?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Varroa mite is one of the pests parasitizing on honey bees inflicting substantial effects on beekeeping subsector worldwide. Since its first report in Ethiopia in 2015, the mite has got distributed to various locations of the country. The purpose of the study was to determine the prevalence, infestation levels and risk factors of varroa mites in South-western Region, Ethiopia. A total of five districts purposively selected based on their potentialities for beekeeping and accessibilities for data collection. Data collection was undertaken during wet and dry seasons from 384 colonies, mite prevalence and infestation data on adult bees and in brood collected following standard protocols. The overall prevalence rate of mite in the area was recorded as 73.81% and 48.44% with higher prevalence rates, 83.33% (p < 0.01) and 54.95% (p < 0.001) during wet seasons for apiary and colony levels respectively. The infestation level (Mean ± SE) of colonies on adults and broods were recorded as 1.367 ± 0.080 versus 1.819 ± 0.095 during dry season, and 2.481 ± 0.151 versus 3.299 ± 0.194 during wet seasons. The Multivariate logistic analysis indicated that agro ecology, season, hive placement sites, and colony status were determinants for its prevalence. Similarly, agro ecology, season, colony management and colony status were determinants for the infestation levels of varroa mites. Detailed investigation on its effect on honey yield, impacts of possible colony management options in minimizing its prevalence and infestation level needs follow up studies.
PUBLIC INTEREST STATEMENT
Beekeeping plays important roles in the livelihoods of huge populations in Ethiopia in general and in the study areas in particular. However, the occurrence of various pests’ attacks is listed as among the major bottlenecks hindering optimization of benefits from the subsector. Because of its widespread distribution and lack of cares, the varroa mite has becoming among the major pest since its first occurrences in 2015ʹs in most parts of the country. However, in relation to the remoteness and lack of any study undertaken so far, clear information about the prevalence and awareness about varroa mite is lacking in the study areas. Hence, the current study was aimed with identifying the prevalence and infestation levels, the magnitudes of distribution, and potential risk factors of the pest, which provides essential information for beekeepers and other stakeholders to undertake possible mitigation options to minimize its effects and pave ways for further investigations.
1. Introduction
The varroa mite is one of the pests parasitizing honey bees’ brood and adult stages, feeding on their hemolymph and fat tissues (Bernandi & Venturino, Citation2016; Ramsey et al., Citation2019) causing varroosis. There are four species: Varroa destructor, V. jacobsoni, V. rindereri and V. under woodi have been identified worldwide (Conte et al., Citation2020; Dietemann et al., Citation2013; Mondet et al., Citation2014; Rosenkranz et al., Citation2010). Of these, Varroa destructor is the most widely distributed worldwide, inflicting serious health consequences on honey bees (Fazier et al., Citation2010; Galindo-Cardona et al., Citation2020; Hristov et al., Citation2020; Locke, Citation2016; Mondet et al., Citation2014), and the species reported to be existing in Ethiopia so far (Dessalegn et al., Citation2016).
Despite its parasitic effects, varroa mite also serves as a major host for various pathogens like acute bee paralysis virus (ABPV), deformed wing virus (DWV), black queen cell virus (BQCV) and Sac brood bee virus (SBBV) (Muli et al., Citation2014; Mondet et al., Citation2014; Bernandi & Venturino, Citation2016; Mendoza et al., Citation2020; Locke et al., Citation2021; Truong et al., Citation2022)Citation2020. It has also been reported to be a host for bacterial and fungal infections (Ball, Citation1997; Gliński & Jarosz, Citation1992; Hubert et al., Citation2017). Hence, the parasitic effects allied with its hosting effects for various pathogens on honey bees, varroa mite becomes one of major causes for huge colony losses worldwide (Highfield et al., Citation2009; Pirk et al., Citation2016; Chantawannakul et al., Citation2016; Martin & Brettell, Citation2019; Hristov et al., Citation2020; Bahreini et al., Citation2021). Furthermore, bee products quality losses due to residual effects of acaricides(chemicals) used in control of the mite becoming the major concern; especially in countries with higher infestation (Abd El-Wahab et al., Citation2021; Bahreini et al., Citation2020; Qadir et al., Citation2021).
