?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Finger millet is an important cereal crop in Africa, where it plays an important role in nutrition and food security. The purpose of this study was to determine the effect of the germination period on the physicochemical properties of elite finger millet varieties. Axum, Meba, Tadesse and Tessema varieties were soaked for 24 h and germinated for 24, 48 and 72 h at room temperature (23.2 ± 2.6°C) and relative humidity (37.91 ± 8.17%), before being oven-dried and milled into four different flour samples. Results showed that as the germination period increased, the shoot length, germination percentage, germination loss, total titratable acidity and protein content increased, while the pH value, ash and fat content decreased from 6.43 to 5.97, 2.41 to 1.67 mg/100 g and 2.41 to 1.67 mg/100 g respectively. In all varieties, color L of germinated flour increased significantly (P < 0.05), but colors a (redness) and b decreased. According to the findings, germination improved the physicochemical properties of elite finger millet flours.
PUBLIC INTEREST STATEMENT
Finger millet plays a significant role in sub-Saharan Africa and other developing countries in the food security system for many poor farmers because of its low price, high nutritional content and excellent storage conditions compared to other cereal crops. It is used to develop different food products like porridge, injera, bread, biscuits and others alone or composite with other crops, but it contains high anti-nutritional factors, which reduce the nutritional content. This study aimed to produce nutritionally rich germinated finger millet flour. According to the current research finding, 24-h germinated finger flour has a good nutritional content relative to other germination periods. Thus, food industries should be encouraged to use 24-h germinated finger millet flour for the development of foods because it is useful for decreasing the cost of importing flour for food applications and germinated finger millet flour is rich in nutritional content that can contribute to tackling malnutrition.
1. Introduction
Finger millet belongs to groups of small seeded species of cereal grains, which are annual plants. The family of the grain is Poaceae, and it originated in Ethiopia (Ramashia et al., Citation2018). Finger millet (Eleusine Coracana) is part of minor tropical cereal crops that adapt to different agro-climatic conditions, which are in dry areas with limited rainfall (Gebreyohannes et al., Citation2021) and cultivated in Nepal, Taiwan, China, Japan, India and United States. Globally, around 55–60% of finger millet is produced in Africa especially in Ethiopia, Kenya, Malawi, Tanzania, Uganda, Zambia, Zimbabwe, Rwanda and Burundi (Ramashia et al., Citation2019). The grain is widely produced in Africa, and it ranks fourth next to sorghum (Sorghum bicolor), pearl millet (Pennisetum glaucum) and foxtail millet (Setaria italica) as stated by Ramashia et al. (Citation2019). The production of finger millet accounts for 11% in all millets worldwide (Opole, Citation2019). Mostly, it is produced in the eastern part of African sub-humid areas (Zewdu et al., Citation2018).
Ethiopia is the major finger millet-producing country in eastern Africa next to Kenya (Semahegn et al., Citation2021). In Ethiopia, finger millet is named Dagussa and a very useful indigenous cereal crop mostly cultivated by small holder farmers. Finger millet is the six important crop in the country next to teff, wheat, maize, sorghum and barley (Simion et al., Citation2020). It is basically cultivated in Amhara, Oromia, Benishangul-Gumuz, Tigray and Southern nations, nationalities and peoples of Ethiopia. It is an important crop in Gojjam, Gonder, Wlollega, Illubabur, Gamo-Gofa and Northern Tigray (Helen & Shimelis, Citation2017). Finger millet is also becoming a prominent crop in areas of Ethiopia’s central rift valley, such as Arsi Negelle, Shashemene and Siraro (Gebreyohannes et al., Citation2021).
Finger millet is a very useful cereal because of its high protein, fat, and carbohydrate content, which is comparable to wheat and higher than rice (Rathore et al., Citation2019). It has high nutritional quality and adapts to different environmental conditions compared to other millet species (Opole, Citation2019). Finger millet is an ideal crop for use as stable food and famine reserve because it can be stored for a long time without insect damage (Amadou et al., Citation2013). Because of its high nutritional value and storage qualities, it serves as a food security crop (Dida Bulbula & Urga, Citation2018). It plays a great role in dietary needs and as a source of income for many rural households in sub-Saharan Africa and other developing countries due to its richness in protein, carbohydrate, fiber, iron, phosphorus, potassium and calcium (Zewdu et al., Citation2018). It is a useful crop in the diets of children and pregnant and breast feeding women and in addressing malnutrition concerns in developing countries (Chandra et al., Citation2016).
The nutrient content of finger millet depends on the processing methods, presence or absence of anti-nutritional factors and possible interaction of nutrients with other food components. Different processing methods like fermentation, roasting, germination, dehulling and cooking are useful to make the product suitable for human consumption. Among these processing technologies, germination is very useful to ensure the nutritional security of the population for developing countries by increasing the nutrient content and reducing the anti-nutrient of finger millet. Germination is a traditional process, which is used to soften the kernel structure and increase the nutritional composition of finger millet grains (Pushparaj & Urooj, Citation2011). Helen and Shimelis (Citation2017) determined the effect of processing on phytonutrient and nutrient composition of finger millet, but the current study focused on the effect of the germination period on the physicochemical properties of elite finger millet varieties.
