 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chenopodium quinoa
Willd (quinoa) is a crop that originated from the Andes and has been recently introduced in Zimbabwe so the agronomic performance is unknown yet. A 3-year field experiment was carried out at the Women’s University in Africa research farm, Marondera, Zimbabwe. The research evaluated the response of quinoa to different cattle manure application rates, plant population ha−1 and growing seasons. A 3 × 3 × 2 factorial in a completely randomized block design with three replicates was used in the experiment. Cherry Vanilla Quinoa variety was established under three cattle manure application rates (0, 9 and 18 t ha−1), three planting densities (29629, 55555 and 80 000 plants ha−1) and two growing seasons per year (summer and winter). Analysis of variance was done to compare the effects of the treatments on quinoa growth parameters. There was significant (P < 0.05) interaction on cattle manure application rates × plant population ha−1 × growing season in all the measured response variables. The vegetative phase was higher on soil fertility amended than unamended plots in summer than winter regardless of the planting density. Highest grain yield (2.5 t ha−1) and thousand grain weight (T.G.W) (2.5 g) were recorded in winter at 9 t ha−1 + 55,555 plants ha−1 while lowest T.G.W (0.2 g) and grain yield (0.1 t ha−1) were noted in 18 t ha−1 + 80,000 plants ha−1 in summer. Quinoa can be grown in winter or summer with 9 t ha−1 + 55555 plants ha−1 for grain or biomass, respectively.
PUBLIC INTEREST STATEMENT
Quinoa is a multi-purpose crop which is native in South America. The grain can be used for food while the produced biomass is a potential source for fodder. The crop is potentially adaptive to many climates other than its traditional conditions. However, its agronomic performance has not been widely evaluated in Zimbabwe. Being a potential crop, its growth and yield performance were evaluated for 3 years in summer and winter at the Women’s University in Africa research farm, Marondera under different planting densities and cattle manure application rates. The results showed that the crop can be successfully grown in both the winter and summer seasons but giving different yield components suited in each season. Grain yield was higher in winter but with poorer biomass production than in summer. The summer season showed to be the best time for quinoa growth if one wants high biomass production, e.g. in fodder production.
1. Introduction
Chenopodium quinoa Willd (quinoa) is a highly nutritious pseudocereal traditionally grown for food in some parts of the world (Elizabeth & Da, Citation2016). The quinoa originated from Andes in South America and currently grown in Bolivia, Peru, United States, Ecuador and Canada as a food crop (Navruz-Varli & Sanlier, Citation2016). Its seed is reported to contain a well-balanced and significant amount of the nine essential amino acids required to fulfill our daily protein requirement (Miranda et al., Citation2012). The seed is also an important source of vitamins, unsaturated fatty acids and carotenoids (Hinojosa et al., Citation2019) which are higher than the local cereals in Zimbabwe. The high nutritional status makes it a most critical crop in addressing the malnutrition and hidden hunger problems in many developing countries. The quinoa grain is highly demanded in the USA, Europe, and Asia (Hinojosa et al., Citation2019); hence, it is a potentially innovative and economically promising export cash crop for many African countries such as Zimbabwe. Additionally, the quinoa has multi-uses, the seeds and leaves are utilized as food, biomass used as animal feed or cover crop, and can serve as a phytoremediation tool for environmental cleaning.
The quinoa has been successfully grown in some African countries such as Kenya, Ethiopia and Malawi but has been recently introduced to Zimbabwe therefore no agronomic evaluations have been done yet. The crop tolerates a wide range of marginal soils with a broad range of pH (4.5 to 9.5) (Hinojosa et al., Citation2018), saline (Koyro et al., Citation2008), heat (Rashid et al., Citation2018), and drought-stressed environments (S.E. Jacobsen et al., Citation2003). According to a research done by S.E. Jacobsen and Bach (Citation1998), the optimal temperature of growth for quinoa is approximately 22°C (Maliro et al., Citation2017). Nevertheless, the crop can withstand temperatures as low as −4°C and high as 35°C and grows within a relative humidity range of 40% to 88% (Martínez et al., Citation2009). The winter season in Zimbabwe is characterized by low temperatures which are comparably similar to those of the Andean region, Bolivia. However, the summer season in Zimbabwe normally experiences average temperatures which are less than 35°C, the maximum growing temperature for quinoa. This necessitated the need to evaluate the quinoa growth performance under both winter and summer seasons in Zimbabwe.
