?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
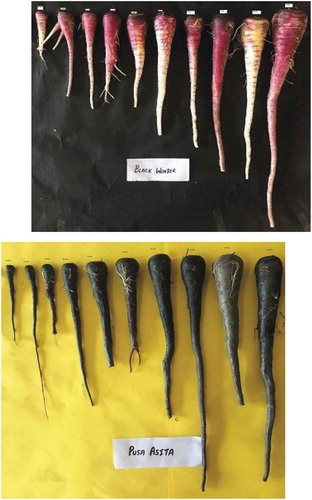
Black carrots cultivars “Pusa Asita” and “Black Wonder” were grown in an experimental field in Amritsar, Punjab, India and harvested after 45 days of sowing at weekly interval. Physical and chemical properties of carrot roots were determined during growth and development. Root weight of “Black Wonder” was higher than “Pusa Asita”. Anthocyanin content of “Pusa Asita” and “Black Wonder” varied from 11.46–519.39 mg/100 g and 0.62–3.29 mg/100 g, respectively. Ascorbic acid content varied from 0.963–4.115 mg/100 g and 0.707–3.437 for “Pusa Asita” and “Black Wonder”, respectively. Total and soluble solids increased marginally with the development of roots. Antioxidant activity of “Pusa Asita” and “Black Wonder” varied from 28.69–91.08% and 4.55–15.62%, respectively. “Pusa Asita” has higher anthocyanin content and antioxidant activity than “Black Wonder” which makes it a potent cultivar for pigment extraction and nutraceutical application.
PUBLIC INTEREST STATEMENT
Carrot is widely consumed vegetable all over the world due to its nutritional value. The colour of carrot varies due to genotype, development stages, environmental conditions, and agricultural practices. The different-coloured carrots include red, orange, yellow, white, purple and black carrot based on their pigment content. Black carrot is popular in eastern countries but it is an underutilized vegetable. Black carrot is an excellent source of Anthocyanins and Antioxidants, which posses anti-cancerous activity. The objective of this research was to study the pigment biosynthesis along with other parameters during various developmental stages and to promote black carrot consumption based on its nutritional superpowers. Black carrot pigment can be used in food where it imparts different shades depending upon the pH of f.
1. Introduction
Carrot is cultivated around the year in temperate region but only during winter season in subtropical region (Haq & Prasad, Citation2015). The important quality attributes of the carrot root are size, shape, uniformity, colour, texture, sensory characteristics and nutritional value (Mazza, Citation1989). Maturity is one of the major factors that affects the quality of the crop (Lee & Kader, Citation2000). Growth of carrot follows a characteristic pattern of rapid expansion on the upper side between 56 and 98 days due to deposition of storage material. Later on, there is a gradual shift of expansion towards lower side (Brown & Gracie, Citation2000). Pigment content of carrot varies due to genotype, development stage, temperature and agronomic practices (Bajaj et al., Citation1980). Among different types of carrots, black carrot is more popular in Eastern countries such as Turkey, Afghanistan, Egypt, Pakistan, India etc (Algarra et al., Citation2014). Black carrot is rich in anthocyanins, which are intensely coloured water-soluble pigments responsible for the orange, red, purple and blue colors. These anthocyanins are highly stable due to presence of acylated group and exist in monomeric form such as cyanidin-3-glucose, galactose and xylose (Tsutsumi et al., Citation2019).
Black carrot is a valuable source of antioxidative, bioactive, and functional components therefore, can be used as natural colour in fruit juices, nectars, soft drinks, preserves, jellies and confectionery (Downham & Collins, Citation2000; Kamiloglu et al., Citation2018). Black carrot plays an important role in health promotion as it provides photoprotection, scavenges free radical and lowers the incidence of cardiovascular disease (Sevimli-Gur et al., Citation2013). The demand of black carrot as salad, juice or pickle has increased because of phytochemicals and anthocyanin content (Akhtar et al., Citation2017). It is a potential source of natural pigments, therefore, present study was conducted to evaluate the pigment biosynthesis along with other physicochemical parameters during various developmental stages.
