?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Dry evergreen Afromontane forests are the most threatened in Ethiopia due to agricultural land expansion as they are exposed to high population pressure and thus, conservation measures should be based on scientific evidence since resources for nature conservation are limited. We evaluated the species composition, structure and regeneration status of woody plant species at Gelawoldie community forest. This was analyzed from 32 quadrats laid down in belt transect, each with 400 m2 and 25 m2 for trees and shrubs, and seedlings and saplings, respectively using systematic sampling method. Diversity was analysed using Shannon-Wiener diversity index. A total of 59 woody plant species in 49 genera and 38 families were identified. Of these, 35.5%, 50.8% and 13.5% were trees, shrubs and climbers, respectively. The diversity and evenness of the forest were 3.8 and 0.9, respectively. Moreover, the forest had total mean stand density of 2016 stems ha−1 and basal area of 93.8 m2ha−1. More than 61% of the woody plant species had lower than 5% importance value index (IVI), while the remaining 39% had ≥ 5%. The overall results of the present study revealed higher number of individuals at the lowest diameter (2.5–10 cm) and height classes and progressively declined numbers in higher classes yielding reverse J-shaped distribution pattern. This shows that the forest had healthy regeneration. However, analysis from individual woody plant structure, and count of seedlings and saplings showed that about 17.7% and 5.3% of the species including the endemic have fair and poor regeneration, respectively. Thus, there is a strong need for conservation measures that have to start by identifying the major drivers of low regeneration and the subsequent loss.
PUBLIC INTEREST STATEMENT
The present study was conducted to evaluate the woody plant diversity and regeneration status of Gelawoldie community forest attributed to the high risk of destruction pressure from agricultural land expansion, overgrazing and selective cutting. The study has found out that the forest had relatively higher diversity, however with different regeneration status. The findings of this study will serve as a baseline data to design and implement data-based and better forest management plans to responsible bodies. Moreover, the findings remind the surrounding community about the status of the forest and the human factors affecting the future and the risk posed on the services derived from the forest. Timely actions from all the stakeholders will contribute a lot for the continuity of the forest.
Competing Interests
The authors declares no competing interests.
1. Introduction
Ethiopia belongs to the 25 biodiversity-rich countries in the world. There are 34 biodiversity hotspots worldwide, of which two; Eastern Afromontane and Horn of Africa are found in Ethiopia. This utmost is ascribed to the great geographical diversity particularly altitude that consequently determines macro and micro climatic variability and creates environments that support a wide variety of flora and fauna (Ethiopia Biodiversity Institute [EBI], Citation2014). Furthermore, Ethiopia harbours a high degree of endemism with 544 endemic plant taxa, representing 10% of the overall flora (Kelbessa & Demissew, Citation2014). Approximately 16.7% of the flora represents woody plants, of which 30% are trees mainly indigenous (Tona, Citation2016). Similar to other biological systems, indigenous plant species have adapted through several years of evolution and serve as wombs for the development of biodiversity and keystone natural resources such as soils and water (Negash, Citation2010).
Historical records indicate that an equivalent of 35–40% of Ethiopia’s land area had once been covered by natural high forests. However, data from airborne and satellite remote sensing have revealed a natural high forest cover of 4.75% and 0.20% in the 1970s and 1980s, respectively (Reusing, Citation2000). According to Food and Agriculture Organization of the United Nations (Citation2010), approximately 11% of the total area of Ethiopia has been covered by forests of all types; high forest, open forest, plantations etc. The forest cover has shown a tremendous decrease from 1990 to 2000 and declined to 4% of the total land area of Ethiopia (EarthTrends, Citation2003). This indicates the sharp and radical decline in the forest cover of the country through years. Consistently, the results of comparative studies have found small closed/high forest cover percentage of the total land area in Amhara, Oromia, Tigray and southern regions (Homeier, Citation2011). The closed/high forest cover showed a 44,000 ha decrease per year from 2005 to 2009, resulting in a 14.6% change (Homeier, Citation2011). However, the reduction is more severe in Amhara and Tigray region while open and mosaic forest covers increased as per forest definition of Mayaux et al. (Citation2003) and Homeier (Citation2011). Among the forest types in Ethiopia, dry evergreen Afromontane forest and grassland complex that occur above 1800 m and below 3000 m cover most of the total land area. This forest is found in areas where Ethiopian highland agriculture developed thousands of years ago and is inhabited by the majority of the population in the country (Friis et al., Citation2010; Gurmessa, Citation2011). The distribution of dry evergreen Afromontane forests and grassland coincides with areas that people have dominantly been practicing cereal-based agriculture. This has resulted in a significant destruction pressure on the forests because of the exponential population growth and the accompanied demand for fuelwood and land for agriculture (Friis et al., Citation2010; Gurmessa, Citation2011).
Similarly, the Amhara National Regional State (ANRS) in Northwestern Ethiopia shares these phenomena. The region has a total area of approximately 170, 052 km2, of which 2%, 27% and 6% is covered by high forest, shrubland and woodland, respectively (Sisay et al., Citation2017). More than 60% of the total land area in ANRS is covered by Afromontane vegetation, of which dry Afromontane forests occupy the largest proportion (Wassie, Citation2017) approximately 18% (Homeier, Citation2011). The dry Afromontane forests in the region are found as remnant forests in the landscape either as protected state or Ethiopian Orthodox church forests (Wassie, Citation2017). These forests are under increasing pressure by human population through farmland expansion, tree harvesting, urbanization and others.
