod borer, Helicoverpa armigera (Hubner). Frontiers in Plant Science, 8, 1–10. doi:10.3389/fpls.2017.01423
- Dinesh, K., Anusha, S., Bharu, R. S., & Dangi, N. L. (2017). Estimation of avoidable yield losses caused by Helicoverpa armigera (Hubner) on chickpea. Journal of Entomology & Zoology Studies, 5(2), 1476–1478.
- Esmaeili, A., & Asgari, A. (2015). In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. International Journal of Biology & Macromolecules, 81, 283–290. doi:10.1016/j.ijbiomac.2015.08.010
- Eyob, T., Azerefegne, A., Tameru, A., Temesgen, A., & Blomme, G. (2010). Studies on the efficacy of some selected botanicals against enset root mealybug (Cataenococcus ensete) Williams and Matile-Fererro (Homptera: Seudococcidae). Tree & Forestry Science & Biotechnology, 4, 91–94.
- Fite, T., Tefera, T., Negeri, M., Damte, T., & Sori, W. (2018). Management of Helicoverpa armigera (Lepidoptera: Noctuidae) by nutritional indices and botanical extracts of Millettia ferruginea and Azadirachta indica. Advance in Entomology, 6, 235–255. doi:10.4236/ae.2018.64019
- Gebre-Amlak, A. (1999). Insecticidal activity of chinaberry, endod and pepper tree against the maize stalk borer (Lepidoptera: Noctuidae) in Southern Ethiopia. International Journal of Pest Management, 45(1), 9–13. doi:10.1080/096708799227987
- Geletu, B., Million, E., & Anbessa, Y. (1996). Improved cultivars and production technology of chickpea in Ethiopia.
- Getu, A., Zemede, A., & Ensermu, K. (2015). Ethnobotanical study of medicinal plants used by local communities of Minjar-Shenkora District, North Shewa zone of Amhara region, Ethiopia. Journal of Medicinal Plants Studies, 3(6), 01–11.
- Habeeb, S. M. (2010). Ethno veterinary knowledge of crude plant extracts and its methods of application traditional and modern for tick control. West African Applied Science Journal, 11(9), 1047–1050.
- Hashemi, Z. S., & Hossain, M. A. (2016). Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pacific Science Review A: Natural Science and Engineering, 18(2), 128–131.
- Hussain, D., Muhammad, S., Ghulam, G., & Muneer, A. (2015). Resistance in field population of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Journal of Entomological Science, 50, 2. doi:10.18474/JES14-24.1
- Hussain, M., Ahmad, K. S., Majeed, M., Mehmood, A., Hamid, A., Yousaf, M. M, & Khan, A. Q. (2016). Integrated management of Helicoverpa armigera on different genotypes of Kabuli chickpea in Punjab, Pakistan. International Journal of Bioscience, 9(2), 110–119.
- Isman, M. B. (2006). Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology, 51(1), 45–66. doi:10.1146/annurev.ento.51.110104.151146
- Jain, P. C., & Bhargava, M. C. (2007). Entomology novel approaches (pp. 165–309). New India Publishing.
- Jallow, M. F. A., Cunningham, J. P., & Zalucki, M. P. (2004). Intra-specific variation for host plant use in Helicoverpa armigera (Hubner) (Lepidoptera: Noctudae): Implications for management. Crop Protection, 23, 955–964. doi:10.1016/j.cropro.2004.02.008
- Jembere, B. (2002). Evaluation of the toxicity potential Millettia ferruginea (Hochest) Baker against Sitophilus zeamais moths. International Journal of Pest Management, 42(1), 29–32.
- Jembere, B., Getahun, D., Negash, M., & Seyoum, M. (2006). Toxicity of Birbira (Milletia ferruginea) seed crude extracts to some insect pests as compared to other botanical and synthetic insecticides. In Natural Products and Drug discovery. Proceedings of the 11th NAPRECA Symposium, 9–12 August 2005, Hoˆ tel Panorama, Antananarivo, Madagascar (pp. 88–96). Nairobi, Kenya: A NAPRECA Publication.