The quinoa is sensitive to different levels of planting densities and fertilizer types and application rates (Siavoshi et al., Citation2010). The planting space rates directly affect plant population per unit area hence the grain yield of the quinoa (Eisa et al., Citation2018). Low planting densities are directly correlated to lower grain yields than plant densities of >150,000 plants ha−1 (Siavoshi et al., Citation2010). Currently, there are no specific recommended planting densities for the quinoa. The variations in the planting densities are due to differences in soil fertility and general soil properties in an area (Maliro et al., Citation2017) hence difficult to recommend an ideal planting density for an area if the crop is newly introduced. Sief et al. (Citation2015) found an ideal spacing rate of 40–80 cm with nitrogen application rates of 120 kg urea ha−1 in non-leaching soils. Nitrogen source influenced the growth and development of quinoa where application rates of 40–160 kg N ha−1 inorganic fertilizers were ideal for high yields (Erley et al., Citation2005). Contrarily, Siavoshi et al. (Citation2010) observed that the quinoa responded slowly to organic manure applied at 5–10 Mt ha−1. These organic manure recommended rates by Siavoshi et al. (Citation2010) are an extremely high side hence need for further research on the application rates and sources of organic manure.
The grain yield of quinoa was reported to vary from region to region with highest (7.50 t ha−1) recorded in Lebanon, followed by Egypt (3.87 t ha−1), and lowest (0.23 t ha−1) recorded in Mauritania (Papastylianou et al., Citation2014). Bertero et al. (Citation1998) noted that the difference in altitudes and temperature variations had a major influence on the grain yielding of quinoa. Low (<22°C) temperature encouraged high grain filling whereas extremely high (>30°C) temperature resulted in poor grain filling (Erley et al., Citation2005). In areas with relatively low >22°C temperatures, the crop took short time to reach the flowering stage and about 40–200 days to reach the physiological maturity depending on the cultivar (Martínez et al., Citation2009). Nevertheless, excessively high temperature during the growth phase is one of the most important abiotic stresses causing yield reduction (Hinojosa et al., Citation2018). In Africa, the temperature had a major influence in adaptation of quinoa to areas of introduction (Maliro et al., Citation2017).
Zimbabwe experiences a savanna type of climate with two major distinct seasons (hot, wet summers and cool, dry winters). The summer is characterized by high temperatures and rainfall hence ideal for most locally grown crops like the millet, groundnuts, sorghum and maize. However, the cool and dry winter conditions are suitable for crops such as wheat that are grown under irrigation. Places like Marondera and Nyanga experience relatively low temperatures and are used as ideal breeding sites for cool-seasoned crops like the Irish potato whereas areas such as Hwange and Beitbridge are characterized by hot weathers throughout the year. With such a diverse climate and ecological zones, there is great potential for the successful introduction of the quinoa in Zimbabwe. S.E. Jacobsen and Mujica (Citation2003) also concluded that the crop can be successfully grown in environments similar to those of the Andean region.
The grain yield of quinoa was observed to be positively correlated to agronomic management and fertilizer type (Maliro et al., Citation2017). An average yield of 3.87 t ha−1 was recorded under good crop management in cool areas (Papastylianou et al., Citation2014). In Zimbabwe, neglected traditional small grain crops like millet and rapoko are mostly grown by resource-poor farmers who cannot easily afford to buy synthetic fertilizers (Siavoshi et al., Citation2010). Generally, the synthetic fertilizers are too expensive to these farmers and their incorrect use is detrimental to the soil health (Martínez et al., Citation2009). The high costs of inputs together with recurrent droughts in Africa are countering the production of staple crops thereby worsening food insecurity. Introducing hardy and widely adaptable crops like the quinoa can be a panacea to food insecurity in the Sub-Saharan region. The quinoa is potentially drought tolerant and nutritious alternative to maize. However, the crop is new in Zimbabwe so no agronomic information, like planting density and fertilizer requirements, is known yet. Therefore, the objectives of this study were to evaluate the quinoa growth performance and grain yield under two main growing seasons (winter and summer) in Zimbabwe and determining the effects of different soil fertility amendment levels + planting densities on the crop productivity. We hypothesized that the quinoa performance does not vary under the two growing seasons (summer and winter) in Zimbabwean.
2. Materials and methods
2.1. The study area
The research was done from 2017 to 2019 at the Women’s University in Africa (WUA) research farm, Marondera, Zimbabwe. The farm is about 78 km east of Harare along the Harare-Mutare highway. It is located between −18° 11ꞌ 6.97ꞌꞌ S and 31° 33ꞌ 6.95ꞌꞌE and it is situated at elevation 1668 m above sea level. The area is characterized by an unimodal wet season receiving rainfall ranging from 750 to 1000 mm yr−1 that fall between October and April. It is the natural farming region (NR) IIa of Zimbabwe. The soils at the WUA research farm are classified as cambisols according to the IUSS Working Group WRB (Citation2015). The soil nutrient status of the research farm was analyzed before establishing the experiment (Table ).