These anthropogenic activities have caused a significant forest cover change over the years. There has been a dramatic decline in forest cover between 1987 and 1999 in ANRS due to the extended transition period soon after the fall of the Derge regime (Alemneh et al., Citation2019). This period was characterized by land grabbing for farm expansion and grazing, encroachment of agriculture in higher elevation and weakening of communal land management practices (Alemneh et al., Citation2019). From 1999 to 2014, the tree cover change was static. The tree cover change has been intense and an overall increase of 3% was reported from 2014 to 2017. However, the increment has been attributed entirely to a shift from mixed crop and livestock agriculture to plantation forestry dominated by eucalyptus (Alemneh et al., Citation2019). Studies have shown that there are about 209, 799 ha of plantations, most of which are covered by eucalyptus (Wassie, Citation2017). The development of eucalyptus plantation has increased rapidly and suppresses the regeneration and growth of indigenous species, causes loss of biodiversity, soil quality and moisture content in natural and sacred church forests in the region (Chanie et al., Citation2013; Mekonnen, Citation2019).
Evidence strongly suggests the need for conservation actions so as to protect and save threatened endemic and indigenous plant species in dry evergreen Afromontane forests of ANRS. The successful conservation and better management of forests require up-to-date information on the forests such as vegetation composition, structure, abundance, diversity and status. This is due to the fact that the efforts invested to protect plant diversity must be prioritized. Moreover, vegetation data are lacking in the study area. The objective of this study was to evaluate woody plant species diversity, structure and regeneration status of Gelawoldie community forest and compare with other forests in ANRS, Northwestern Ethiopia.
2. Materials and methods
2.1. Description of the study area
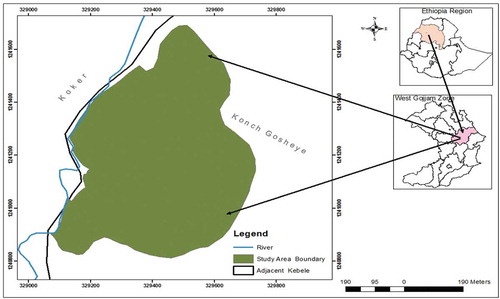
The study was undertaken at Gelawoldie community forest (Figure ), which is found in Yilmana Densa district of West Gojjam zone of the ANRS, Northwestern Ethiopia. Geographically, the district is situated at 11°29ʹ59.99” N latitude and 37° 19ʹ 60.00” E longitude. The district covers a total area of 99 180, of which 56% undulating, 20% mountainous, 8% gorge and 16% plateau with the highest plateau of Mount Adama (Taddege, Citation2019).
Gelawoldie community forest is 45 km from Bahir Dar, the capital city of ANRS. It is found in Konch Gosheye kebele of Yilmana Densa district and bordered by Kotet river in the north (Figure ). The coordinates of the forest are between 12°41ʹ14.4”–12°41ʹ27.32” N latitude and 32°94ʹ80.0”–32° 95ʹ52.76” E longitude. It covers over 36.9 ha. The forest has an average altitude of 2343 metre above sea level (m.a.s.l.)
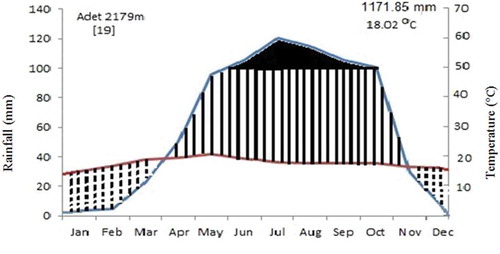
Rainfall and temperature data for Yilmana Densa district for the past almost two decades (2000–2018) were obtained from Western Amhara Meteorology Service Center (Citation2018) in Bahir Dar. According to the data, the mean annual maximum temperature is 25.6°C and the mean minimum temperature is 10.4°C (Figure ). The hottest month is May with maximum temperature of 28.4°C and the coldest month is January with minimum temperature of 6.1°C. The district has unimodal rainy season from June to September and has an average annual rainfall of about 1171.9 mm (Figure )
Figure 2. Clima diagram of Yilmana Densa district (2000–2018), Data source:.Western Amhara Meteorology Service Center (Citation2018)
2.2. Sampling design
Reconnaissance survey was conducted through field visits and physical observation in order to get the general view of site conditions and identify sampling sites. Systematic sampling method was employed to lay the quadrats for inventory of woody species in the study area. A total of six belt transects each with 100 m spacing were laid down along an altitudinal gradient from the bottom to the top of the forest. The first belt transect was laid out randomly at one side of the forest along the gradient. A total of 32 quadrats of 20 m × 20 m (400 m2), 4–6 per belt transect were established. Quadrats were 50 m far apart. Each belt transect consisted of different numbers of quadrats depending on their length ranging from 230 to 420 m. Physiographic variables such as altitude, aspect and slope were recorded for each quadrat. However, variations in species composition were not recorded which might be due to narrow altitudinal range (Data not shown). Data on diversity of trees, shrubs and woody climbers were collected following Kent and Coker (Citation1992). Diversity was determined using Shannon-Wiener diversity index which measures both species richness and evenness/abundance. This technique was selected as it is not sensitive to sample size. Sub-quadrats of 5 m× 5 m were laid down at corners and centre of each 20 m × 20 m quadrat to collect data on seedlings and sapling.