- Jembere, B., Namukobe, J., Benard, T. K., & Dagne, E. (2007). Extracts of Millettia ferruginea, Tephrosia vogellii and Tephrosia pentaphylla against the bean weevil Zabrotes subfaciatus (Boheman). Sinet: Ethiopian Journal of Science, 30, 49–54.
- Junhirun, P., Wanchai, P., Thitaree, Y., Torranis, R., Opender, K., & Vasakorn, B. (2018). The study of isolated alkane compounds and crude extracts from Sphagneticola trilobata (Asterales: Asteraceae) as a candidate botanical insecticide for lepidopteran larvae. Journal of Economic Entomology, 111(6), 2699–2705. doi:10.1093/jee/toy246
- Koul, O. (2016). The handbook of naturally occurring insecticidal toxins (pp. 864). United Kingdom: CABI Wallingford.
- Li, C., Qinqin, W., Haoliang, Q., Qiyuan, W., Huizhu, Y., & Changhui, R. (2018). Resistance selection of indoxacarb in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae): Cross- resistance, biochemical mechanisms and associated fitness costs. Pest Management Science, 74, 2636–2644. doi:10.1002/ps.5056
- Lulie, N., & Raja, N. (2012). Evaluation of certain botanical preparations against African bollworm, Helicoverpa armigera Hubner (Lepidoptera: Noctuidae) and non target organisms in chickpea, Cicer arietinum L. Journal of Biofertilizer & Biopesticides, 3, 130.
- Mironidis, G., Kapantaidaki, D., Bentila, M., Morou, E., Savopoulou-Soultani, M., & Vontas, J. (2013). Resurgence of the cotton bollworm Helicoverpa armigera in northern Greece associated with insecticide resistance. Insect Science, 20, 505–512. doi:10.1111/j.1744-7917.2012.01528.x
- Mkenda, P., Mwanauta, R., Stevenson, P. C., Ndakidemi, P., Mtei, K., & Belmain, S. R. (2015). Extracts from field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. PloS One, 10, e0143530. doi:10.1371/journal.pone.0143530
- Mulatu, B. (2007). Contact bioassay of an endemic plant to Ethiopia on three aphid species. Ethiopian Journal of Biological Science, 6(1), 51–62.
- Mulatu, B., & Gabremedhin, T. (2000). Oviposition deterrent and toxic effects of various botanicals on the Adzuki Bean Beetle, Callosobruchus chinensis. Insect Science & Its Application, 1, 33–38.
- Pande, S., Sharma, S. B., & Ramkrishna, A. (2000). Biotic stresses affecting legumes production in the Indi-Gangetic Plain. In Constraints and Opportunities (pp. 128–155). Hyderabad, India: International Crop Research Institute for Semi-Arid Tropics.
- Patil, S. B., Goya, A., Satish, S., Ch., I., Shiv, K., & Mustapha, B. (2017). Sustainable management of chickpea pod borer. A review. Agronomy & Sustainable Development, 37, 20. doi:10.1007/s13593-017-0428-8
- Pawar, C. S. (1998). Helicoverpa, a national problem which needs a national policy and commitment for its management. Pestology, 22, 51–59.
- R Development Core Team. (2018). A language and environment for statistical computing. Vienna, Austria: R Foundation for statistical computing.
- Rahman, A. K., Haque, M. H., Alam, S. N., Mahmudunnabi, M., & Dutta, N. K. (2014). Efficacy of botanicals against Helicoverpa armigera (Hubner) in tomato. The Agriculturists, 12(1), 131–139. doi:10.3329/agric.v12i1.19868
- Reddy, G. V. P., & Manjunatha, M. (2003). Laboratory and field studies on the integrated pest management of Helicoverpa armigera (Hübner) in cotton, based on pheromone trap catch threshold level. Applied Entomology, 124(5–6), 213–221. doi:10.1046/j.1439-0418.2000.00466.x
- Revathi, N., Ravikumar, G., Kalaiselvi, M., Gomathi, D., & Uma, C. (2011). Pathogenicity of three entomopathogenic fungi against Helicoverpa armigera. Journal of Plant Pathology & Microbiology, 2, 114. doi:10.4172/2157-7471.1000114
- Sarwar, M. (2012). Competency of natural and synthetic chemicals in controlling gram pod borer, Helicoverpa armigera (Hubner) on chicken crop. International Journal of Agricultural Sciences, 2(4), 132–135.