2. Materials and methods
2.1. Plant materials
Black Carrot (Daucus carota.L) cultivars “Pusa Asita” and “Black Wonder” were grown in the first week of October in an experimental field in Amritsar, India following the recommendations of Punjab Agricultural University, Ludhiana, India. Atmospheric temperature and soil pH ranged between 7.2 and 23.9°C and 6.0–7.0, respectively. Deep and well-drained soil was converted into ridges with 25–30 cm spacing. First irrigation was given immediately after sowing, furthermore, 3–4 irrigations were given till harvesting. Carrots were randomly harvested after 45 days of sowing manually at an interval of 7 days till roots reached maturity up to 108 days of sowing. Black carrots were stored at −18°C for further analysis after measuring their physical properties.
2.2. Chemicals
Chemicals used for analysis were of analytical or HPLC grade and supplied by Sisco Research Laboratories (SRL) Pvt Ltd, Mumbai, India; Thomas Baker (Chemical) Ltd, Mumbai, India; Qualigens Fine Chemicals, Mumbai, India; Central Drug House Pvt Ltd (CDH), New Delhi, India and Spectrochem Pvt Ltd, Mumbai, India.
2.3. Physical parameters
Root weight was determined with weighing balance whereas its length and breadth was determined with thread and ruler. Firmness was measured by Fruit Pressure Tester (Facchini SRL, Alfonsine, Italy).
2.4. Moisture content
The moisture content was determined in a vacuum oven (Narang Scientific Pvt Ltd., New Delhi, India) at 60 ± 2̊C and 13.33 KPa pressure for 24 h (AOAC, Citation1990). 10 g sample was taken in the pre-weighed petriplate and then placed in the vacuum oven for 24 hrs. Sample was kept in the desiccator for 15 min and then weight was noted down. Analysis was done in triplicate.
2.5. Total soluble solids and total solids
Total soluble solids were quantified by using hand refractometer (Erma, Tokyo, Japan). Approximately 10 g sample was crushed in the pestle and mortar till it became fine paste, then juice was squeezed using muslin cloth. 1–2 drops of clear juice were poured over prism to check the total soluble solids. Total solids content was determined by subtracting moisture content from hundred. Readings were taken in triplicate.
2.6. Ascorbic acid
Approximately 2–5 g sample and 30 ml of metaphosphoric acid (3%) were blended and centrifuged. The extract was filtered through charcoal bed to remove the pigments. Dye factor (mg of ascorbic acid/ml of dye solution) was calculated by titrating standard L-ascorbic acid (0.1 mg/ml) with dye solution (50 mg of sodium salt of 2,6-dicholorophenol-indophenol + 42 mg sodium bicarbonate and diluted up to 200 ml). The extract was titrated against standardized dye solution to a pink colour end point, which persisted for 15s.
2.7. Total, reducing and non reducing sugars
Reducing and total sugars were determined with Dinitrosalicyclic acid method (Ranganna, Citation1986). Non- reducing sugar was calculated from difference of total sugars and reducing sugars.
2.8. Anthocyanin content analysis
Sample was extracted with ethanolic HCl (85% of ethanol-95% and 15% of 0.1 N HCl) in a pestle mortar till the residue become colourless and absorbance of extract was measured at 535 nm (Ranganna, Citation1986).
Extinction Factor = 98.2
2.9. Colour values
The colour was measured using Hunter Color Lab (Hunter Associates Laboratory, Reston) in terms of L [lightness], a [redness (+) and greenness (-)] and b [yellowness (+) and blueness (-)]. The instrument was calibrated with a standard black tile and then standard white tile (L = 90.55, a = −0.71, b = 0.39). A sample-handling dish was charged with ground carrot samples, placed on the analyzing port and noted the L, a, b values. The hue angle (h⁰ = tan−1 b/a) represents the maximum degree of redness at 0⁰, yellowness at 90⁰, greenness at 180⁰ and blueness at 270⁰ (Patras. et al., Citation2011). The chroma (C = √a2 + b2) indicates the colour intensity or saturation.