2.3. Vegetation data collection
All the woody plant species in each quadrat were recorded and documented in vernacular names. For plant species that were difficult to identify in the field, plant samples with all parts were collected, pressed, dried and mounted. The voucher specimens were identified at a species level using authenticated specimens, published flora volumes of Ethiopia and Eritrea, and experts available in the National Herbarium, Addis Ababa University, Ethiopia. The structural attributes of woody species in the forest were evaluated in terms of DBH, height, density and basal area. Diameter was measured for every individual tree and shrub with DBH greater than 2.5 cm at breast height or 1.3 m above ground using a diameter tape. Where the tree was branched at breast height or below, the diameter was measured separately for the branches and averaged. In cases where tree trunk buttressed, diameter measurements were taken just above the buttresses. Height was measured for every individual tree and shrub with DBH > 2.5 cm and height > 3 m using Suunto clinometer. Where measuring tree and shrub height with clinometer was a challenge, these parameters were estimated visually. Seedlings (height = 1.0 m) and saplings (height greater than 1.0 m and less than 3 m) were also counted and recorded as described by Senbeta and Teketay (Citation2001). DBH values were converted into diameter to calculate the basal area of the species. In each quadrat, the species list and number of trees, seedlings and saplings were recorded to determine the regeneration status.
2.4. Data analysis
2.4.1. Diversity analysis
Floristic data were analyzed using the Shannon–Wiener’s diversity index (H’) as follows:
Where; p is the proportion (n/N) of individuals of one particular species found (n) divided by the total number of individuals found (N), ln is the natural log, Σ is the sum of the calculations, and s is the number of species (Shannon & Wiener, Citation1949).
Shannon’s Equitability (EH) or Evenness was determined by:
Where; S is the number of species recorded (Shannon & Wiener, Citation1949).
2.4.2. Analysis of vegetation structure
The structure of the vegetation was analyzed by computing species density, DBH, height, basal area, frequency and important value index (IVI). The DBH and tree height were classified into DBH and height classes as described in Kent and Coker (Citation1992) and, Kuma and Shibru (Citation2015).
DBH = (C/π) where, C is circumference and π is ≈ 3.14
Basal area (BA) = πD2/4 = (DBH/2)2π = C2/4π C = Circumference, D = diameter, DBH is diameter at breast height, π ≈ 3.14
Dominance (DO) = total cover or basal area of species A/area sampled.
Importance values index (IVI) is a unitless score calculated by adding the relative dominance (RDO), relative density (RD) and relative frequency (RF) of a species (Kent & Coker, Citation1992; Mueller- Dombois & Ellenberge, Citation1974). It is useful to compare the ecological significance of a species (Lamprecht, Citation1989), and a good index for summarizing vegetation characteristics and ranking species for management and conservation practices.
2.4.3. Regeneration status analysis
The regeneration status of the trees, shrubs and woody climbers was determined by computing density ratios between seedlings and mature individuals, seedlings and saplings, and sapling and mature individuals (Dhaulkhandi et al., Citation2008).
2.4.4. Similarity and dissimilarity analysis
Sorensen is the most common binary similarity coefficient used to analyze similarity between different forests. Sorensen’s coefficient was calculated as:
(Sorensen, Citation1948)
Ss = Sorensen’s similarity coefficient; a = number of species common to both forests; b and c are number of species in forests b and c
3. Results and discussion
3.1. Diversity of woody plant species
A total of 59 woody plant species belonging to 49 genera and 38 families were recorded and identified in the Gelawoldie community forest. The life form distribution of these species was 21(35.6%) trees, 3(50.8%) shrubs and 8(13.5%) woody climbers (Table ). Nearly similar numbers of woody plant species were reported by Fisaha et al. (Citation2013) in Wof Washa natural forest, Tadele et al. (Citation2014) in Zengena forest, Birhanu et al. (Citation2018) in Amoro forest and Ayanaw and Dalle (Citation2018) in Yemrehane Kirstos forest. The study forest has greater number of species than Shello Giorgis (Ayalew et al. Citation2020), Awi zone (Gebeyehu et al., Citation2019), Weiramba (Teshager et al., Citation2018) and Wanzaye (Getnet 2018) dry Afromontane forests of Amhara Region. However, Gelawoldie forest has considerably lower species richness than Tara Gedam and Abebaye (Zegeye et al., Citation2011) and Mahbere Sillassie Monastery forests (Habtamu, Citation2017). Fabaceae and Asteraceae were the most species-rich families comprising of 5(8.78%) species each followed by Capparidaceae and Euphorbiaceae with 3(5.26%) species each (Table ). On the other hand, the genus Vernonia had the richest species in the study area followed by Acacia, Acanthus, Jasminum and Maytenus. Mekonen et al. (Citation2015) and Habtamu (Citation2017) have found Fabaceae as the dominant family in Woynwuha natural forest and Mahbere Sillassie Monastery forest, respectively. The variations in species composition are probably due to differences in extent of protection, geographical location and different level of awareness in the communities. This could be inferred from the higher species richness in Tara Gedam and Mahbere Sillassie Monastery which received a relatively better protection. In Gelawoldie community forest, Juniperus procera and Eucalyptus camaldulensis were found outside the sampling quadrats, which might be an indication for potential expansion of exotic species to the forest. This calls for swift conservation intervention actions. From the total woody species, Acanthus sennii, Clemanths longicauda, Laggera tomentosa categorized under near threatened (NT) and Vernonia leopoldii classified under least concern (LC) category were endemic to Ethiopia (Vivero et al., Citation2005). The results are in agreement with those of Zewdie (Citation2013) and lower than those reported in Sesa Mariam Monastery (Meshesha et al., Citation2015). The relatively high endemics in Gelawoldie community forest may be due to the fact that the forest shares some characteristics of both moist Afromontane and dry Afromontane forests (Friis et al., Citation2010).