The first occurrence of varroa mite was reported in Eastern honey bees, Apis cerana (Jack & Ellis, Citation2021; Rosenkranz et al., Citation2010), which then jumped host over to the Western honeybees (Apis mellifera) in the 1970ʹs (Dietemann et al., Citation2019; Roth et al., Citation2020). The first occurrence of varroa mite in Africa (South Africa) was reported in 1997 (Allsopp et al., Citation1997); subsequently in Eastern Africa in 2009 (Fazier et al., Citation2010). In Ethiopia, the first occurrence of varroa mite was reported in 2015 (Dessalegne, Citation2015). Nonetheless, it has got a wide range of distribution in the continent, whose occurrence was reported in various countries like Angola, Kenya, Morocco, Egypt, Senegal, Niger, Nigeria, and son on (Pirk et al., Citation2016).
In relation to the very suitable natural resources available in the South-Western region of the country, beekeeping plays crucial economic roles for huge communities in the areas. However, the currently obtained benefits from the subsector are very minimum compared to the existing potentials. Though various factors are listed contributing to it, existence of pests, predators, and diseases are listed as the major ones. So far, though clear evidences on the effects of pathogens is lacking, pests’ and predators’ attacks take the lead in devastating honey bees and their products.
Due to its difficulties for identification allied with lack of detailed investigations on it, the general awareness about varroa mite, its existence, and possible prevention mechanisms are not clearly recognized by most beekeepers and other stakeholders in the areas. Hence, the study was aimed with investigating the prevalence of varroa and its potential risk factors, determining its distribution and infestation levels on honey bees (Apis mellifera scutellata L.) in focus with selected areas of the South-Western Region of Ethiopia.
2. Materials and methods
2.1. Description of the study areas and site selection
The study was conducted in five selected districts of the South-Western Region, Ethiopia (the newly delineated region, not appeared on google maps); namely Gimbo, Gesha, and Chena, Semien Benchi and Debub Benchi districts (). Purposive sampling method was used both for selection of study districts and peasant associations (PAs) based on their beekeeping potentials, agroecology, and accessibility for data collection. Accordingly, three PAs were selected from each district, and a total of fifteen peasant associations considered for the study.
2.2. Sampling and data collection
The number of honey bee colonies required for the study was determined using random sampling procedures at 50% expected prevalence with 95% confidence interval and 5% absolute precision, using Thrusfield (Citation2005) formulas (1).
Where n: required sample size; Pexp: expected prevalence (50%); d: desired absolute precision (5%).
Hence, a total of 384 colonies were considered, which were systematically sampled from a total of 84 apiaries, considering the possible factors related to colony conditions, environmental conditions, and beekeeper-related variabilities into account. A total of 10 to 65 colonies per each peasant association (PA) and a minimum of 3 colonies per apiary were inspected for sample collection. Number of sampled apiary sites per each district were 20,16, 14,19, and 15 from Gesha, Gimbo, Semein Benchi, Chena, and Debub Benchi encompassing 80, 95, 37,120, and 52 colonies respectively. Sample collection and examination were done both during the dry (December to February) and wet (May-July) seasons of the year 2021. In terms of forage (pollen and nectar) availabilities, the first season is considered a dearth and the latter as a major active season. Available data were collected both from adult bees and brood combs cells using Dietemann et al. (Citation2013) standard protocols. Accordingly, approximately 300–400 adult bees were taken from brood combs; similarly, brood combs containing about 300–400 sealed cells of either drone or worker broods were taken as a sample from each colony. The sampled brood and adult bees were coded and put in sealable containers till examination.