2.10. Free radical scavenging activity
The sample was extracted with 5 ml of methanol for 2 h in an orbital shaker (LabTech, Namyangju) and centrifuged at 10,000 xg for 10 min. 100 μl of supernatant was added to 3.9 ml of 0.1 mMol DPPH solution in brown glass vial. The reaction mixture was shaken and incubated in dark for 30 min. Absorbance was taken at 515 nm (Genesys 10S UV-VIS, Thermo Scientific, Massachusetts) against the blank. The percentage of scavenging was determined as follows.
2.11. Statistical analysis
One way ANOVA was applied on the data of root weight, root length, breadth and length-breadth ratio, anthocyanin content, free radical scavenging activity, total sugars, reducing sugars and non-reducing sugars and colour parameters. Least significant difference values were calculated to find significant difference at p ≤ 0.05. Mean values, standard deviation and ANOVA were computed using Minitab Software (Minitab Inc., State College, USA).
3. Results and discussion
Two Black carrot cultivars were studied for physicochemical changes during growth and development of root.
3.1. Physical parameters
Length and Breadth of root increased from 3.20 to 38.13 cm and 0.80–5.70 cm for “Black Wonder” whereas 9.71–32.37 cm and 1.03–4.10 cm for “Pusa Asita,” respectively, during 45–108 days after sowing (Table ). Data on length and breadth revealed that growth rate of “Pusa Asita” was higher than “Black Wonder” in first 45 days after sowing but after that “Black Wonder” showed higher growth rate during 45–108 days after sowing (Figure ). L/B ratio of “Black Wonder” first decreased and then increased during the development process whereas “Pusa Asita” showed opposite trend with first increased and then decreased. Decrease in L/B ratio indicates increase in breadth whereas increase in L/B ratio indicates increase in length of the root. Length, breadth and L/B ratio describes the growth pattern which was different in “Pusa Asita” and “Black Wonder”. Weight of root increased gradually from 2.00 to 148.51 g in “Black Wonder” and 3.58–78.89 g “Pusa Asita” during the same period. At maturity, mass of root was almost double in “Black Wonder” as compared to “Pusa Asita” which indicated high biomass accumulation in the former cultivar. Firmness of carrot root increased from 16.77 to 27.87 lb for “Black Wonder” and 21.17–31.30 lb for “Pusa Asita” during 73 to 108 days after sowing. Firmness could not be determined in the beginning due to small size of root. There was increase in firmness in both the cultivars but “Pusa Asita” had harder texture than “Black Wonder”.
Table 1. Physical Parameters of Black Carrots during growth of root (n = 3)
Length, breadth, weight and firmness of carrots increased significantly (p ≤ 0.05) with the growth and development of roots. Statistical analysis revealed a significant (p ≤ 0.05) change in L/B ratio of both the cultivars. Length and diameter of black carrot varied between 25.62 and 25.78 cm and 3.3–3.38 cm (Singla et al., Citation2020). Da Silva et al. (Citation2007) observed that weight, length and diameter of carrot root were in the range of 58.3–70.9 g, 15.6–18 cm and 2.0–2.4 cm respectively. Size of root increased due to secondary growth of the vascular cambium producing xylem to the interior (core) and phloem to the exterior (cortex) (Esau, Citation1940). The average root length varied with uniformity of seeds and plant density. Expansion of the storage tissue in the root begins at the top of carrot and moves down till it reaches a portion of the root below which rapid expansion cannot take place (Brown & Gracie, Citation2000). Carrot root centre is rich in xylem vessels, will undergo secondary thickening and lignification during development. During root swelling, there is an increase in epidermal and sub-epidermal parenchyma cells in surrounding tissues (Esau, Citation1940). Ng et al. (Citation1998) also reported that fresh weight, dry weight and total root length increased during maturation and the firmness of matured carrot was 26.01 lb. The main root grew longer but remained thin for another one month (Brett & Waldron, Citation1996). Root length increases rapidly up to 54 days and then becomes slow (Phan & Hsu, Citation1973). Previous studies have reported similar increase in size and firmness parameters.