2. Materials and methods
2.1. Location of the study
The physicochemical properties were investigated at the Melkassa Agricultural Research Center in Food Science and Nutrition research and Soil research laboratories. The crude fat and crude fiber were analyzed at the Ethiopia Institute of Agricultural Research (Head quarter laboratory) and the Debre Zeit Agricultural Research Center, respectively.
2.2. Sample collection and preparation
Axum, Meba, Tadesse and Tessema finger millet varieties were collected from Arsi Negelle Agricultural Research, which is a subcenter of the Melkassa Agricultural Research Center. The finger millet varieties were grown in 2020/2021 season. The collected samples were transported to the Melkassa Agricultural Research Center. The samples were cleaned and sorted to remove stone, dust particles and broken, undersized and immature grains. About 2.4 kilograms of finger millet grains were taken from each variety and washed three to four times using tap water. Among this, 600 g of finger millet grains were sun dried and served as control. The remaining cleaned and washed finger millet grains were soaked in a ratio of seeds to water of 1:3 for 24 h at room temperature (23.2 ± 2.6°C) and relative humidity (37.91 ± 8.17%), as indicated in Derbew and Moges (Citation2017) with minor modifications. The steeping water and grain were separated using plastic sieve, and the grains were placed in muslin cloths. The soaked and washed grains were germinated for 24 h, 48 h and 72 h in plastic bags at room temperature (23.2 ± 2.6°C) and relative humidity (37.91 ± 8.17%). Then, the germinated finger millet grains were dried using oven dry at 60°C for 6 h, milled using a miller (3010–019, USA) and sieved at 1 mm. The flour was packed using an airtight polyethylene bag and stored at room temperature (23.2 ± 2.6°C) and relative humidity (37.91 ± 8.17%).
2.3. Physicochemical properties of the flour
2.3.1. Determination of germination loss
The germination loss was determined as stated by Nirmala et al. (Citation2000). The weight of grain before germination (A) and the weight of grain after germination (B) were recorded. Total germination loss was calculated based on the following formula:
where
A is the weight of grain before germination and
B is the weight of grain after germination.
2.3.2. Determination of the shoot length
The shoot length of germinated finger millet was measured in centimeters as indicated by Harding (Citation2012). Finger millet grains were germinated in 1:3 grain to water ratio for 24 h, 48 h and 72 h. Among the germinated finger millet grains, 10 finger millet grains were selected randomly and the shoot length was measured using a Caliper (EC000157, China).
2.3.3. Determination of the germination percentage
The germination percentage was determined according to Anjum and Bajwa (Citation2005). The total number of seeds (B) and the number of germinated seeds (A) were counted manually. The germination percentage was calculated according to the following formula:
where
A is the number of germinated seeds and
B is the number of total seeds.
2.3.4. Determination of pH
The pH of finger millet flour was determined using a pH meter as indicated by Y. Kumar et al. (Citation2020). Ten grams of finger millet flour was taken, mixed with 100 ml of distilled water and left for 30 min at room temperature. The pH meter (HANNA instrument) was calibrated using standard buffer solutions of pH 7 and 4. Then, a probe meter was immersed into the supernatant and the readings were taken after 30 seconds.
2.3.5. Determination of total titratable acidity
Total titratable acidity (TTA) of finger millet flour was determined according to Nakarani et al. (Citation2021). Sodium hydroxide (0.1 N) solution was standardized, and the solution was put into a 2500 ml digital burette. Ten grams of finger millet flour was taken and mixed with 100 ml of distilled water in a 250 ml flask. Then, 10 ml of sample (B) was taken from the solution and diluted with 90 ml of distilled water. Four drops of phenolphthalein solution were added and mixed, and the solution was put on a magnetic stirrer and titrated with standardized sodium hydroxide solution (A) until pink color was displayed. Finally, the volume of sodium hydroxide was recorded and TTA was expressed as the lactic acid percentage.
Total titratable acidity was calculated according to the following formula:
where
A is ml of 0.1 NaOH required for the titration and
B is ml of samples taken for the test.
2.3.6. Color analysis of the flour
The color of the flour was analyzed using a Hunter Lab Scan XE Spectrophotometer (LSXE-2, Virginia, USA). The colors L, a and b of the flour were recorded.
2.3.7. Determination of the proximate composition of finger millet flour
The moisture content of the flour was analyzed according to AOAC (Citation2005) using the official method 925.10 by the oven dry method. The ash content of the flour was determined by AOAC (Citation2005) using the official method 923.03 by the muffle furnace method. The crude protein content was analyzed according to AOAC (Citation2005) using the official method 960.52 by the Kjeldahl method. The crude fiber content of the flour was analyzed by the crude fiber method according to the official methods of AOAC (Citation2005). The crude fat content of the finger millet flour was determined according to the Soxhlet extraction method described in AOAC (Citation2005). The total carbohydrate content was determined by the difference method as stated by Manzi et al. (Citation2004), and total energy was calculated using Atwater factors as stated by Mouquet-Rivier et al. (Citation2008).