The count of phoretic mites on adult bees was performed after dislodging the mite by vigorously shaking the bees in a 70%-ethyl alcohol mixed with 10 ml of 1% detergent-water solution in a flask tube for about 5 minutes, then the shacked bees were filtered on a mesh sized 3–4 mm, which is small enough to hold the bees but large enough to let mites through, and washing. Then the solution with mites was let pass through a fine gauze with pores of smaller enough sizes (<0.5 mm) to hold the mites back (Dietemann et al., Citation2013); then the mites were counted on the sieves carefully or by turning them down on to white papers.
Examination of mites on sampled broods was done by opening the cell capes and carefully removing the larvae from the cell using a pin, and keenly observing the mites in each opened cells and on larvae bodies. Hence, infestation level entailing how the severity a colony be infected with the pest which could be determined via comparing mite counts with adult bees or broods examined multiplied with hundred (2)
3.3. Data management and analysis
The collected data were recorded in Microsoft Excel sheets and analyzed using STATA-15 software. Possible analytical tools such as descriptive statistics of epidemiology, ANOVA, Odds ratio were used. Prior of modelling, the auto-collinearity checkup of variables was undertaken, and one of variables having strong collinearity scores (≤-0.6 and ≥ 0.6) were excluded from the model to avoid multicollinearity effects on dependent variable and variables having p < 0.25 under univariate analysis were incorporated for multivariate analysis. Accordingly, out of ten variables considered in the data sets, seven of them were considered for the model.
3. Results
3.1. Prevalence of varroa mite
The overall prevalence of varroa mite at apiary and colony levels in the areas were found to be 73.21% and 48.44% respectively. The higher distribution level, 83.33% (p < 0.01), and prevalence rates, 54.95% (p < 0.001) occurs during wet seasons (Table ). The current result revealed relatively less prevalence level of the mite obtained compared to most previous studies conducted in Central, Western and Northern parts of the country, which mostly ranging from 80 to 100% of prevalence (Dessalegne, Citation2015; Mengistu et al., Citation2016; Mezgabu et al., Citation2016).
Table 1. Varroa prevalence rates of the areas
3.2. Odds ratio of risk factors of varroa prevalence
The Multivariate logistic regression analysis of risk factors revealed that agro ecology, season, hive placement sites, and colony status were determinants for the prevalence of varroa mite (Table ).
Table 2. Odds ratio of risk factors for varroa prevalence
Accordingly, significantly less prevalence was recorded (at p < 0.001) in lowlands compared to midland and highland areas which was lower by about 67.3% than highland areas (Table ). However, no significant variation between midland and high land areas. Higher prevalence rate (at p < 0.001) was also recorded during wet seasons than dry seasons by about 174.6% (Table ). There was significant variation on mite prevalence based on hive placement sites. Accordingly, colonies placed in forests were found with less prevalence rates compared to those placed at back yard and apiary sites (at p < 0.001). However, there is no significant variation between hives placed at backyards and apiary sites. The status of colony strength has a significant effect on the prevalence rates of mites being significantly less prevalence at p < 0.001 recorded in weak colonies compared to stronger ones (Table ).
3.3. Infestation level by season
The infestation level of a colony indicates the number of mites in a colony (proportion of mites per number of examined bees or broods), which is an indicator for determining whether the infection has reached its threshold (economically injurious) level or not (Floris et al., Citation2020), usually expressed in percentages.
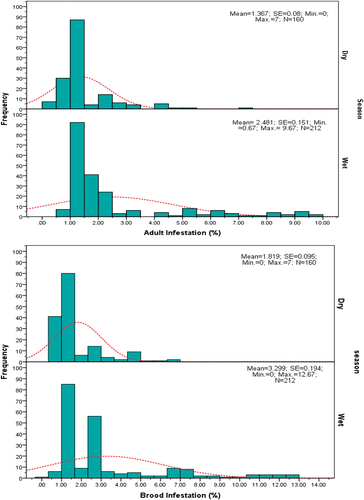
The infestation level (Mean ± SE) of varroa mite on adult bees was recorded as 1.367 ± 0.080 (dry season), and 2.481 ± 0.151 (wet season), ranging from 0% to 9.67%. Whereas, 1.819 ± 0.095 (dry season) and 3.299 ± 0.194 (wet season) in brood cells, ranging from 0% to 12.67% (Figure ). The result indicated a significantly higher infestation level at p < 0.001 during wet seasons in both parameters.