Table 1. List of woody plant species in Gelawoldie community forest with their family name, mean densities, relative densities (%), frequency, relative frequency (%), dominance (Do), relative dominance (%), IVI (%) and habit
3.2. Species diversity and evenness
Although the study forest is managed mainly by the community and had higher exposure to anthropogenic activities such as selective tree cutting, grazing and agricultural expansion, the overall Shannon–Wiener diversity and evenness indices were high; 3.8 and 0.9, respectively (Table ). The diversity and abundance results of the present study are relatively larger than other similar forests such as Sinko community forest (Zewdie, Citation2013), Yemrehane Kirstos church forest (Ayanaw & Dalle, Citation2018) and Amoro forest (Birhanu et al., Citation2018). This high diversity and evenness reflect improved forest management in the community. Comparison of the species composition of Gelawoldie community forest to other dry evergreen Afromontane forests showed a range of similarity indices (Table ). The resemblance was found generally moderate. The strongest similarity was recorded with Amoro forest (Birhanu et al., Citation2018) followed by Angada (Alemu, Citation2011) and Zengena forests (Tadele et al., Citation2014). The weakest similarity was found with Yemrehane Kirstos church forest (Table ). The strongest similarity with Amoro forest might be attributed to geographical proximity and resemblance in climatic factors (Friis et al., Citation2010). The two forests are found in adjacent districts and seed dispersal by animals can occur.
Table 2. Comparison of species diversity, evenness indices and Sorenson similarity index among different dry evergreen Afromontane forests in Ethiopia
3.3. Analysis of structural characteristics
The total mean stand density of woody species was 2016 individuals ha−1, of which 631 were trees with DBH > 2.5 cm. B. abyssinica was the most abundant species followed by C. aurea, C. edulis and R. abyssinica (Table ). Individuals with DBH class ≥10 and ≤ 20 cm (a) were 170 ha-1 and DBH >20 cm (b) were 73 ha-1. The ratio described as a/b is taken as the measure of size class distribution (Breitenbach, Citation1963). Accordingly, the ratio of the study forest was 2.3 (Table ). This showed that the proportion of medium-sized individuals (DBH ≥10 and ≤ 20 cm) is greater than the large-sized individuals (DBH>20 cm). Gelawoldie community forest has more trees in the lower DBH classes than in the higher classes when compared to Sinko community (Zewdie, Citation2013), Angada (Alemu, Citation2011) and Denkoro Chaka (A. Ayalew et al., Citation2006) forest. However, the ratio is relatively smaller than the results obtained from Chilmo forest (Beche, Citation2011). The proportion of small-sized individuals (DBH<10 cm) was much larger (46.8%). This indicates that there was selective cutting in the community forest.
Table 3. Stand densities of Gelawoldie community forest and other Afromontane forests at two DBH classes
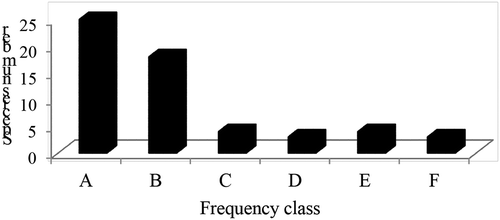
According to this study, D. angustifolia, D. abyssinica and V. amygdalina were the dominant species after E. capensis (Table ). Likewise, individuals of C. macrostachyus and B. abyssinica were more frequently available in the study forest followed by R. abyssinica, A. schimperiana, O. quadripartita, C. aurea and A. abyssinica (Table ).The least frequent species in the study area were F. carica, L. tomentosa, O. tomentosa, P. schimperi, J. schimperiana and S. kunthianum, which cover approximately 6.3% of the area. The woody species in this study were classified into six frequency classes as A = 1–5; B = 6–10; C = 11–15; D = 16–20; E = 21–25 and F = 26–30 according to Lamprecht (Citation1989). Most species approximately 43.9% occurred in 1–5 of the study quadrats followed by 6–10. The remaining species were evenly distributed to other frequency classes (Figure ). The Gelawoldie community forest showed high values in the lower frequency classes and low values in the higher frequency classes which indicates high degree of plant species heterogeneity (Figure ). This agrees with the findings of Bantiwalu (Citation2010), Alemu (Citation2011), and Achiso (Citation2014) on Angada, Sanka-Meda and Choke mountain forests, respectively.