3.2. Moisture content, total and soluble solids
During 45 to 108 days of sowing, moisture content of black carrot “Black Wonder” and “Pusa Asita” varied from 88.87–91.96 and 88.71–90.15, respectively. Lowest moisture content reported was 88.17 at 73 DAS for “Pusa Asita”. However, soluble solids increased from 5.03–8.60 and 6.27–9.47 °B for “Black Wonder” and “Pusa Asita”, respectively (Table ). Maximum total soluble solids reported were 8.6 and 9.46°B in “Black Wonder” and “Pusa Asita” at 108 DAS. Total solids or dry matter content varied from 8.03–11.31 and 9.48–11.28% for “Black Wonder” and “Pusa Asita,” respectively. Highest total solids content reported was 11.28 for “Pusa Asita” at 73 DAS and 11.31 for “Black Wonder” at 87 DAS. The total solids first increased due to increase in insoluble and soluble solids but then decreased to degradation of insoluble constituents like cellulose, hemicellolose etc as evident by decrease in fibre content (Bhattacherjee et al., Citation2020). Soluble solids and total solids were higher in “Pusa Asita” as compared to “Black Wonder” which is an important parameter for drying. Statistical analysis showed a significant (p ≤ 0.05) change in moisture, soluble solids and total solids with development and growth. Singla et al. (Citation2020) found that TSS of black carrot was 8.20°B. TSS of “Pusa Asita” was 7.77 °B whereas in other black carrot cultivars varied between 7.73° and 7.77°B (Koley et al., Citation2014). Gajewski et al. (Citation2009) recorded that dry matter of black carrot cultivars increased from 9.63 to 12.37% after 133 days. Present results showed lower values for total and soluble solids than previously reported values which might be due to variation in cultivar, climate and testing procedures.
Table 2. Moisture content, Total soluble solids (̊B)and Total solid content of Black Carrot during growth of root (n = 3)
3.3. Anthocyanin content
The major objective of the investigation was to monitor change in pigment content during development stages. On 45th day of sowing, “Black Wonder” and “Pusa Asita” had 0.624 and 11.46 mg/100 g anthocyanin content, respectively (Table ). Anthocyanin content increased to 519.39 and 3.29 in “Pusa Asita” and “Black Wonder,” respectively, after 108 days of sowing. The increase in anthocyanin content during growth and development was statistically significant (p ≤ 0.05). “Black Wonder” had lower anthocyanin content because outer skin was pigmented and inner core was creamish white. Kammerer et al. (Citation2004) revealed that acylated anthocyanins contributed 55–99% of the total anthocyanin content in different black carrot varieties. Acylated cyanidin derivatives are more stable than non-acylated anthocyanins. Purple carrot has been reported to contain 350 mg/100 g anthocyanin content (Arscott & Tanumihardjo, Citation2010). Total monomeric anthocyanin content of black carrot varied from 7.33 to 83.40 mg Cyanidin 3 Glucoside/100 g (Koley et al., Citation2014). Anthocyanin content of black carrot cultivars ranged from 1.5–17.7 mg/100 g FW (Kammerer et al., Citation2004). These results showed that “Pusa Asita” had very high anthocyanin content than the “Black Wonder” and those reported in the literature. This difference may be due to genotype, maturity, growing conditions and season.
Table 3. Anthocyanin content and antioxidant activity of Black Carrots during growth of root. (n = 3)
3.4. Ascorbic acid
Ascorbic acid varied from 0.96–4.11 and 0.7–3.44 mg/100 g for “Pusa Asita” and “Black Wonder”, respectively (Table ). During the initial stages of root formation, the ascorbic acid content was lower but in later stages it increased significantly (p ≤ 0.05) in both the cultivars. Nicolle et al. (Citation2004) reported ascorbic content of 2.5–4.7 mg/100 g in the fresh purple carrot. Wang et al. (Citation2015) reported that the ascorbic acid content increased during development of carrot roots and was highest at breaker stage then decreased. Favell (Citation1998) reported ascorbic acid content of 2.8–4.5 mg/100 g in fresh carrots. Results revealed that ascorbic acid content of two cultivars under investigation was within the range of values reported in the literature.
3.5. Free radical scavenging activity
“Pusa Asita” showed an increase in the free radical scavenging activity from 28.69 to 91.08% whereas it varied from 4.55 to 15.62% in “Black Wonder” during 45–108 days after sowing (Table ). Leja et al. (Citation2013) reported that high antiradical activity in purple root extracts was due to presence of anthocyanins. Gajewski et al. (Citation2007), found higher antioxidant capacity in methanolic extracts from purple carrot. Present findings are in accordance with previous results which indicated that the increase in anthocyanin content resulted in higher antioxidant activity.