Figure 1. The average rainfall (mm) and temperature (oC) recorded at the WUA research farm, Marondera during the period 2017–2019.
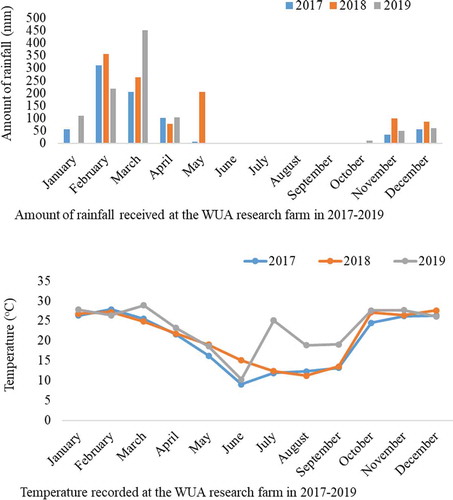
Table 1. The initial chemical properties of the soil at the WUA research farm and cattle manure used in the study
The WUA research farm has two distinct growing seasons in a year that are hot, wet summers (October to April) and cool dry, winters (May to August). The average monthly summer and winter temperatures for the WUA research farm during the study period were 26.1°C and 12.3°C, respectively (Figure ). The area received an average monthly summer rainfall of 108.98 mm while winter rainfall ranged from 0 to 5.12 mm per month (Figure ).
2.2. Soil and cattle manure analysis
Six soil samples were taken to a depth of 200 mm using a graduated soil auger in January 2017 before first sowing of the quinoa. Sampling was taken per plot and then randomly mixed to make a composite soil sample for analyses. Cattle manure was sourced from the WUA farm cattle pens in October 2017 and sun-dried for 1 week to homogenize the moisture content. After the drying, a 1 kg sample was randomly taken for analysis and the bulky stored for use.
2.2.1. Total carbon (C) and Nitrogen (N) determination
The C & N in both the cattle manure and soil were determined using a method described by Parwada and Van Tol (Citation2018).
2.2.2. Olsen extractable P
A solution of 0.5 M Sodium bicarbonate adjusted to a pH of 8.5 using 1 M Sodium hydroxide was used for the extraction. A 2.5 g sample of soil was extracted with 50 mL of the 0.5 M NaHCO3 solution on a horizontal shaker for 0.5 h (Schoenau & O’Halloran, Citation2006). After shaking, the suspensions were filtered through WhatmanTM No. 42 filter paper and the filtrates were then adjusted to pH 5 with 2.5 M sulfuric acid before analysis. The orthophosphate in the extracts was automatically determined using a continuous flow analyzer (San 2++ Skalar CFA, Skalar Analytical B.V. The Netherlands) employing the ammonium molybdate – antimony potassium tartrate – ascorbic acid method.
2.2.3. Exchangeable ammonium and nitrate and nitrite
The inorganic nitrogen NH4 (N), NO3 (N) and NO2 (N) were extracted using 50 mL of 0.5 M potassium sulfate (1:10 w/v). A 5 g homogenous sample was weighed into a 150-mL plastic bottle and shaken on a reciprocating shaker for 30 min at 160 rpm. Then, the suspension was filtered using WhatmanTM No. 42 filter paper (Maynard et al., Citation2006). The ammonium concentration was determined based on the Berthelot reaction involving salicylate while the nitrate and nitrite concentrations were determined based on the cadmium reduction method; all done automatically on a continuous flow analyzer (San 2++ Skalar CFA, Skalar Analytical B.V. The Netherlands).
The soil was inherently low in nutrient content compared to the cattle manure (Table ). The soil had an acidic pH (5.46) coupled with an electrical conductivity value of 4.15. In addition, the soil had lower total C, N, P and Ca which were consistent with the lower cation exchange capacity compared to cattle manure. However, the soil had a considerable higher quantity of K and Mg (Table ).
2.3. Experimental design
The study was carried out using a 3 × 3 × 2 factorial experiment in a randomised complete block design with three replications. Positive and negative controls of no cattle manure and compound D (inorganic fertilizer) applications were included in the treatments. The compound D was applied using a blanket-recommended rate (250 kg ha−1) in the study area. The factors were three cattle manure application rates (0, 9 and 18 t ha−1), plant spacing of 75 × 45 cm, 60 × 30 cm, 50 × 25 cm (inter row × in row) were used to give plant populations of 29629, 55556 and 80000 plants ha−1, respectively, and two growing seasons (Table ). The compound D supplied 17.5 kg ha−1 of N whereas the 9 and 18 t ha−1 of cattle manure added 11.5 and 23 kg of N ha−1 respectively (Table ). A plot was 2 × 2 m with a 1 m space in between the plots to give a total area of 280 m2 for the whole experimental site. The growing seasons were winter and summer. The crop was grown in early January and early May for summer and winter, respectively. Irrigation was done regularly to maintain the soil moisture at field capacity in the winter and once during prolonged dry periods in summer.