3.4. Vegetation structure
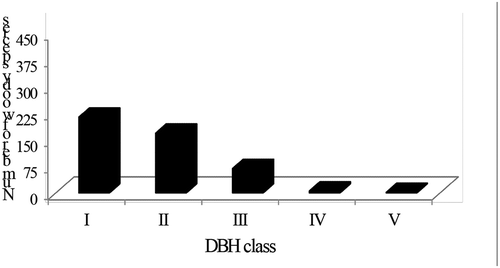
3.4.1. Diameter at breast height (DBH)
The distribution of woody plant species in different DBH classes is shown in Figure . A total of 457 individuals with height >2 m and DBH >2.5 cm was recorded in the study forest. Matured/trees woody plants of the study area were classified into five DBH classes as: I = 2.5−10 cm; II = 10.01–20 cm; III = 20.01–30 cm; IV = 30.01–40 cm and V = 40.01–50 cm. The first DBH class had the highest species density ha-1. About 83% of the tree density ha-1 was in the I and II DBH classes but only 1.57% to DBH classes IV and V (Figure ). This shows that the total number of trees decreased with an increasing tree diameter (Figure ). A. abyssinica and A. schimperiana were the only species found in the higher two DBH classes (IV and V) and C. macrostachyus, P. viridiflorum and B. micrantha were dominant in the middle DBH classes. The DBH distribution pattern of woody plant species indicates the general trend of population dynamics and recruitment status of the species (Zegeye et al., Citation2011). Thus, the DBH distribution of woody plant species of the study area indicates almost a reverse J-shape which is usually an indicator of healthy population status of a species. The result concurs with DBH frequency distribution of Sesa Mariam Monastery forest (Meshesha et al., Citation2015), Wanzaye natural forest (Asfaw, Citation2018) and Amoro forest (Birhanu et al., Citation2018) of the Amhara Region. Contrary to the present study, Teshager et al. (Citation2018) and Gebeyehu et al. (Citation2019) have found bell-shaped and irregular J-shaped woody plant population frequency distribution patterns. The similarities and variations among these forests might be attributed to the different management practices and anthropogenic activities in and around the forests.
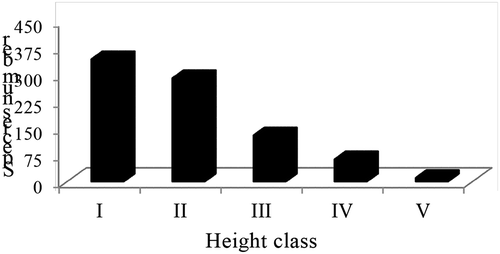
3.5. Height distribution
The height class frequency distribution of trees and shrubs showed similar pattern to DBH distribution (Figure ).The frequency distribution of height classes of trees and shrubs in the study forest was described using 25 woody species with 837 individuals and classified into five height classes according to Ayanaw and Dalle (Citation2018) with little modification as I = 2.0–5.0 m; II = 5.01–10.0 m; III = 10.01–15.0 m; IV = 15.01–20.0 m and V = 20.01–25 m. Slightly more than 75.6% of the individual woody plants were in height classes I and II (Figure ). The results agree with those of Alemu (Citation2011), Zewdie (Citation2013), and Birhanu et al. (Citation2018) in Angada, Sinko community and Amoro forests, respectively. The lower number of large-sized individuals in the upper classes of Gelawoldie community forest implies the presence of small number of adult trees for reproduction (Lamprecht, Citation1989). On the other hand, the dominance of small-sized individuals is an indicator for the presence of good regeneration but low recruitment which might have been caused by anthropogenic activities. This coincides with the findings of Zewdie (Citation2013) at Sinko community forest.
3.6. Basal area
The total basal area of woody species in Gelawoldie community forest with DBH > 2.5 cm was 93.8m2ha-1 (Table ). The study forest had relatively larger total basal area than Chilmo (Bekele, Citation1994), Denkoro (A. Ayalew et al., Citation2006), Sanka Meda (Bantiwalu, Citation2010), Angada (Alemu, Citation2011) and Sinko community forests but lower than Wof Washa forest (Bekele, Citation1994). Most of the basal area was contributed by A. abyssinica (306.0 m2/ha), A. shimperiana (287.9 m2/ha), A. lahai (154.3 m2/ha), C. macrostachyus (135.9 m2/ha), S. kunthianum (131.7 m2/ha), V. amygdalina (126.5 m2/ha) and P. viridiflorum (122.6 m2/ha). Basal area provides the measure of the relative importance of the species rather than simple stem count (Lamprecht, Citation1989). Species with higher basal area could be considered as the most important species in the study forest. In this study, basal area analysis across individual species revealed that there was high domination by very few or small woody species. Accordingly, A. abyssinica, A. schimperiana, A. lahai, C. macrostachyus, S. kunthianum, V. amygdalina and, P. viridiflorum, were the most dominant in descending order and important species in terms of their basal area. They accounted for 57.5% of the total basal area. The contribution of small-sized individuals to the basal area was relatively small. Gelawoldie community forest had basal area higher than most forests in Ethiopia. This suggests that the study forest has better growth and potential to retain higher biomass.