3.6. Total, reducing and non- reducing sugars
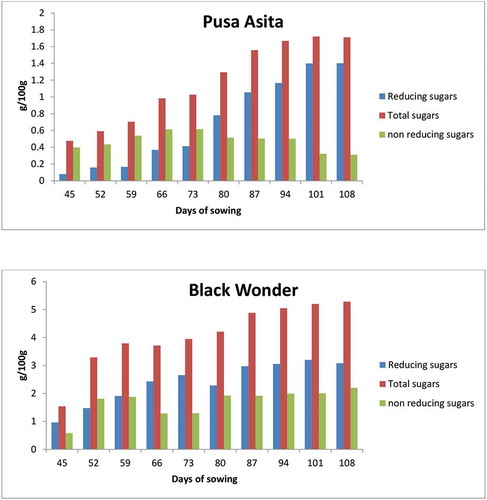
Carrot quality is generally determined by its sugar content which contributes to sweetness. Sugar and terpenoids are most important sensory indicators for consumer acceptability (Simon et al., Citation1980). Total, reducing and non-reducing sugars content were 1.54–5.28 g/100 g, 0.96–3.09 g/100 g and 0.58–2.20 g/100 g for “Black Wonder” whereas 0.48–1.71 g/100 g, 0.08–1.40 g/100 g and 0.31–0.62 g/100 g for “Pusa Asita” during 45–108 days of sowing (Figure ). Statistical analysis revealed a significant (p ≤ 0.05) increase in the total and reducing sugar content for both cultivars.
Ricardo and Rees (Citation1970) reported that carrot roots synthesized acid, alkaline or neutral invertases. Alkaline or neutral invertases activity yielded high sucrose content in mature carrots whereas acid invertase caused hydrolysis of sucrose to glucose and fructose. “Pusa Asita” showed higher non-reducing sugars or sucrose between 66–73 days of sowing and then decline with the growth of roots. Sugar content in carrots varied between 3.88 and 4.91 mg/100 g (Dhillon et al., Citation2016). The purple-coloured carrots have been reported to contain 5.38, 4.11, 0.69, 0.58 g/100gof total sugars, sucrose, glucose and fructose, respectively (Alasalvar et al., Citation2001). Simon (Citation1985) reported that total sugar content ranges from 3–8% with more quantity of sucrose and lesser amount of glucose and fructose. Substantial accumulation of sucrose and reducing sugars in the roots occurred after 50 days (Alasalvar et al., Citation2001). Sugar content of carrot root increased during its growth and reached the maximum level after 3 months of sowing. “Black Wonder” cultivar contained sugar content as reported in literature but “Pusa Asita” had lower reducing and non-reducing sugar contents.
3.7. Colour values
Presence of different and additional acylating groups of anthocyanins might be responsible for colour difference in black carrots (Montilla et al., Citation2011). “L” value of “Pusa Asita” decreased from 27.96 to 22.05 indicating that roots were becoming darker whereas “a” and “b” value varied between 0.17–1.54 and −0.51 to −1.80, respectively, during 45–108 days of sowing (Table ). Negative value of “b” showed that color of carrot became blue. “Black Wonder” had “L” value 33.84 after 45 days of sowing and lowered to 29.85 after 180 days of sowing while “a” and “b” value decreased from 2.12–0.59 and 2.75–0.63, respectively. Significant (p ≤ 0.05) decrease was observed in the “L”, “a”, “b” values for both cultivars. “L”, “a”, and “b” values of black carrot were 41.93, 3.50, and −2.80 (Singla et al., Citation2020) and 26.95, 3.35 and −0.73, respectively (Ersus & Yurdagel, Citation2006). Another study on black carrot reported that “L” and “b” values in the range of 30.1 to 30.19 and −13.64 to −14.44, respectively (Montilla et al., Citation2011). Results showed that “Pusa Asita” had higher pigment content and therefore low “L” value.