Table 2. Treatment combinations used and response variables measured in the experiment
2.4. Data collection
All the measured response variables are shown in Table . The plant height (PH) (m) and dry biomass yield were measured at physiological maturity stage (when the plant leaves started to dry and fall off) in each treatment plot. The total dry biomass weight (TDBW) was calculated as follows:
where = Total dry biomass weight (t ha−1),
= Total dry weight of the sample (t) and
= Area per plot (ha).
A number of branches per plant were counted physically at physiological maturity stage. The grain yield (t ha−1) and thousand grain weight (T.G.W) were also measured. The T.G.W was obtained by weighing 1000 grains of quinoa on a balance scale and recorded the weight in grams. The harvest index (HI) was defined as the ratio of grain yield and the above-ground biomass at maturity
:
where and
was the straw weight at maturity of the C. quinoa Willd.
2.5. Statistical analysis
The observations were independent of each other; data followed a normal distribution and homoscedasticity, and thus, a three-factor analysis of variance (ANOVA) was run to compare the growth parameter of the C. quinoa Willd under different cattle manure application rates, planting density and growing seasons. Correlation analyzes were done to determine the associations between the seasonal temperature and the response variables. The growing season (S) was the main effects in the study. All data were analyzed using JMP version 11.0.0 statistical software (SAS Institute, 2010).
3. RESULTS
There were significant (P < 0.05) interactions of cattle manure × planting density × growing season on all the measured C. quinoa response variables (Table ). Also, year (Y) and season (S) × year interactions had insignificant (P > 0.05) effects on all the response variables (Table ).
Table 3. The ANOVA summary for the mean square values of the C. quinoa Willd growth parameters and yield during the three years
3.1. Effects of cattle manure application rates × planting density × growing season combinations on the C. quinoa Willd vegetative growth parameters
In summer, all soil fertility-amended plots had taller plants relative to the unamended (control) plots, regardless of planting density. However, in winter, the planting density significantly (P < 0.05) influenced the C. quinoa height (Table ). Tallest (1.27 m) C. quinoa plants were observed in summer at 9 t ha−1 cattle manure application rate + 55555 plants ha−1 (Table ). Shortest (0.20 m) plants were noted in winter under 0 t ha−1 cattle manure application rate + 29629 plants ha−1 (Table ).
Table 4. Effects of cattle manure application rates × planting density × growing season on the C. quinoa Willd vegetative growth parameters
Significantly (P < 0.05) longest days (54) to flowering were observed in summer under 9 t ha−1 cattle manure application rate and compound D applied plots at 29629 and 55555 plants ha−1 (Table ). Shortest days (23 days) to flowering were noted in winter at 18 t ha−1 cattle manure application rate + 80,000 plants ha−1. The days to flowering (DF) were general decreasing as the planting density was increasing in the cattle manure amended plots regardless of the quantity (Table ).
The branching characteristics of the C. quinoa Willd significantly (P < 0.05) varied with the cattle manure application rates + plant density (Table ). The quinoa plants developed more branches in summer than winter. Summer plants under 18 t ha−1 + 55555 plants ha−1 and winter plants under compound D + 29269 plants ha−1 developed most (65) and least (21) number of branches per plant, respectively (Table ). Summer crop grown at 9 t ha−1 + 55555 plants ha−1 and winter crop at 0 t ha−1 cattle manure application rate + 29629 plants ha−1 recorded the highest (10.41 t ha−1) and lowest (3.08 t ha−1) TDBW, respectively (Table ).
Days to physiological maturity (DPM) were significantly (P < 0.05) longer in summer on <80000 plants ha−1 + >0 t ha−1 cattle manure application rates (Table ). Generally, shorter days to maturity were noted in winter under all the soil fertility amendments than summer (Table ). Longest (91 days) and shortest time (35 days) to reach physiological maturity were noted in 9 t ha−1 cattle manure+55555 plants ha−1 in summer and 0 t ha−1 cattle manure + 80000 plants ha−1 in winter, respectively (Table ).