Table 4. Basal area comparison of Gelawoldie Community Forest with other forests
3.7. Importance value index (IVI)
The important value index (IVI) of woody plant species varied greatly, ranging from nil to 16.6% (Table ). More than 61% of the woody plant species had lower IVI; lower than 5% while the remaining 39% of the species had IVI ≥ 5%. The ecologically most important plant species with higher IVI values in descending order were B. abyssinica, C. edulis, R. abyssinica, O. quadripartita, A. lahai, A. abyssinica, C. macrostachyus, A. schimperiana, E. capensis, P. viridiflorum and B. polystachya. These species represent more than 48% of all species in the study area. Almost similar IVI values are found in Denkoro forest for M. arbutifolia, M. africana and J. abyssinicum (Mekonen et al., Citation2015). C. edulis, P. viridiflorum, C. aurea, B. abyssinica and C. macrostachyus have lower IVI values in Denkoro forest than Gelawoldie community forest (Mekonen et al., Citation2015). According to Shibru and Balcha (Citation2004), the greatest IVI value reflects the presence and extent of dominant species in relation to other species in the structure of a forest stand. Moreover, IVI is used as a criterion to prioritize a species for conservation actions (Kacholi, Citation2013). In this regard, most of the species including the endemic ones in the present study require high priority for conservation efforts.
3.8. Regeneration of woody species
A total of 758 seedlings, 627 saplings and 632 matured individuals were recorded in the study area (Appendix 1). The ratio of seedlings and saplings to the matured woody plants was 1.2 and 1, respectively. Approximately 77%, 17.7% and 5.3% of the species had good, fair and poor regeneration status, respectively (Appendix 1). A. lahai, B. abyssinica, C. aurea, C. edulis, C. macrostachyus, E. capensis, O. quadripartita, M. arbutifolia, P. viridiflorum, R. abyssinica and other species had higher number of seedlings and saplings compared to matured plants (Appendix 1). The densities of seedlings were higher than those of saplings and mature woody plants which resulted in an overall good regeneration. Ayalew et al. (Citation2006), Birhanu et al. (Citation2018) and Getnet (2018) have found good regeneration status of B. abyssinica in Denkoro, Amoro and Wanzaye forests, respectively. Likewise, M. arbutifolia in Wanzaye (Getnet et al. 2018), Yemrehene (Ayanaw et al. 2018) and Yegof Washa (Woldearegay et al. Citation2018) forests found to have good regeneration. The good regeneration in some species might be due to un-palatability by herbivores.
This also agrees with the findings of Gebeyehu et al. (Citation2019) who have assessed regeneration status of five forests in Awi zone. Contrary to the present study, A. Ayalew et al. (Citation2006) have reported poor regeneration status of M. arbutfolia, D. abyssinica and E. capensis in Denkoro forest. On the other hand, A. abyssinica, A. sennii, B. spekeana, C. anisata, C. longicauda, D. angustifolia, D. abyssinica, F. carica, O. rochetiana and some others showed fair regeneration. In the present study, poor regenerations were recorded for 5.3% of the species. This is due to the fact that the study area is under high overgrazing and browsing pressure by domestic animals.
4. Conclusion
The present study provides valuable information about the present conditions of woody plant species diversity, structure and regeneration status of Gelawoldie community forest. The results revealed that the diversity in the forest is high, evenly distributed and also harbours reasonable number of endemic plants. The overall stand densities expressed as DBH and height classes and number of seedlings, saplings and matured plants showed inverse J-shaped distribution pattern which suggests a stable population structure of the forest. However, a small number of species were poorly represented or missed either in the lower or higher DBH and height classes and this included the endemic plant species. Therefore, evidence-based and well-planned long-term conservation measures are commendable.
Declaration of interest statement
The authors declare that there is no conflict of interest and agree the submission of the manuscript.
Additional information
Funding
Notes on contributors
Getahun Yemata
Getie Mucheye is a botanist by specialization currently working as a Wildlife Development and Protection expert in Amhara National Regional State Environment, Forest and Wildlife Protection and Development Authority. The author has long years of experience in Meteorology, Culture, Tourism and Parks Development. He is more enthusiastic to conduct research and pursue his further study in the fields of plant diversity analysis, effect of climate change on diversity, conservation and ecosystem services.
References
- Achiso, Z. (2014). Distribution of woody vegetation along the altitudinal range from Abay (Blue Nile) gorge to Choke Mountain, East Gojjam zone. Addis Ababa University.
- Alemneh, T., Zaitchik, B. F., Simane, B., & Ambelu, A. (2019). Changing patterns of tree cover in a tropical highland region and implications for food, energy, and water resources. Frontiers in Environmental Science, 7, 1. https://doi.org/10.3389/fenvs.2019.00001
- Alemu, S. (2011). Woody species composition, diversity and structural analysis of Angada forest in Merti district, Arsi zone of Oromia region. Addis Ababa University.
- Asfaw, A. G. (2018). Woody species composition, diversity and vegetation structure of dry Afromontane forest, Ethiopia. Journal of Agriculture and Ecology Research International, 16(3), 1–21.
- Ayalew, A., Bekele, T., & Demissew, S. (2006). The undifferentiated Afromontane forest of Denkoro in the central highland of Ethiopia: A floristic and structural analysis. SINET: Ethiopian Journal of Science, 29, 45–56.