Table 4. Hunter “L”, “a”, “b” values of Black Carrot during growth of root (n = 3)
Previously reported “L” values are higher than “Pusa Asita” but lower than “Black wonder”. It further affirms that “Pusa Asita” can be explored for pigment extraction. The hue angle at mature stage of “Pusa Asita” and “Black Wonder” was −44.06 and 48.17, respectively (Table ). Significant (p ≤ 0.05) change was observed in the hue angle values of both cultivars at developmental stages. Accumulation of cyanidin derivatives gives wide colour spectrum characterized by hue angle differences of about 25° between the most bluish and red colour (Montilla et al., Citation2011). Hue angle ranged from −3.68 to −23.96 corresponding to bluish hue (Ersus & Yurdagel, Citation2006). The Chroma and color difference (ΔE) values increased significantly (p ≤ 0.05) during growth of carrots. It indicates the change in colour during development stages which was also supported by change in anthocyanin content.
Table 5. Hue angle, Chroma and ΔE values of Black Carrot during growth of root (n = 3)
4. Conclusion
“Pusa Asita” showed longer, thin and firm roots whereas “Black Wonder” had broader head and higher root weight during growth from 45 DAS to 108 DAS. Moisture content, ascorbic acid, total, reducing and non-reducing sugars were higher in “Black Wonder” as compared to “Pusa Asita”. Total solids, total soluble solids, anthocyanin content and free radical scavenging activity were higher in “Pusa Asita”. Length, breadth, firmness, sugars, ascorbic acid, anthocyanin content and total soluble solids increased with the development of roots from 45 days of sowing to 108 days of sowing. “Pusa Asita” was dark coloured with lower “L” and “a” values and a negative “b” value indicating blue colour. Study indicated continous growth pattern and pigment content in the selected cultivars, moreover, the best time for the harvesting of black carrot was 108 days of sowing as it had reached maturity and contained maximum pigment content. “Pusa Asita” can be explored commercially to produce biopigments.
Additional information
Funding
Notes on contributors
Amritpal Kaur
Amritpal Kaur is a PhD scholar in the Department of Food Science and Technology, Guru Nanak Dev University, India. Her research areas include Cultivation of fruits and vegetables, Postharvest analysis, Food Product Development, Physico-Chemical analysis of Food Products, Pigment Extraction and Incorporation of extracted pigments in Foods.
Dalbir Singh Sogi
Dr. Dalbir Singh Sogi is a Professor in the Department of Food Science and Technology, Guru Nanak Dev University. His area of specialization includes Plant Proteins, Bio-pigments, Fruits and Vegetables Technology, Waste Management.
References
- Akhtar, S., Rauf, A., Imran, M., Qamar, M., Riaz, M., & Mubarak, M. S. (2017). Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends in Food Science and Technology, 66, 36–15. https://doi.org/10.1016/j.tifs.2017.05.004
- Alasalvar, C., Grigor, J. M., Zhang, D., Quantick, P. C., & Fereidoon, S. (2001). Comparison of volatiles, phenolics, sugars, antioxidant vitamins and sensory quality of different coloured carrot varieties. Journal of Agricultural and Food Chemistry, 49(3), 1410–1416. https://doi.org/10.1021/jf000595h
- Algarra, M., Fernandes, A., Mateus, N., de Freitas, V., Esteves da Silva, J. C. G., & Casado, J. (2014). Anthocyanin profile and antioxidant capacity of black carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. Journal of Food Composition and Analysis, 33(1), 71–76. https://doi.org/10.1016/j.jfca.2013.11.005
- AOAC. (1990). Official methods of analysis (15th ed.). The Association of official Analytical Chemist.
- Arscott, S. A., & Tanumihardjo, S. A. (2010). Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Comprehensive Reviews in Food Science and Food Safety, 9(2), 223–239. https://doi.org/10.1111/j.1541-4337.2009.00103.x
- Bajaj, K. L., Kaur, G., & Sukhija, B. S. (1980). Chemical composition and some plant characteristics in relation to quality of some promising cultivars of carrot (Daucus carota L.). Plant Foods for Human Nutrition, 30(2), 97–107. https://doi.org/10.1007/BF01099047
- Bhattacherjee, A. K., Dikshit, A., & Tandon, D. K. (2020). Nutraceutical changes during ripening of bael (Aegle marmelos L. Correa) fruits harvested at different maturity periods. Indian Journal of Traditional Knowledge, 19(2), 416–422. http://op.niscair.res.in/index.php/IJTK/article/view/35359/465477536
- Brett, C., & Waldron, K. W. (1996). Physiology and biochemistry of plant cell walls (2nd ed.). Chapman and Hall.