2.4. Statistical analysis
The variation between the mean levels of all treatments was analyzed by one-way ANOVA using SPSS version 23.0 (SPSS Inc. Illinois, and USA). Sample treatment comparisons were analyzed statistically using Duncan’s multiple range post hoc test, with a probability P < 0.05 significantly different. All measurements were performed in triplicate, and the results were recorded as mean ± standard deviation (SD).
3. Results and discussion
3.1. Effect of the germination period on the physicochemical composition of finger millet varieties
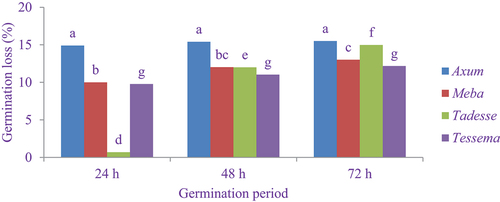
shows the germination loss of finger millet varieties. The highest germination loss (15.5%) was recorded in the Axum variety at a germination period of 72 h, whereas the lowest germination loss (0.69%) was obtained in the Tadesse variety at a germination period of 24 h. The germination loss significantly increased (P < 0.05) in Meba and Tadesse varieties. This might be due to leaching of water soluble and metabolism of carbohydrate during germination. On the other hand, there was no significant difference (P < 0.05) between Axum and Tessema varieties at 24 h, 48 h and 72 h germination. Similar observation has been reported by Malleshi et al. (Citation1986) who indicated that the germination loss in maize and finger millet ranged between 2.9 to 6% and 5.5 to 16.2% at 48 h and 96 h germination, respectively. The VL Mandua-315 finger millet variety loss of germination was 35.39% after 96 h germination, but there was no significant difference at 12 h germination (Ashwani Kumar et al., Citation2021). The malting losses in native and the Indaf-15 finger millet varieties were 30% and 32.5%, respectively, as reported by Ashwani Kumar et al. (Citation2021). The germination loss increases as the germination period increases, which increases metabolic activity, resulting in carbohydrate breakdown of starch and weight loss (Ashwani Kumar et al., Citation2021).
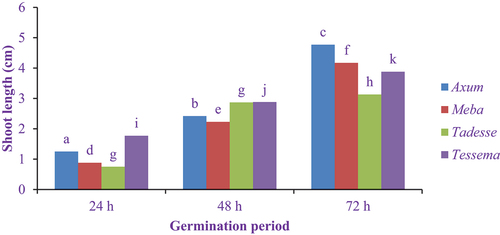
The shoot length of germinated elite finger millet flours is shown in . The maximum (4.77 cm) and minimum (0.75 cm) shoot lengths were obtained at germination periods of 72 h and 24 h in Axum and Tadesse varieties. The shoot length increased significantly (P < 0.05) at all germination periods except the germination period of 48 h and 72 h for the Tadesse variety, which showed no significant difference (P < 0.005). Similar observations were made by Devi et al. (Citation2014) who reported that the shoot length increased from 0.67 to 2.25 cm for PL-1, 0.91 to 3.32 cm for PL-2 and 0.67 to 2.49 cm for PL-3 cowpea genotypes as the germination period extended from 24 to 72 h. After proper steeping, the sprout length of brown rice, oat, sorghum and millet seeds was clearly increased with the increasing germination time because steeping is important to activate the enzymes needed for germination (Li et al., Citation2020).
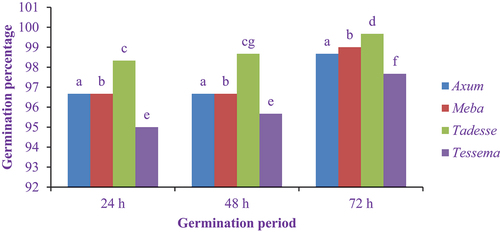
The germination percentage of elite finger millet varieties ranged from 96.67 to 98.67% for Axum, 96.67 to 99% for Meba, 98.33 to 99.67% for Tadesse and 95 to 97.67% for Tessema varieties as indicated in . The result of this research finding showed no significant difference in Axum and Meba varieties at all germination periods, but the germination percentage of Tadesse and Tessema varieties has showed an significant increase (P < 0.05) at 48 h and 72 h relative to 24 h germination periods. Similar findings were observed by Devi et al. (Citation2014), whereby the germination capacity of cowpea genotypes increased from 7.33 to 21.67% for PL-1, 44.67 to 53% for PL-2 and 15 to 27% for PL-3.
Figure 3. Effect of the germination period on the germination percentage of finger millet varieties.