3.4. Relationship of infestation levels (adult and brood stages)
The infestation level on adult bees has a strong relationship, as shown in Table , with a positive correlation coefficient of 0.979 (p < 0.001). This revealed that a change in one of these variables will most likely result in a change in the other variable, with similar trends and directions. Similarly, they have a strong regression relationship at p < 0.001. Though each variable has no sense of dependence one on another, they have a significant regression coefficient. Accordingly, for instance, if we consider the infestation level of adult bees to estimate the infestation level in brood cells, it was noted that the infestation level in brood cells has a regression coefficient of 1.254 and a constant/intercept of 0.153 with standard errors of 0.013 and 0.037, respectively. In contrast, an estimate of adult infestation levels from brood infestation levels indicated that an adult infestation regresses with 0.765 and constant, −0.034, with standard errors of 0.008 and 0.029, respectively (Table ). According to Marin and Zlatko (Citation2020), the total varroa count in a colony might precisely predicted as a function of mite counts on adult bees, in brood cells, and naturally falling mites.
Table 3. Relationship of infestation levels
3.5. Infestation level by risk factors
The univariate analysis of infestation levels using the General Linear Model (GLM) indicated agro-ecology, season, colony status, and colony management were determinants for mite infestations both for broods and adults (Table ). The result revealed that number of mites in a colony attributing for infestation levels will be significantly determined by the listed these factors which majorly determining the its successful perpetuation in the hives in related with favorable environmental conditions, and brood availability which directly related with colony strengths. On the other hands colony managements like removal of empty combs and other debris in the hives might creating opportunities for removal of mite from staying longer hidden in combs; few beekeepers also reported practicing removing/reducing brood combs and smoking herbs during its’ sever occurrences. Similarly, Hillayova et al., (Citation2022), proved that apt colony management practices found significantly reducing their infestations by mite as it influencing the number of varroa mites failing off of the bees.
Table 4. Mean separation of varroa infestation
N: Number of colonies with +ve cases of varroa; SE- Standard Error; Min.: Minimum; Max.: Maximum; values with different superscripts indicate significant variation of infestation levels (mean) at P<0.05.
4. Discussions
Due to its biological characteristics, the prevalence of varroa mite has been reported to be strongly positively correlated (at p < 0.001) with elevations (Muli et al., Citation2014), whereas it is negatively correlated with hot and dry conditions (Maggi et al., Citation2016). The higher prevalence of mites in colonies kept at back yards and apiaries are more likely exposed to contamination of healthy colonies with diseased ones due to drifting, robing, and other means like feeding as a relatively greater number of colonies are placed at a closer distance (Aubert et al., Citation2008; Bordier et al., Citation2017). Contamination of colonies via sharing resources (FAO (Food and Agriculture Organization), Citation2018) and introducing new colonies to apiaries from unknown sources without any precaution measures can also hasten mite infections (Dessalegne, Citation2015; Namayanja et al., Citation2016). As noted from beekeepers’ responses, most of them are placing colonies in close proximity to each other, basically to ease protection against pest attacks, mainly ants, the major grievance for beekeeping, resulting in huge economic devastation on the beekeeping subsector (Dereje et al., Citation2020; Nuru et al., Citation2014; Tesfu & Dawit, Citation2021). Perhaps some beekeepers also reported having a practice of exchanging resources (brood, pollen, and honey combs) among colonies without any precautions in order to maintain their strength and sometimes during splitting for queen rearing purposes. Similarly, Haftom et al. (Citation2019) and Mancuso et al. (Citation2020) also reported that physical contacts of colonies mediated by beekeepers are among the major roots of disease and pest transmission that most beekeepers are less concerned about. Though the varroa mite parasitizes both broods and adults, the availability of broods is critical for its perpetuation because this is where an adult female mite lays her eggs and phoretic mites develop fully (Gregorc & Sampson, Citation2019; Conte et al., Citation2020; Underwood & Lopez-Uribe, Citation2022). In this regard, the amount of brood in a colony is directly related to the status of its strength (existence of a prolific queen and a huge number of foragers and drones) which is highly determined by availabilities of ample forages source (El-Niweiri & El- Sarrag, Citation2006).