- Brown, P., & Gracie, P. (2000). Final report: Factors influencing carrot size and shape. Tasmanian Institute of Agricultural Research. Horticultural Australia Ltd.
- Da Silva, E. A., Vieira, M. A., Vieira, E. A., Amboni, R. D. D. M. C., Amante, E. R., & Teixeira, E. (2007). Chemical, physical and sensory parameters of different carrot varieties. Journal of Food Process Engineering, 30(6), 746–756. https://doi.org/10.1111/j.1745-4530.2007.00125.x
- Dhillon, H. S., Dhillon, T. S., & Devi, R. (2016). Quality characterization in carrot (Daucus carota L.) Germplasm. Indian Journal of Ecology, 43(1), 330–332. http://indianecologicalsociety.com/society/full-journals/
- Downham, A., & Collins, P. (2000). Colouring our foods in the last and next millennium. International Journal of Food Science & Technology, 35(1), 5–22. https://doi.org/10.1046/j.1365-2621.2000.00373.x
- Ersus, S., & Yurdagel, U. (2006). Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. Journal of Food Engineering, 80(3), 805–812. https://doi.org/10.1016/j.jfoodeng.2006.07.009
- Esau, K. (1940). Developmental anatomy of the fleshy storage organ of Daucus carota. Hilgardia, 13(5), 175–256. https://doi.org/10.3733/hilg.v13n05p175
- Favell, D. J. (1998). A comparison of the vitamin C content of fresh and frozen vegetables. Food Chemistry, 62(1), 59–64. https://doi.org/10.1016/S0308-8146(97)00165-9
- Gajewski, M., Syzmczak, P., Elkner, K., Dabrowska, A., Kret, A., & Danilcenko, H. (2007). Some aspects of nutritive and biological value of carrot cultivars of orange, yellow and purple colored roots. Vegetable Crops Research Bulletin, 67(1), 149–160. https://doi.org/10.2478/v10032-007-0039-z
- Gajewski, M., Szymczak, P., & Bajer, M. (2009). The accumulation of chemical compounds in storage roots by carrots of different cultivars during vegetation period. Acta Scientiarum Polonorum Hortorum Cultus, 8(4), 69–78. http://www.hortorumcultus.actapol.net/pub/8_4_69.pdf
- Haq, R., & Prasad, V. (2015). Nutritional and processing aspects of carrot (Daucus carota) - A review. South Asian Journal of Food Technology and Environment, 1(1), 1–14. https://doi.org/10.46370/sajfte.2015.v01i01.01
- Kamiloglu, S., Camp, J. V., & Capanoglu, E. (2018). Black carrot polyphenols: Effect of processing, storage and digestion- an overview. Phytochemistry Reviews, 17(2), 379–395. https://doi.org/10.1007/s11101-017-9539-8
- Kammerer, D., Carle, R., & Schieber, A. (2004). Quantification of anthocyanins in black carrot extracts (Daucus carota ssp. sativus var. atrorubens Alef.) and evaluation of their color properties. European Food Research and Technology, 219(5), 479–486. https://doi.org/10.1007/s00217-004-0976-4
- Koley, T. N., Singh, S., Khemariya, P., Sarkar, A., Kaur, C., Chaurasia, S. N. S., & Naik, P. (2014). Evaluation of bioactive properties of Indian carrot (Daucus carota L.): A chemometric approach. Food Research International, 60, 76–85. https://doi.org/10.1016/j.foodres.2013.12.006
- Lee, S. K., & Kader, A. A. (2000). Pre-harvest and post-harvest factors influencing vitamin C content of horticultural crops. Postharvest Biology and Technology, 20(3), 207–220. https://doi.org/10.1016/S0925-5214(00)00133-2
- Leja, M., Kaminska, I., Kramer, M., Maksylewicz-Kaul, A., Kammerer, D., Carle, R., & Baranski, R. (2013). The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant Foods for Human Nutrition, 68(2), 163–170. https://doi.org/10.1007/s11130-013-0351-3
- Mazza, G. (1989). Carrots. In N. A. Eskin (Ed.), Quality and preservation of vegetables (pp. 75–119). CRC Press.