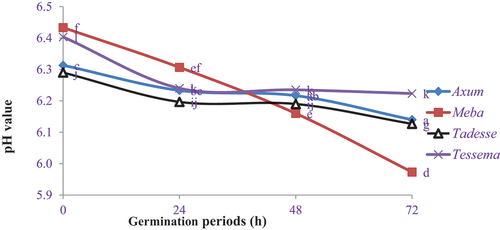
shows the pH values of germinated finger millet flours. The maximum pH value (6.43) was obtained from the ungerminated Meba variety, whereas the minimum pH value (5.97) was recorded in the Meba variety at 72 h germination. The pH value of germinated finger millet flour significantly decreased (P < 0.05) at 48 h and 72 h germination in Axum, Meba and Tessema varieties, but it had no significant difference (P < 0.05) in the Tadesse variety at 48 h germination as compared to ungerminated Axum flour. The germination period has a negative correlation with the pH value of finger millet varieties. The pH value decreased due to the production of organic acids during germination, which activates the synthesis of hydrolytic enzymes that degrade starches and proteins into sugars and amino acids. These sugars are utilized by lactic acid bacteria and yeast, which convert sugars into carbon dioxide, ethanol and lactic acid bacteria (Nefale & Mashau, Citation2018). Similar findings were observed by Ocheme and Chinma (Citation2008) and Nefale and Mashau (Citation2018) whereby germination decreased the pH value of finger millet flour. According to the findings of Derbew and Moges (Citation2017), the pH value decreased significantly (P < 0.05) in both Girana and Misker sorghum varieties as the germination time increased from 24 h to 72 h. The pH value of finger millet and pearl millet decreased from 7.5 to 6.2% and 7.5 to 6.1% at 24 h and 72 h germination, respectively (Owheruo et al., Citation2019). Udeh et al. (Citation2018) also showed that malting had decreased the pH value of finger millet varieties and sorghum grain flours.
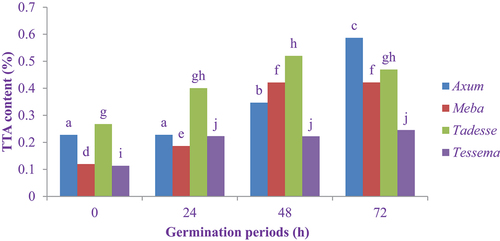
The effect of the germination period on the total titratable acidity of finger millet flour is shown in . The highest total titratable acidity (0.59%) was recorded in the Axum variety at 72 h germination, but the lowest content (0.11%) was recorded in ungerminated Tessema variety flour. The total titratable acidity of Axum, Meba and Tessema varieties flours increased significantly (P < 0.05) at 24, 48 and 72 h germination as compared to ungerminated flour. The germination period has a positive correlation with total titratable acidity of finger millet flour. The increment of total titratable acidity might be due to the production of acid by beneficial microorganisms like lactic acid bacteria, which break down sugars to produce lactic acid and other by-products (Adesokan et al., Citation2011). So an increase in acidity during germination could be an indication of the complex compounds like proteins and lipids hydrolyzed into amino acids and fatty acids (Adedeji et al., Citation2014) and also an indication of free fatty acid products during soaking and germination (Ogori et al., Citation2013). Similar results were reported by Nefale and Mashau (Citation2018) and Ocheme and Chinma (Citation2008) on the content of total titratable acidity of finger millet, which increased as the germination period increased. Derbew and Moges (Citation2017) also reported that total titratable acidity increased significantly (P < 0.05) from 0.19 to 0.29 and 0.25 to 0.30 in Girana and Misker varieties at 72 h germination, respectively. Owheruo et al. (Citation2019) also indicated that the content of total titratable acidity increased as the germination period increased both in African finger millet and pearl millet.
Results of lightness (L*), redness (a*) and yellowish (b*) of the finger millet varieties flour are shown in . The highest value of L* (78.41) was found in the Tadesse variety at 72 h germination, whereas the lowest value (27.73) was also observed in the ungerminated Tessema variety flour. The lightness (L*) of germinated finger millet flour significantly (P < 0.05) increased in all varieties as compared to ungerminated finger millet flour. The L* value of ungerminated finger millet flour was lower than that of germinated finger millet flour, due to the removal of bran layers during soaking and germination. The result of the current research study is in line with the result of Nefale and Mashau (Citation2018) who reported that the lightness (L*) values of ungerminated and 72-h germinated finger millet were 70.10 and 72.83, respectively. The lightness (L*) of germinated finger millet flour increased as compared to that of ungerminated finger millet flour (Hejazi & Orsat, Citation2016). Finger millet flour that germinated for 24 h and 48 h had no significant difference (P < 0.05) relative to ungerminated finger millet (Nefale & Mashau, Citation2018).
Table 1. Effect of the germination period on the color of finger millet flour
The highest redness (a*) value (21.44) was found in the ungerminated Tessema variety, but the lowest value (1.85) was obtained in the Tadesse variety at 72 h germination. The redness (a*) value of ungerminated Axum, Meba, Tadesse and Tessema flour significantly decreased (P < 0.05) at 24, 48 and 72 h germination, but there was no significant difference (P < 0.05) between germination 48 and 72 h in all varieties except the flour of Tadesse variety as indicated in . This finding is similar to the report of Hejazi and Orsat (Citation2016) who indicated that the redness (a*) value of germinated finger millet flour decreased relative to ungerminated finger millet flour. This report contradicts with the report of Nefale and Mashau (Citation2018) who showed that there was no significant difference (P < 0.05) between germinated (24, 48 and 72 h) and ungerminated finger millet flour.