- Ayalew, T., Merawi, E., & Alemu, S. (2020). Woody plant species diversity of Shello Giorgis dry Afromontane forest, Farita district, west Amhara, Ethiopia. Biodiversity International Journal, 4(1), 59–65.
- Ayanaw, A., & Dalle, G. (2018). Woody Species diversity, structure and regeneration status of Yemrehane Kirstos church forest of Lasta Woreda, north Wollo Zone, Amhara region, Ethiopia. International Journal of Forests Research. https://doi.org/10.1155/2018/5302523
- Bantiwalu, S. (2010). Floristic composition, structure and regeneration status plant species in Sanka Meda Forest, Guna District, Arsi zone of Oromia region, southeast Ethiopia. Addis Ababa University.
- Beche, D. (2011). Floristic composition, diversity and structure of woody plant species in Menagesha Suba State Forest, Central Ethiopia. Addis Ababa University.
- Bekele, T. (1994). Phytosociology and ecology of a humid Afromontane forest on the central plateau of Ethiopia. Journal of Vegetation Science, 5(1), 87–98. https://doi.org/10.2307/3235642
- Birhanu, L., Bekele, T., & Demissew, S. (2018). Woody species composition and structure of Amoro Forest in West Gojjam Zone, northwestern Ethiopia. Journal of Ecology and the Natural Environment, 10(4), 53–64. https://doi.org/10.5897/JENE2018.0688
- Breitenbach, V. F. (1963). Forests and woodlands of Ethiopia: A geobotanical contribution to the knowledge of the principal plant communities of Ethiopia with special regard to forestry. Journal of Life, Earth & Health Sciences, 1, 5–16.
- Chanie, T., Collick, A. S., Adgo, E., Lehmann, C. J., & Steenhuis, T. S. (2013). Eco-hydrological impacts of Eucalyptus in the semi humid Ethiopian highlands: The Lake Tana Plain. Journal of Hydrology and Hydromechanics, 61(1), 21–29. https://doi.org/10.2478/johh-2013-0004
- Dhaulkhandi, M., Dobhal, A., Batt, S., & Kumar, M. (2008). Community structures and regeneration potential of natural forest site in Gangotri, India. Journal of Basic and Applied Sciences, 4(1), 49–52.
- EarthTrends. 2003. Forests, grasslands, and dry lands. Ethiopia-Country profile. http://earthtrends.wri.org.
- Ethiopia Biodiversity Institute (EBI). (2014). Ethiopia’s 5th National report to the convention on biological diversity.
- Fisaha, G., Hundera, K., & Dalle, G. (2013). Woody plants diversity, structural analysis and regeneration status of Wof Washa natural forest, Northeast Ethiopia. African Journal of Ecology, 51(4), 599–608. https://doi.org/10.1111/aje.12071
- Food and Agriculture Organization of the United Nations. (2010). Global Forest Resources Assessment 2010. FAO Forestry Paper 163. Rome.
- Friis, I., Demissew, S., & Breugel, V. P. (2010). Atlas of the potential vegetation of Ethiopia. The Royal Danish Academy of Sciences and Letters.
- Gebeyehu, G., Soromessa, T., Bekele, T., & Teketay, D. (2019). Species composition, stand structure, and regeneration status of tree species in dry Afromontane forests of Awi Zone, northwestern Ethiopia. Ecosystem Health and Sustainability, 5(1), 199–215. https://doi.org/10.1080/20964129.2019.1664938
- Gurmessa, F. (2011). Chapter 5, Dry evergreen montane forests of Ethiopia. In Forest types in Ethiopia: Status, potential contribution and challenges. Forum for Environment 2011 (pp. 79–102).
- Habtamu, B. (2017). Woody species diversity, structure and regeneration status of Mahbere Sillassie Monastry forest, Northwest Ethiopia. University of Gondar.
- Homeier, D. (2011). Chapter 2, Evaluation of forest cover change between 2005 and 2009 in four regional states of Ethiopia. In Environmental Policy Review 2011: Key issues in Ethiopia 2011 (pp. 38–66).
- Kacholi, D. S. (2013). Effects of habitat fragmentation on biodiversity of Uluguru mountain forests in Morogoro region, Tanzania. August University.
- Kelbessa, E., & Demissew, S. (2014). Diversity of vascular plant taxa of the flora of Ethiopia and Eritrea. Ethiopian Journal of Biological Sciences, 13(Supp.), 37–45.
- Kent, M., & Coker, P. (1992). Vegetation description and analysis. Ambo practical approach.
- Kuma, M., & Shibru, S. (2015). Floristic composition, vegetation structure, and regeneration status of woody plant species of Oda forest of Humbo Carbon Project, Wolaita, Ethiopia. Hind. Journal of Botany, 1–9.
- Lamprecht, H. (1989). Silviculture in the tropics.Tropical forest ecosystems and their tree species- possibilities and methods in the long-term utilization.T2 Verlagsgesellschaft, GmbH, Rob Dort.