Forest-placed colonies are commonly in traditional hives, seasonally hung to catch swarms, usually certain days/months before the onset of major honey flow seasons, and a complete removal of all resources (honey, brood, and pollen) is done during harvesting. This probably creates unfavorable conditions for the phoretic mite in accessing broods for prolonged periods of time to reproduce itself. In addition, colonies are relatively placed dispersedly, making them less prone to contamination via drifting and robbing. Hence, these might be the possible reasons for its less prevalence of mites in colonies placed in forests compared to those placed in backyards and apiary sites. Due to the high tolerance and coexistence of Africanized bees with disease and pest attacks, it is usually not an easy task to extrapolate the exact effect of each pathogen or pest on bees and their products only from simple survey results.
Similarly, Curie (Citation2008) and Paray and Gupta (Citation2017) stated that there is an inconsistency in determining the minimum economic threshold infestation levels of varroa mites due to season and multiple infection effects. However, with these facts, mostly colonies with 2% infestation level under ethyl alcohol wash tests are more likely considered to be reaching minimum threshold levels (Jack & Ellis, Citation2021; Spivak & Reuter, Citation2016). With this into account, from the current study, about 36(22.5%) and 61(38.13%) colonies were identified as reaching over 2% infestation levels during the dry and wet seasons, respectively (Figure ).
The overall infestation level of colonies with varroa in the current study, 1.924 found to be similar with the report in Kenya of same species (Apis mellifera scutellata) of adult stages reported to be 3.67% (Fazier et al., Citation2010). Similarly, nearly equivalent results, 2.67% infestation levels were reported in a study conducted at Toke-kutaye district of West Shoa zone of Oromia Region (Mengistu et al., Citation2016). The highest infestation level, 15.73% (on adult bees) and 18.07% (in broods), reported in Central part of the country, Walmara district (Mezgabu et al., Citation2016). Similarly, higher prevalence, 82% with 15% infestation level (on adults) was recorded in Northern parts of the country, Tigray Region. The higher prevalence and infestation in these areas might be resulted from environmental conditions, colony management practices, and honey bee race differences; being Apis mellifera bandansi and A.m. monticola races in Central and Northern parts of the country respectively, which are characterized with less hygienic behaviors compared to Apis mellifera scutellata race.
The mean comparison (ANOVA) test of adult with brood infestation indicated that the mean adult infestation level of colonies was significantly lower than that of broods at p < 0.05 during both seasons. Similarly, a strong correlation, but no significant variation of infestation levels between brood and adult stages (at p < 0.05%) reported in a study conducted in Sudan (El-Niweiri & El- Sarrag, Citation2006).