The value of yellowish (b*) significantly decreased (P < 0.05) as relative to ungerminated finger millet flour. There was no significant difference (P < 0.05) between germination 24 and 48 h in Axum and Tadesse varieties. The reason for increasing lightness and decreasing redness and yellowness of the flour may be due to the removal of bran layers during soaking and germination. Similar results were reported by Hejazi and Orsat (Citation2016) that the yellowish (b*) value of germinated finger millet flour decreased as compared to that of ungerminated finger millet flour. However, the yellowish (b*) result of finger millet flour increased significantly as the germination period increased according to the findings of Nefale and Mashau (Citation2018).
3.2. Effect of the germination period on the proximate composition of finger millet varieties
shows the proximate composition of germinated finger millet varieties flour. The moisture contents of Axum, Meba, Tadesse and Tessema varieties were increased at 24 h germination but decreased at 48 and 72 h germination relative to ungerminated flour. The highest moisture content (10.30 mg/100 g) was recorded in the Tadesse variety at 24 h germination, and the lowest moisture content (4.50 mg/100 g) was obtained in the Tadesse variety at 48 h germination. The moisture content increased at 24 h germination periods due to hydration of finger millet seeds during soaking and germination. The other reason for increment of moisture content during germination is that the structure (Sorption isotherm) may change and attract more water to it. The result obtained in this study is in line with the results obtained by Banusha and Vasantharuba (Citation2013) where the moisture content of finger millet increased during malting. Onwurafor et al. (Citation2020) reported similar observation during malting of Mungbean grain. Obadina et al. (Citation2017) also reported the increment of the moisture content of pearl millet with the increase with germination periods.
Table 2. Effect of the germination period on the proximate composition of elite finger millet varieties
The maximum ash content (2.41 mg/100 g) was found in the ungerminated Tadesse variety, and also, the minimum ash content (1.67 mg/100 g) was recorded in the Tadesse variety at 72 h germination. The ash content of the finger millet flour decreased as the germination period increased. The reason for the decrease in the ash content of germinated finger millet flour might be the removal of shoots, roots and bran layers, and also, some minerals in seeds might be used for sprouting metabolism (Ashwani Kumar et al., Citation2021). Another reason for the reduction of ash content during germination might be the leaching of minerals during steeping and washing. Similar results were stated by Ashwani Kumar et al. (Citation2021) who reported the ash content of finger millet flour, which decreased from 2.27% to 1.24% in non-germinated and 96 h germination period, respectively.
The highest (2.12 mg/100 g) and lowest fat (0.91 mg/100 g) content was obtained in the Axum variety at 0 h and 72 h germination, respectively. There was no significant difference (P < 0.05) in the fat content of the Tadesse variety in all germination periods. The fat content significantly decreased (P < 0.05) as the germination period increased from 0 h to 72 h in all finger millet varieties flour except Axum variety. This significant reduction in fat content could be due to increased activity of enzymes, and also, the fat served as an energy source during germination. The reduction of the fat content in malted flour might increase the shelf life by decreasing rancidity, which is most likely due to enzymes released in the flour. Similar observation was reported by Owheruo et al. (Citation2019) in finger millet and pearl millet flours. Moreover, the fat content decreased significantly (P < 0.05) in pearl millet flour as indicated by Ocheme and Chinma (Citation2008).
The highest protein content (10.47 mg/100 g) was recorded in the Tessema variety at a germination period of 48 h, whereas the Tessema variety had a lowest protein content (3.70 mg/100 g) at 72 h germination. The statistical analysis showed that the protein content of Axum and Tessema varieties increased significantly (P < 0.05) at 48 h and 72 h of germination, and the Meba variety also increased significantly (P < 0.05) at 24 h and 72 h germination relative to ungerminated flour, but the germination period had no significant effect on the protein content of the Tadesse variety. The protein content of finger millet flour increases as the germination time increased due to the activity of the protease, an increase that degrades peptides into amino acids (Nkhata et al., Citation2018). However, the protein content of Tadesse and Tessema varieties decreased at 72 h germination. This might be due to the limited amount of seed nitrogenous matter moving from seed to growing embryo. The result of the current research study is in line with the research finding of Swami et al. (Citation2013) who reported that the germination period of 8 to 24 h increased the protein content of finger millet flour from 14.7 to 17%. The current research study result is consistent with the research finding of Derbew and Moges (Citation2017) who showed that the protein content increased in the case of germination time. However, Ashwani Kumar et al. (Citation2021) reported that the protein content of finger millet flour decreased from 6.04 % to 3.41% at 96 h germination.
There was no significant difference (P < 0.05) in the crude fiber content of the Axum variety at 24 h and 48 h germination and also in the Tadesse variety at 24 h germination as compared to ungerminated flour. The content of crude fiber did not increase significantly (P < 0.05) at all germination periods in Meba and Tessema varieties, but the content of fiber significantly increased (P < 0.05) at 72 h germination of Axum and Tadesse varieties relative to ungerminated flours. This is due to the synthesis of structural components like hemicellulose and cellulose during germination (Obadina et al., Citation2017) and the breakdown of starch during germination (Ocheme & Chinma, Citation2008). Similar observations were reported by Banusha and Vasantharuba (Citation2013), Ocheme and Chinma (Citation2008), Obadina et al. (Citation2017), Auta et al. (Citation2014) and Agbor Asuk et al. (Citation2020) in finger millet, pearl millet, pearl millet, pearl millet and sorghum, respectively.