- Mayaux, P., Bartholome, E., Massart, M., Van Cutsem, C., Cabral, A., Nonguierma, A., Diallo, O., Pretorius, C., Thompson, M., Cherlet, M., Pekel, J. F., Defourny, P., Vasconcelos, M., Di Gregorio, A., Fritz, S., De Grandi, G., Elvidge, C., Vogt, P., & Belward, A. (2003). A land cover map of Africa: Carte de L’occupation du sol de L’Afrique. European Commission: Joint Research Center.
- Mekonen, T., Ayele, B., & Ashagrie, Y. (2015). Woody plant species diversity, structure and regeneration status of Woynwuha natural forest, northwest Ethiopia. Asian Journal of Ethnopharmacology and Medicinal Foods, 1(1), 7–19.
- Mekonnen, A. B. (2019). Distribution and ecological impact of exotic woody plant species inside sacred groves of Northwestern Ethiopia. Biodiversity and Conservation, 28(11), 1–15. https://doi.org/10.1007/s10531-019-01799-4
- Meshesha, B. W., Tsegaye, B. A., & Telake, B. B. (2015). Survey on composition of perennial vegetation in Sesa Mariam Monastery, northwestern Ethiopia. BMC Research Notes, 8(1), 622. https://doi.org/10.1186/s13104-015-1562-5
- Mueller- Dombois, D., & Ellenberge, H. (1974). Aims and methods of vegetation ecology. John Wiley & Sons.
- Negash, L. (2010). . A selection of Ethiopia’s indigenous trees: Biology, uses and propagation techniques. Addis Ababa University Press.
- Reusing, M. (2000). Change detection of natural high forests in Ethiopia using remote sensing and GIS techniques. International Archives of the Photogrammetry and Remote Sensing, 33(B7/3; PART 7), 1253–1258.
- Senbeta, F., & Teketay, D. (2001). Regeneration of indigenous woody species under the canopies of tree plantations in Central Ethiopia. Tropical Ecology, 42, 175–185.
- Shannon, C. E., & Wiener, W. (1949). The mathematical theory of communication. University of Illions Press.
- Shibru, S., & Balcha, G. (2004). Composition, structure and regeneration status of woody plant species in Dindin natural forest, southeast Ethiopia: An Implication for conservation. Ethiopian Journal of Science, 2(1), 31–48.
- Sisay, K., Thurnher, C., Belay, B., Lindner, G., & Hasenauer, H. (2017). Volume and carbon estimates for the forest area of the Amhara Region in Northwestern Ethiopia. Forests, 8(4), 122. https://doi.org/10.3390/f8040122
- Sorensen, T. A. (1948). A method of establishing groups of equal amplitude in plant sociology loased on similarity of species content, and its application to analyses of the vegetation on Denish Commous. Kongelige Danske Videnskabernes Selskabs Skrifter, 5, 1–34.
- Taddege, T. (2019). Compiled body of works in the field of epidemiology: Field residency outputs in the Ethiopian field epidemiology and laboratory training program (EFELTP). University of Gondar.
- Tadele, D., Lulekal, E., Damtie, D., & Assefa, A. (2014). Floristic diversity and regeneration status of woody plants in Zengena forest, a remnant montane forest patch in northwestern Ethiopia. Journal of Forestry Research, 25(2), 329–336. https://doi.org/10.1007/s11676-013-0420-3
- Teshager, Z., Argaw, M., & Eshete, A. (2018). Woody species diversity, structure and regeneration status in Weiramba forest of Amhara Region, Ethiopia: Implications of managing forests for biodiversity conservation. Journal of Natural Sciences Research, 8(5), 16–31.
- Tona, B. (2016). Review on woody plant species of Ethiopian high forests. Journal of Resources Development and Management, 27, 7–16.
- Vivero, J. L., Kelbessa, E., & Demissew, S. (2005). The Red list of endemic trees and shrubs of Ethiopia and Eritrea. Cambridge.
- Wassie, A. (2017). Chapter 15, Forest resources in Amhara: Brief description, distribution and status. In Social and ecological system dynamics: Characteristics, trends and integration in Lake Tana Basin (pp. 231–243). Springer International Publishing. https://doi.org/10.1007/978-3-319-45755-0-15
- Western Amhara Meteorology Service Center. (2018). The temperature and rainfall data of Addet station, Bahir Dar, Ethiopia.
- Woldearegay, M., Woldu, Z., & Lulekal, E. (2018). Species diversity, population structure and regeneration status of woody plants in Yegof dry Afromontane forest, north eastern Ethiopia. European Journal of Advanced Research in Biological and Life Sciences. www.idpublications.org
- Yirga, F., Marie, M., Kassa, S., & Haile, M. (2019). Impact of altitude and anthropogenic disturbance on plant species composition, diversity, and structure at the Wof-Washa highlands of Ethiopia. Helyon, 5. https://doi.org/10.1016/j.heliyon.2019.e02284
- Zegeye, H., Teketay, D., & Kelbessa, E. (2011). Diversity and regeneration status of woody species in Tara Gedam and Abebaye forests, Northwestern Ethiopia. . Journal of Forestry Research, 22(3), 315. https://doi.org/10.1007/s11676-011-0176-6
- Zewdie, A. (2013). Assessment of diversity and structure of woody plant species and land cover changes of Sinko community forest, Fogera district, Northwestern Ethiopia. Bahir Dar University.
Appendix
Density of seedling, sapling and matured tree species/ha in Gelawoldie community forest