Table 5. Effects of soil fertility amendments within different growing season × planting densities combinations on the C. quinoa the days to physiological maturity and yield parameters
T.G.W, grain yield and HI were higher in winter than summer season. Highest (2.5 g) and lowest (0.2 g) T.G.W were noted in winter and summer at 9 t ha−1 cattle manure + 29296 plants ha−1 and D + 80000 plants ha−1, respectively (Table ). In winter, all soil fertility-amended plots planted with 29629 and 55555 plants ha−1 recorded higher grain yield than the unamended plots. Highest (2.5 t ha−1) grain yield was obtained in winter with 9 t ha−1 cattle manure + 55555 plants ha−1 and lowest (0.1 t ha−1) in summer with D + 80000 plants ha−1 (Table ). HI was highest (0.2) in winter at 9 t ha−1 cattle manure + 55555 plants ha−1 and lowest under 80000 plants ha−1 regardless of the season and quantity of cattle manure applied (Table ).
Temperature was positively correlated to PH (0.80), TDBW (0.65), DF (0.70), branch number (BN) (0.60) and DPM (0.89) but negatively correlated to grain yield, T.G.W and HI (Table ). Meaning that the PH, TDBW, DF, BN and DPM were higher in summer, but the grain yield, T.G.W and HI were higher in winter (low temperatures). PH was positively correlated to the TDBW (0.75), yield (0.86), T.G.W (0.79) and HI (0.58). DPM were positively correlated to yield (0.81) and T.G.W (0.59) (Table ).
Table 6. Correlation analysis for seasonal temperature (T°C) and response variables of the quinoa grown in summer and winter, Marondera, Zimbabwe
4. Discussion
The PH, TDBW, DF, DPM and BN were higher under high (summer) than low (winter) temperatures. However, the T.G.W, grain yield and HI were higher during low than high temperatures. The results indicated that temperature was important in the growth and development of the quinoa. The PH, DPM, T.G.W, grain yield and the HI were higher at <80000 plants ha−1 planting densities regardless of the fertility levels. We did not measure the crop nutrient use efficient (NUE) directly so we assumed that a high plant performance corresponded to high NUE. Basing on this, the quinoa plants showed to utilize nutrients more efficiently at <80000 plant ha−1 in all the fertility-amended plots.
In summer, the grain yield ranged from 0.1 t ha−1 under compound D + 80000 plants ha−1 to 0.57 t ha−1 at compound D + 55555 plants ha−1 and varied from 0.2 t ha−1 on 0 t ha−1 cattle manure+29629 plants ha−1 to 2.55 t ha−1 at 9 t ha−1 cattle manure + 55555 plants ha−1 in winter. The recorded quinoa grain yields for winter were similar to Jacobsen (2003) who reported quinoa yields of 1.1–1.7 t ha−1 at 15–30°C temperature range. Notably, in this study, the grain yields and BN were started to decline at a planting density of 80000 plants ha−1 regardless of the fertility levels. The results are consistent with previous reports (Siavoshi et al., Citation2010; Sief et al., Citation2015) which highlighted a decline in quinoa performance after a certain optimal planting population per unit areas. The declining grain yield at the planting density of 80000 plants ha−1 was caused by the limited supply of carbon and nitrogen under intense interplant competition for intercepted radiation, soil nutrient and water. This would also have caused barrenness and decreased the flowering and seed filling hence the low T.G.W and HI. Resultantly, an optimal planting density exists for maximizing the utilization of available resources and achieving the maximum yield per unit area by coordinating crop population and individual development (Bertero et al., Citation1998).
The vegetative growth parameters (PH, BN) increased with increase in fertility from 0 to 9 t ha−1 cattle manure application rates then declined at 18 t ha−1 cattle manure application rates at all the planting densities. Nitrogen is very important for the vegetative growth in plant so raising the available soil nitrogen from 83.47 kg ha−1 (initial N content in soils) to 95 kg ha−1 (9 t ha−1 cattle manure) enhanced the quinoa vegetative growth. Nevertheless, further increase in nitrogen to 106.47 kg ha−1 (18 t ha−1 cattle manure) caused low growth performance. We could not ascertain a reason for this observation but suspected toxicity from increased levels of micronutrients at 18 t ha−1 cattle manure application rate and further research is required in this area.
Our results showed 55555 plants ha−1 as the optimal quinoa planting density, however, other studies have reported optimal planting densities above the 55555 plants ha−1 (Bazile et al., Citation2016; Sief et al., Citation2015). These differences may be due to the varying in specific field conditions like water and soil fertility. The effect of plant density on growth habits and yield could vary due to genotypes in question and growing seasons in the same geographical areas (Hirich et al., Citation2014). Hence, the existing relationship between the grain yield with growth performance and yield under different planting densities is important in understanding the basic mechanism of yield-plant density relationship. This would also help in optimizing plant density for improving yield.