A maximum carbohydrate content (83.50 mg/100 g) was observed in the Tessema variety at 72 h of germination, but the minimum carbohydrate content (73.05 mg/100 g) was found at 24 h germinated Axum flour. The carbohydrate content of Axum, Tadesse and Tessema varieties decreased significantly (P < 0.05) at 24 h germination as compared to ungerminated flour. However, the carbohydrate content of the Tadesse variety increased significantly (P < 0.05) at 48 h and 72 h of germination. There was no significant difference (P < 0.05) in the content of carbohydrate at 48 h and 72 h germination of Axum and Meba varieties. The variation in carbohydrate content could be due to rise and reduction of other food components such as moisture, fat, protein, ash and crude fiber during germination (Derbew & Moges, Citation2017). The result of the current study especially at 24 h germination is similar to the suggestion of Obadina et al. (Citation2017) in pearl millet flour. This result is not in line with the report of Ocheme and Chinma (Citation2008) in pearl millet flour at 48 h germination and also Owheruo et al. (Citation2019) in finger millet, but similar results have been reported in pearl millet at 3 days of germination. In contrary, Derbew and Moges (Citation2017) also showed that the carbohydrate content of sorghum flour decreased at 48 h and 72 h of germination.
The highest energy value (363.79 kcal/100 g) was observed in the Tadesse variety at 72 h germination, whereas the lowest energy value (340.15 kcal/100 g) was found in the Axum variety at 24 h of germination. There was no significant difference (P < 0.05) in the energy value of Axum, Meba and Tessema varieties at 48 and 72 h of germination as compared to ungerminated flour. The value of energy decreased significantly (P < 0.05) in Axum, Meba, Tadesse and Tessema varieties at 24 h of germination relative to ungerminated flour. This is due to the breakdown of starch granules by amylase into simple sugars, which are more soluble in water (Senhofa et al., Citation2016). The simple sugar is used as an energy source for growing embryo during germination. The reduction of the fat content also contributed for the lower energy value. However, the value of energy significantly increased in Tadesse varieties at 48 h and 72 h of germination relative to ungerminated flour. This increment could be due to the reduction of the moisture content at 48 h and 72 h of germination, which resulted in a rise of carbohydrate content of the Tadesse variety.
Obadina et al. (Citation2017) reported that the energy value of pearl millet flour decreased with the increment of germination periods, which is more similar to the current result of 24 h of germination but opposite to the energy value of 48 h and 72 h of germination. Ocheme and Chinma (Citation2008) reported that the energy value of pearl millet flour decreased significantly at 48 h germination, which was not similar to the current finding at 48 h germination in all varieties except in the Tessema variety. Derbew and Moges (Citation2017) also reported that the energy value of sorghum flour decreased as the germination period increased.
4. Conclusions
The germination period increased the germination percentage, germination loss and shoot length of finger millet grain and also improved the nutritional content of finger millet flour. Specifically, it increased the protein content, fiber content, total titratable acidity and color L value, while color a, color b, pH value, fat content and carbohydrate content of finger millet flour decreased. However, prolonged germination resulted in a reduction in fat content and high malt loss. Hence, 24 h germinated finger millet flour has excellent physicochemical properties. The current research study will encourage food industries to use 24 h germinated finger millet flours for the production of weaning foods.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adedeji, O. E., Oyinloye, O. D., & Ocheme, O. B. (2014). Effects of germination time on the functional properties of maize flour and the degree of gelatinization of its cookies. African Journal of Food Science, 8(1), 42–14. https://doi.org/10.5897/AJFS2013.1106
- Adesokan, I. A., Odetoyinbo, B. B., Ekanola, Y. A., Avanrenren, R. E., & Fakorede, S. (2011). Production of Nigerian nono using lactic starter cultures. Pakistan Journal of Nutrition, 10(3), 203–207. https://doi.org/10.3923/pjn.2011.203.207
- Agbor Asuk, A., Nnaemeka Ugwu, M., & Idole, B. (2020). The effect of different malting periods on the nutritional composition of malted sorghum-soy composite flour. Journal of Food Science and Nutrition Research, 3(3), 217–230. https://doi.org/10.26502/jfsnr.2642-11000051
- Amadou, I., Gounga, M. E., & Le, G. W. (2013). Millets: Nutritional composition, some health benefits and processing - A review. Emirates Journal of Food and Agriculture, 25(7), 501–508. https://doi.org/10.9755/ejfa.v25i7.12045
- Anjum, T., & Bajwa, R. (2005). Importance of germination indices in interpretation of allelochemical effects. International Journal of Agriculture and Biology, 7(1), 417–419. https://doi.org/10.1016/j.phytochem.2005.07.007
- AOAC. (2005). AOAC official method 941.15. Association of official analytical chemists. In P. Cunniff (Ed.), Association of official analytical chemists, Gaithersburg, USA.