- Sarwar, M., Ahmad, N., & Tofique, M. (2011). Identification of susceptible and tolerant gram (Cicer arietinum L.) genotypes against gram pod borer (Helicoverpa armigera) (Hubner). Pakistan Journal of Botany, 43(2), 1265–1270.
- Schmutter, H. (1990). Properties and potential natural pesticides from the neem tree, Azadirachta indica. Annual Review of Entomology, 35, 271–297. doi:10.1146/annurev.en.35.010190.001415
- Shabozoi, N. U. K., Abro, G. H., Syed, T. S., & Awan, M. S. (2011). Economic appraisal of pest management options in Okra. Pakistan Journal of Zoology, 43, 5.
- Shapiro, S. S., & Wilk, M. B. (1965). An analysis of variance test for normality (complete samples). Biometrika, 52, 591–611. doi:10.1093/biomet/52.3-4.591
- Sharma, H., Gowda, C., Stevenson, P., Ridsdill-Smith, T., Clement, S., & Rao, G. R. (2007). Host plant resistance and insect pest management in chickpea. In S. S. Yadav, R. J. Redden, W. Chen, & B. Sharma (Eds.), Chickpea breeding and management (pp. 520–537). Wallingford, Oxon, UK: CABI.
- Silva, R. S., Tomaz, A. C., Lopes, M. C., Martins, J. C., Xavier, V. M., & Picanço, M. C. (2015). Toxicity of botanical insecticides on Diaphania hyalinata, their selectivity for the predatory ant Paratre china sp., and their potential phytotoxicity on pumpkin. International Journal of Pest Management, 62(2), 95–104. doi:10.1080/09670874.2015.1111466
- Singh, M., Bisht, I., & Dutta, M. (2014). Broadening the genetic base of grain legumes. New Delhi: Springer India. doi:10.1007/978-81-322-2023-7_3.
- Singh, S., Kumar, N., Maniraj, R., Lakshmikanth, R., Rao, K., Muralimohan, N., & Sreevathsa, R. (2018). Expression of Cry2Aa, a Bacillus thuringiensis insecticidal protein in transgenic pigeon pea confers resistance to gram pod borer, Helicoverpa armigera. Scientific Reports, 8(1), 1–12.
- Tilahun, B., Azerefegn, F., & Reddy, K. M. S. (2009). Bioassay and residual toxicity of Baker tree Millettia ferruginea (Hochst) seed crude water extract against maize stalk borer Busseola fusca (fuller) (Lepidoptera: Noctuidae) (1st ed., pp. 12). Dilla: Office of Research and Publication, Dilla University.
- Tilahun, B., & Azerefegne, F. (2013). Efficacy of the aqueous crude seed extract of Millettia ferruginea (Fabaceae) on the maize stemborer Busseola fusca (Lepidoptera: Noctuidae) in the field. International Journal of Tropical Insect Science, 33(4), 256–263. doi:10.1017/S1742758413000258
- Wakil, W., Ashfaq, M., Ghazanfar, M., Afzal, M., & Riasat, T. (2009). Integrated management of Helicoverpa armigera in chickpea in rain fed areas of Punjab, Pakistan. Phytoparasitica, 37, 415–420. doi:10.1007/s12600-009-0059-y
- Younas, A., Waqas, W., Zaeema, K., Muhammad, S., & Sean, M. P. (2016). The efficacy of Beauveria bassiana, jasmonic acid and chlorantraniliprole on larval populations of Helicoverpa armigera in chickpea crop ecosystems. Pest Management Science, 73(2), 418–424. doi:10.1002/ps.4297
- Zanuncio, J., Sheila, M., Luis, C., Wilcken, C., Ramalho, F., Angelica, P., … Jose, S. (2016). Toxic effects of the neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus (Heteroptera: Pentatomidae). Scientific Report, 6, 30261. doi:10.1038/srep30261
- Zuleta-Castro, C., Rios, D., Hoyos, R., & Rozco-Sanchez, F. (2017). First formulation of a botanical active substance extracted from neem cell culture for controlling the armyworm. Agronomy for Sustainable Development, 37, 5–1.