The summer temperatures favoured the vegetative phase of the quinoa but negatively correlated to the grain yield. The hot summers and planting densities (<80000 plants ha−1) lengthen the PH, DPM, TDBW and which also increased with an increase in cattle manure up to 9 t ha−1 application rate. The added cattle manure increased the available nitrogen for the plants after mineralization. High temperatures (summer) promoted high soil microbial activity so accelerated the mineralization process leasing more available nitrogen to the crop. This resulted in luxurious consumption of the nitrogen hence continued vegetative growth at the expense of reproduction (flowering) and maturation. Since this was a preliminary work on the introduction of quinoa in Zimbabwe, we did not quantify the effects of temperature and cattle manure on the microbial activities. The DF were shorter during the winter than summer. This is because mostly quinoa is a facultative short-day plant so took shorter DF during the long nights (winter) than short nights (summer). In the facultative short-day plants, flowering may occur under any photoperiod but photoperiods longer than 12 h (summer conditions) will disrupt seed filling and maturation and result in the low T.G.W, grain yield and HI in the summer seasons (Fawy et al., Citation2017).
Walters et al. (Citation2016) reported a reduction in total biomass of quinoa grain weight by 35% and 23% to 78% at temperatures >22°C. Therefore, the winter temperatures promoted the deposition of more carbohydrates to the grain than straw leading to higher grain yields, T.G.W and HI than summer (Table ), whereas during the summer, grain development was poor as more photosynthates were deposited in the straw than grain. This could have caused by a luxurious uptake of the nitrogen during the summer season because the nitrogen could be abundant as a result of high mineralization of the organic manure. In another study, Peterson and Murphy (Citation2015) reported negative effects of high temperatures (>22°C) during flowering and seed fill stages of quinoa hence significant reductions in grain yield. Walters et al. (Citation2016) observed that inflorescences either lacked seeds or contained empty seeds when the temperature increased above 22°C (Siavoshi et al., Citation2010).
The T.G.W is a factor of weight of grains and the number of grains (1000). This implied that the T.G.W was dependent on the weight of individual seeds therefore the low T.G.W of summer quinoa seeds indicated poor grain filling and environmental conditions experienced by the crop before harvesting. This 1000 grain weight was a very important measure of the quinoa seed quality. Again the grain yield was associated with seed weight meaning that any factor influencing grain filling would influence the grain yield. The winter conditions together with soil fertility amendments with <80000 planting density enhanced high grain filling leading to higher T.G.W values than in summer (Table ). Temperature had influenced the quinoa grain yields through effects on radiation interception, radiation use, yield component and/or carbohydrates partitioning.
The HI range of 0.17–0.23 and 0.08–0.14 in winter and summer, respectively (Table ). The HI is an excellent parameter to assess the dry matter partitioning and efficiency of plants for mobilization of photo-assimilates. The results are similar to Spehar and Santos (Citation2005) who noted positive associations among dry matter production, plant height and grain yield which was translated to maturity period. Spehar and Santos (Citation2005) noted variations in the quinoa plant height due to genotype, where the late-maturing varieties grew taller than the ones that matured early and were also shown to be superior in other yield components. Nevertheless, the observed differences in plant heights between the winter and summer were due to differences in temperatures and not genotype (Table ).
4.1. Conclusion and recommendation
This study provided essential preliminary information on the appropriate season to grow quinoa in Zimbabwe and forms the basis for further evaluations. A combination of high planting density (80000 plants ha−1)+ high cattle manure application rate (18 t ha−1) caused poor quinoa growth performance and grain yield in both summer and winter seasons. The results showed that the phasic development was high during the summer season though associated with low grain yield, T.G.W and HI. Generally, the vegetative phase responded well to improved soil fertility and planting population of 55555 plants ha−1 in summer than winter. The reproductive phase was best during winter with highest grain yield at 55555 plants ha−1 + 9 t cattle manure ha−1. Hence, the 55555 plants ha−1 + 9 t cattle manure ha−1 resulted in best quinoa performance both in summer and winter. However, the best time to grow the quinoa in Zimbabwe can be dependent on the purpose of the crop. If grown for grain yields, winter is the best season, but if production is targeting at high biomass, e.g. for fodder, then summer conditions are ideal. Nevertheless, further evaluation researches on agronomic practices such as crop water requirements are necessary. The evaluations have to be repeated and spread to other agro-ecological zones of Zimbabwe.
Competing interests
The authors declare that they have no competing interests.
correction
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgements
The research did not receive any specific funding but was performed as part of employment at the Women’s University in Africa, Zimbabwe. The authors gratefully acknowledge the Women’s University in Africa, Department of Horticulture for the resources to carry this study at the University Farm.