- Auta, Y. I., Hadi, A. S., & Ismail, M. B. (2014). Malting duration on yield and composition of fura produced with the grains of Pennisetum typhoides. Journal of Food Science and Quality Management, 27(1990), 33–39. http://repository.futminna.edu.ng:8080/jspui/handle/123456789/7433
- Banusha, S., & Vasantharuba, S. (2013). Effect of malting on nutritional contents of finger millet and mung bean. American-Eurasian Journal of Agriculture and Environmental Science, 13(12), 1642–1646. https://doi.org/10.5829/idosi.aejaes.2013.13.12.12285.
- Chandra, D., Chandra, S., Pallavi, & Sharma, A. K. (2016). Review of finger millet (Eleusine coracana (L.) Gaertn): A power house of health benefiting nutrients. Food Science and Human Wellness, 5(3), 149–155. https://doi.org/10.1016/j.fshw.2016.05.004
- Derbew, H., & Moges, D. (2017). Effect of germination duration on nutritional and functional properties of sorghum (Sorghum bicolor): The case of Girana and Miskr varieties. Ethiopian Journal of Science and Technology, 10(3), 165–180. https://doi.org/10.4314/ejst.v10i3.2
- Devi, P. B., Vijayabharathi, R., Sathyabama, S., Malleshi, N. G., & Priyadarisini, V. B. (2014). Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. Journal of Food Science and Technology, 51(6), 1021–1040. https://doi.org/10.1007/s13197-011-0584-9
- Dida Bulbula, D., & Urga, K. (2018). Study on the effect of traditional processing methods on nutritional composition and anti nutritional factors in chickpea (Cicer arietinum). Cogent Food & Agriculture, 4(1), 1422370. https://doi.org/10.1080/23311932.2017.1422370
- Gebreyohannes, A., Shimelis, H., Laing, M., Mathew, I., Odeny, D. A., & Ojulong, H. (2021). Finger millet production in Ethiopia: Opportunities, problem diagnosis, key challenges and recommendations for breeding. Sustainability, 13(23), 13463. https://doi.org/10.3390/su132313463
- Harding, S. (2012). Effect of gamma rays on seed germination, seedling height, survival percentage and tiller production in some rice varieties cultivated in Sierra Leone. American Journal of Experimental Agriculture, 2(2), 247–255. https://doi.org/10.9734/AJEA/2012/820
- Hejazi, S. N., & Orsat, V. (2016). Malting process optimization for protein digestibility enhancement in finger millet grain. Journal of Food Science and Technology, 53(4), 1929–1938. https://doi.org/10.1007/s13197-016-2188-x
- Helen, W., & Shimelis, A. (2017). Effect of processing on phytonutrient and nutrient composition of finger millet. Ethiopian Journal of Crop Science, 8, 117–136.
- Kumar, Y., Sharanagat, V. S., Singh, L., & Mani, S. (2020). Effect of germination and roasting on the proximate composition, total phenolics, and functional properties of black chickpea (Cicer arietinum). Legume Science, 2(1), 1–7. https://doi.org/10.1002/leg3.20
- Kumar, A., Kaur, A., Gupta, K., Gat, Y., & Kumar, V. (2021). Assessment of germination time of finger millet for value addition in functional foods. Current Science, 120(2), 406–413. https://doi.org/10.18520/cs/v120/i2/406-413
- Li, C., Jeong, D., Lee, J. H., & Chung, H. J. (2020). Influence of germination on physicochemical properties of flours from brown rice, oat, sorghum, and millet. Food Science and Biotechnology, 29(9), 1223–1231. https://doi.org/10.1007/s10068-020-00770-2
- Malleshi, N. G., Desikachar, H. S. R., & Tharanathan, R. N. (1986). Physico‐chemical properties of native and malted finger millet, pearl millet and foxtail millet Starches. Starch - Stärke, 38(6), 202–205. https://doi.org/10.1002/star.19860380608
- Manzi, P., Marconi, S., Aguzzi, A., & Pizzoferrato, L. (2004). Commercial mushrooms: Nutritional quality and effect of cooking. Food Chemistry, 84(2), 201–206. https://doi.org/10.1016/S0308-8146(03)00202-4
- Mouquet-Rivier, C., Icard-Vernière, C., Guyot, J. P., Hassane Tou, E., Rochette, I., & Trêche, S. (2008). Consumption pattern, biochemical composition and nutritional value of fermented pearl millet gruels in Burkina Faso. International Journal of Food Sciences and Nutrition, 59(7–8), 716–729. https://doi.org/10.1080/09637480802206389
- Nakarani, U. M., Singh, D., Suthar, K. P., Karmakar, N., Faldu, P., & Patil, H. E. (2021). Nutritional and phytochemical profiling of nutracereal finger millet (Eleusine coracana L.) genotypes. Food Chemistry, 341, 128271. https://doi.org/10.1016/j.foodchem.2020.128271
- Nefale, F. E., & Mashau, M. E. (2018). Effect of germination period on the physicochemical, functional and sensory properties of finger millet flour and porridge. Asian Journal of Applied Sciences, 6(5), 360–367. https://doi.org/10.24203/ajas.v6i5.5466
- Nirmala, M., Rao, M. S., & Muralikrishna, G. (2000). Carbohydrates and their degrading enzymes from native and malted finger millet (Eleusine coracana). Food Chemistry, 69(2), 175–180. https://doi.org/10.1016/S0308-8146(99)00250-2
- Nkhata, S. G., Ayua, E., Kamau, E. H., & Shingiro, J. B. (2018). Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Science & Nutrition, 6(8), 2446–2458. https://doi.org/10.1002/fsn3.846
- Obadina, A. O., Arogbokun, C. A., Soares, A. O., de Carvalho, C. W. P., Barboza, H. T., & Adekoya, I. O. (2017). Changes in nutritional and physico-chemical properties of pearl millet (Pennisetum glaucum) Ex-Borno variety flour as a result of malting. Journal of Food Science and Technology, 54(13), 4442–4451. https://doi.org/10.1007/s13197-017-2922-z
- Ocheme, O. B., & Chinma, C. E. (2008). Effects of soaking and germination on some physicochemical properties of millet flour for porridge production. Journal of Food Technology, 6(5), 185–188.