- Montilla, E. C., Arzaba, M. R., Hillebrand, S., & Winterhalter, P. (2011). Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars antonina, beta sweet, deep purple, and purple haze. Journal of Agricultural and Food Chemistry, 59(7), 3385–3390. https://doi.org/10.1021/jf104724k
- Ng, A., Parr, A. J., Ingham, I. M., Rigby, N. M., & Waldron, K. W. (1998). Cell Wall Chemistry of Carrots (Daucus carota Cv. Amstrong) during Maturation and Storage. Journal of Agricultural and Food Chemistry, 46(8), 2933–2939. https://doi.org/10.1021/jf9709921
- Nicolle, C., Simon, G., Rock, E., Amouroux, P., & Remesy, C. (2004). Genetic variability influences carotenoid, vitamin, phenolic and mineral content in white, yellow, purple, orange and dark orange carrot cultivars. Journal of the American Society for Horticultural Science, 129(4), 523–529. https://doi.org/10.21273/JASHS.129.4.0523
- Patras., A., Brunton, N. P., Tiwari, B. K., & Butler, F. (2011). Stability and degradation kinetics of bioactive compounds and color in strawberry jam during storage. Food and Bioprocess Technology, 4(7), 1245–1252. https://doi.org/10.1007/s11947-009-0226-7
- Phan, C. T., & Hsu, H. (1973). Physical and chemical changes occuring in the carrot root during growth. Canadian Journal of Plant Science, 53(3), 629–634. https://doi.org/10.4141/cjps73-123
- Ranganna, S. (1986). Handbook of Analysis and quality Control for Fruits and Vegetable Products (2nd ed.). Tata Mc-graw hill. 12-15, 88-90.
- Ricardo, C. P. P., & Rees, T. A. (1970). Invertase activity during development of carrot roots. Phytochemistry, 9(2), 239–247. https://doi.org/10.1016/S0031-9422(00)85130-4
- Sevimli-Gur, C., Cetin, B., Akay, S., Gulce-Iz, S., & Yesil-Celiktas, O. (2013). Extracts from black carrot tissue culture as potent anticancer agents. Plant Foods for Human Nutrition, 68(3), 293–298. https://doi.org/10.1007/s11130-013-0371-z
- Simon, P. W. (1985). Carrot flavor: Effects of genotype, growing conditions, storage and processing. In H. E. Pattee (Ed.), evaluation of quality of Fruits and vegetables (pp. 315–322). AVI publishing.
- Simon, P. W., Peterson, C. E., & Lindsay, R. C. (1980). Correlations between sensory and objective parameters of carrot flavor. Journal of Agricultural and Food Chemistry, 28(3), 559–562. https://doi.org/10.1021/jf60229a041
- Singla, M., Kumar, A., Kaur, P., & Goraya, R. K. (2020). Respiratory properties of fresh black carrot (Daucus carota L.) based upon non-linear enzyme kinetics approach. Journal of Food Science and Technology, 57(10), 3903–3912. https://doi.org/10.1007/s13197-020-04422-5
- Tsutsumi, A., Horikoshi, Y., Fushimi, T., Saito, A., Koizumi, R., & Fujii, Y. (2019). Acylated anthocyanins derived from purple carrot (Daucus carota L.) induce elevation of blood flow in rat cremaster arteriole. Food & Function, 10(3), 1726–1735. https://doi.org/10.1039/C8FO02125B
- Wang, G. L., Xu, Z. S., Wang, F., Lee, M. Y., Tan, G. F., & Xiong, A. S. (2015). Regulation of ascorbic acid biosynthesis and recycling during root development in carrot (Daucus carota L.). Plant Physiology and Biochemistry, 94, 10–18. https://doi.org/10.1016/j.plaphy.2015.04.014