Data availability statement
The raw data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
Notes on contributors
C. Parwada
Dr. Cosmas Parwada is a senior lecturer at the Women’s University in Africa. He is soil scientist with sound background in agronomy. He has published many articles related to crop production and soil fertility and conservation.
Professor Ronald Mandumbu is a seasoned lecturer at the Bindura University of Science Education. He is a renowned researcher in crop production and crop protection.
Dr. Handsen Tibugari is a lecturer at the Gwanda State University. He has extensively researched on potential utilization of sorghum allelopathy for weed management. He has vastly published articles related to crop production and crop protection.
Doreen Badze is a recent graduate from the Women’s University in Africa. She has graduated with a BSc (Hons) in Horticulture and is a prominent practising horticulturist in Zimbabwe.
Somerset Mhungu is a lecturer at the Women’s University in Africa. He has researched and published in crop production.
References
- Bazile, D., Pulvento, C., Verniau, A., Al-Nusairi, M. S., Ba, D., Breidy, J., Hassan, L., Mohammed, M. I., Mambetov, O., Otambekova, M., Sepahvand, N. A., Shams, A., Souici, D., Miri, K., & Padulosi, S. (2016). Worldwide evaluations of Quinoa: Preliminary results from post international year of Quinoa FAO projects in nine countries. Frontiers in Plant Science, 7(8), Article 226. https://doi.org/10.3389/fpls.2016.00850
- Bertero, H. D., King, R. W., & Hall, A. J. (1998). Photoperiod-sensitive development phases in quinoa (Chenopodium quinoa Willd.). Field Crops Research, 60(3), 231–16. https://doi.org/10.1016/S0378-4290(98)00128-2
- Eisa, S., El-Samad, E. H., Hussin, S., Ali, E., Ebrahim, M., González Sanchez, J., Ordano, M., Erazzú, L., El-Bordeny, N., & Abdel-Ati, A. (2018). Quinoa in Egypt - Plant density effects on seed yield and nutritional quality in marginal regions. Middle East Journal of Applied Sciences, 8(02), 515–522. http://www.curreswed.com/mejas/me jas/2018/515-522 pdf
- Elizabeth, G.-B., & Da, D. R. (2016). Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: An integrative review. Journal of Nutrition & Food Sciences, 6(3),152-168. doi: 10.4172/2155-9600.1000497
- Erley, G., Kruse, M., & Aufhammer, W. 2005. Yield and nitrogen utilization efficiency of the pseudocereals amaranth, Quinoa, and buckwheat under differing nitrogen fertilization. European Journal of Agronomy. https://www.researchgate.net/publication/223165628
- Fawy, H., Attia, M., & Hagab, R. (2017). Effect of nitrogen fertilization and organic acids on grains productivity and biochemical contents of Quinoa plant grown under soil conditions of Ras Sader-Sinai. Egyptian Journal of Desert Research, 67(1), 169–183. https://doi.org/10.21608/ejdr.2017.5851
- Hinojosa, L., González, J., Barrios-Masias, F., Fuentes, F., & Murphy, K. (2018). Quinoa abiotic stress responses: A review. Plants, 7(4), 106. https://doi.org/10.3390/plants7040106
- Hinojosa, L., Matanguihan, J., & Murphy, K. M. (2019). Effect of high temperature on pollen morphology, plant growth and seed yield in quinoa (Chenopodium quinoa Willd.). Journal of Agronomy and Crop Science, 205(1), 33–45. https://doi.org/10.1111/jac.12302
- Hirich, A., Choukr-Allah, R., & Jacobsen, S. E. (2014). Deficit irrigation and organic compost improve growth and yield of quinoa and pea. Journal of Agronomy and Crop Science, 200(5), 390–398. https://doi.org/10.1111/jac.12073
- IUSS Working Group WRB. 2015. World reference base for soil resources 2014, update 2015, International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No 106, FAO.
- Jacobsen, S. E., & Bach, A. P. (1998). The influence of temperature on seed germination rate in quinoa (Chenopodium quinoa Willd). Science and Technology, 26, 515–523.
- Jacobsen, S. E., & Mujica, A. (2003). The worldwide potential for Quinoa (Chenopodium quinoa Willd.). Food Reviews International, 19(1–2), 167–177. https://doi.org/10.1081/FRI-120018883
- Jacobsen, S. E., Mujica, A., & Jensen, C. R. (2003). The resistance of Quinoa (Chenopodium quinoa Willd.) to Adverse Abiotic Factors. Food Reviews International, 19(1–2), 99–109. https://doi.org/10.1081/FRI-120018872
- Koyro, H. W., Lieth, H., & Eisa, S. (2008). Salt tolerance of Chenopodium quinoa willd, grains of the Andes: Influence of salinity on biomass production, yield, composition of reserves in the seeds, water and solute relations.STasks for vegetation sciences, 42(3), 133–145). http://dx.doi.org/10.1007/978-1-4020-6720-4-13.