- Ogori, A. F., Jatua, M. K., Apeh, M. O., & Adamu, L. (2013). Chemical and functional characteristics of flours from blends of millet grain flour from distilled water soaking and malting (Pennisetum glaucum). International Research Journal of Food Science and Technology, 1(1), 1–7.
- Onwurafor, E. U., Uzodinma, E. O., Uchegbu, N. N., Ani, J. C., Umunnakwe, I. L., & Ziegler, G. (2020). Effect of malting periods on the nutrient composition, antinutrient content and pasting properties of mungbean flour. Agricultural Science, 19(1), 18–24.
- Opole, R. A. (2019). Opportunities for enhancing production, utilization and marketing of finger millet in Africa. African Journal of Food, Agriculture, Nutrition and Development, 19(1), 13863–13882. https://doi.org/10.18697/ajfand.84.BLFB1004
- Owheruo, J. O., Ifesan, B. O. T., & Kolawole, A. O. (2019). Physicochemical properties of malted finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Science and Nutrition, 7(2), 476–482. https://doi.org/10.1002/fsn3.816
- Pushparaj, F. S., & Urooj, A. (2011). Influence of processing on dietary fiber, tannin and in vitro protein digestibility of pearl millet. Food and Nutrition Sciences, 2(8), 895–900. https://doi.org/10.4236/fns.2011.28122
- Ramashia, S. E., Gwata, E. T., Meddows-Taylor, S., Anyasi, T. A., & Jideani, A. I. O. (2018). Some physical and functional properties of finger millet (Eleusine coracana) obtained in sub-Saharan Africa. Food Research International, 104, 110–118. https://doi.org/10.1016/j.foodres.2017.09.065
- Ramashia, S. E., Anyasi, T. A., Gwata, E. T., Meddows-Taylor, S., & Jideani, A. I. O. (2019). Processing, nutritional composition and health benefits of finger millet in sub-Saharan Africa. Food Science and Technology, 39(2), 253–266. https://doi.org/10.1590/fst.25017
- Rathore, T., Singh, R., Kamble, D. B., Upadhyay, A., & Thangalakshmi, S. (2019). Review on finger millet: Processing and value addition. The Pharma Innovation Journal, 8(4), 283–291.
- Semahegn, Z., Teressa, T., & Bejiga, T. (2021). Finger millet [Eleusinecoracana (L) Gaertn] breeding in Ethiopia : A review article. International Journal of Research Studies in Agricultural Sciences, 7, 38–42.
- Senhofa, S., Kince, T., Galoburda, R., Cinkmanis, I., Sabovics, M., & Sturite, I. (2016). Effects of germination on chemical composition of hull -less spring cereals. Research for Rural Development, 1, 91–97.
- Simion, T., Markos, S., & Samuel, T. (2020). Evaluation of finger millet (Eleusine coracana (L). Gaertn.) varieties for grain yield in lowland areas of southern Ethiopia. Cogent Food & Agriculture, 6(1), 1788895. https://doi.org/10.1080/23311932.2020.1788895
- Swami, S. B., Thakor, N. J., & Gurav, H. S. (2013). Effect of soaking and malting on finger millet (EleusineCoracana) grain. Agricultural Engineering International: CIGR Journal, 15(1), 194–200.
- Udeh, H. O., Duodu, K. G., & Jideani, A. I. O. (2018). Effect of malting period on physicochemical properties, minerals, and phytic acid of finger millet (Eleusine coracana) flour varieties. Food Science and Nutrition, 6(7), 1858–1869. https://doi.org/10.1002/fsn3.696
- Zewdu, A., Gemechu, F., & Babu, M. (2018). Pre-Scaling up of improved finger millet technologies: The case of daro lebu and habro districts of west hararghe zone, oromia national regional state, Ethiopia. Journal of Agricultural Education and Extension, 4(2), 131–139.