- Maliro, M., Guwela, V., Nyaika, J., & Murphy, K. M. (2017). Preliminary studies of the performance of Quinoa (Chenopodium quinoa Willd.) Genotypes under irrigated and rainfed conditions of central Malawi. Frontiers in Plant Science, 8, 227. https://doi.org/10.3389/fpls.2017.00227
- Martínez, E. A., Veas, E., Jorquera, C., San Martín, R., & Jara, P. (2009). Re-Introduction of quinoa into Arid Chile: Cultivation of two lowland races under extremely low irrigation. Journal of Agronomy and Crop Science, 195(1), 1–10. https://doi.org/10.1111/j.1439-037X.2008.00332.x
- Maynard, D. G., Kalra, Y. P., & Crumbaugh, J. A. (2006). Nitrate and exchangeable ammonium nitrogen. In M. R. Carter et al (Ed.), Soil sampling and methods of analysis 2nd (pp. 71–80). Canadian Society of Soil Science.
- Miranda, M., Vega-Gálvez, A., Quispe-Fuentes, I., Rodríguez, M. J., Maureira, H., & Martínez, E. A. (2012). Nutritional aspects of six Quinoa (Chenopodium quinoa Willd.) Ecotypes from three geographical areas of Chile. Chilean Journal of Agricultural Research, 72(2), 175–181. https://doi.org/10.4067/S0718-58392012000200002
- Navruz-Varli, S., & Sanlier, N. (2016). Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). Journal of Cereal Science, 69, 371–376. https://doi.org/10.1016/j.jcs.2016.05.004
- Papastylianou, P., Kakabouki, I., Tsiplakou, E., Bilalis, D., Hela, D., Chachalis, D., Anogiatis, G., & Zervas, G. (2014). Effect of fertilization on yield and quality of biomass of Quinoa (Chenopodium quinoa Willd) and Green Amaranth (Amaranthus retroflexus L). Bulletin UASVM Horticulture, 71(2), 288–292. doi: 10.15835//buasvmcn-hort: 10411
- Parwada, C., & Van Tol, J. (2018). Effects of litter source on the dynamics of particulate organic matter fractions and rates of macroaggregate turnover in different soil horizons. European Journal of Soil Science, 69(6), 1126–1136. https://doi.org/10.1111/ejss.12726
- Peterson, A., & Murphy, K. M. (2015). Tolerance of lowland quinoa cultivars to sodium chloride and sodium sulfate salinity. Crop Science, 55(1), 331–338. https://doi.org/10.2135/cropsci2014.04.0271
- Rashid, N., Basra, S. M. A., Shahbaz, M., Iqbal, S., & Hafeez, M. B. (2018). Foliar applied moringa leaf extract induces terminal heat tolerance in Quinoa. International Journal of Agricultural and Biologica, 20(1), 157‒164. doi: 10.17957/IJAB/15: 0469
- Schoenau, J. J., & O’Halloran, I. P. (2006). Sodium bicarbonate-extractable phosphorus. In M. R. Carter & Gregorich, E.R (Ed.), Soil sampling and methods of analysis 2nd (pp. 89–95). Canadian Society of Soil Science.
- Siavoshi, M., Nasiri, A., & Lawre, S. (2010). Effect of organic fertilizer on growth and yield components in rice (Oryza sativa L.). The Journal of Agricultural Science, 3, 15-28. doi: 10.5539/jas.v3n3p217
- Sief, A., El-Deepah, H., Kamel, A., & Ibrahim, J. (2015). Effect of various inter and intra spaces on the yield and quality of Quinoa (Chenopodium quinoa Willd). Journal of Plant Production, 6(3), 371–383. https://doi.org/10.21608/jpp.2015.49331
- Spehar, C. R., & Santos, R. L. B. (2005). Agronomic performance of quinoa selected in the Brazilian Savannah. Pesquisa Agropecuária Brasileira, 40(6), 609–612. https://doi.org/10.1590/S0100-204X2005000600012
- Walters, H., Carpenter-Boggs, L., Desta, K., Yan, L., Matanguihan, G. J., & Murphy, K. M. (2016). Effect of irrigation, intercrop and cultivar on agronomic and nutritional characteristics of quinoa. Agroecology and Sustainable Food Systems, 40(8), 783–803. https://doi.org/10.1080/21683565.2016.1177805