Studies in the Tigray region indicated a lower infestation level was recorded during the dry season than during the wet season (Haftom et al., Citation2019). According to Muli et al. (Citation2014) and (Chemurot et al., Citation2016), the infestation level of varroa mite has a strong correlation with colony strength (at p < 0.013), elevation (at p < 0.001). Similarly, Dessalegn et al. (Citation2016) also reported that the number of varroa mites recovered from adults and broods is significantly positively correlated with colony strength, which is defined by the number of adult bees, brood areas, and nectar and pollen stores. Galindo-Cardona et al. (Citation2020) discovered that varroa mite infestation increases in areas with cool temperatures and higher humidity, where such environmental conditions are deterring the hygienic behavior of honey bees, which is inversely correlated with infestation levels (Masaquiza et al., Citation2021). Similarly, Van der Zee et al. (Citation2015); Rondeau et al. (Citation2019); Underwood and Lopez-Uribe (Citation2022); and Cauia and Cauia (Citation2022) also stipulated that application of apt mite prevention measures during the early part of winter, when low infestation occurs, is more appropriate to complement treatments during winter and falls. The determinants of varroa infestation can be classified into two broad categories: the first being environmental factors like climates, colony density, availability of forages and management; and secondly, the colony characteristics like population dynamics, flight activities, brood characteristics (attractiveness, stimuli, post-caping duration), hygienic and grooming behaviors (Mancuso et al., Citation2020; Nganso et al., Citation2017; Tsuruda et al., Citation2012). The resistance characteristics of honey bees to varroa mites are highly related to the types of races, being tropical (Africanized) honey bees are more resistant than temperate ones due to their peculiar hygienic and grooming behaviors (Mendoza et al., Citation2020; Pirk et al., Citation2016). Moreover, other mechanisms like shorter duration of brood emergency, the highly suppression efficiencies on mites’ reproductive successes, entombing of infested drone broods, are also considerable roles in lowering the infestation levels of tropical honey bees with varroa mite compared to the temperate ones (El-Niweiri & El- Sarrag, Citation2006; Mendoza et al., Citation2020; Conte et al., Citation2020). Nowadays, in related to increased drug resistance of mites, and its implications on product qualities, following a wholistic integrated pest management (IPM) options like selecting resistant colonies, brood break, using small cell combs, applying organic chemicals, spraying powdered sugar, and using screened bottom board (Conte et al., Citation2020; Gregorc & Sampson, Citation2019; Guichard1 et al., Citation2020; Jack & Ellis, Citation2021; Rondeau et al., Citation2019; De Souza et al., Citation2022; Tsuruda et al., Citation2012; Underwood & Currie, Citation2007; Underwood & Lopez-Uribe, Citation2022), disinfecting hives with woodenware materials, supplementing with protein and sugar substitutes (Giacobino et al., Citation2014) play crucial roles to minimize the infestation level of varroa mites below its economically injurious level. In this regard, the protection measures targeted at varroa are not practiced by most beekeepers due to awareness problems with its existence and adverse effects on honey bees.
5. Conclusions
Though the prevalence and infestation levels of the mite are found to be determined by seasons, altitudinal gradients, colony status, hive placement sites, and colony management, the overall infestation level of the mite in the areas is found to be lower to the threshold levels. However, with its current distribution trends, it may have a huge impact on honey bees in future unless possible mitigation options are undertaken. In this regard, its prevalence and possible effects on honey bees are less recognized by most beekeepers. As a result, raising awareness about varroa mites, identifying them, and developing possible prevention mechanisms such as following appropriate hive placements that minimize drifting and robing, selecting resistant colonies, using brood breaks, and avoiding contamination through resource exchange are critical to halting their full-scale spreading. A detailed investigation on possible economic losses caused by the parasite is paramount. The prevalence rates and infestation levels of the mite in relation to hygienic and grooming behaviors of colonies, types of mite species, and prevalence of varroa-hosted pathogens need follow-up studies.
The Author contribution Statement
Tesfu Shegaw, Asrat Arke: Conceived and Designed the study, data collection, prepared write ups.
Nahom Belay, Dawit Habte giorgis: Data analysis, Inputs preparation, sample examination, and supervision.
Acknowledgements
The Authors cordially acknowledge the Beekeepers, District and PA-experts, and others for their wise collaboration during data collections.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors

Tesfu Shegaw
Tesfu Shegaw is a researcher in South-Western Agricultural Research Institute (SWARI), Bonga Agricultural Research Center. He has been conducting researches on Apiculture, and has published various research findings in various peer reviewed journals and other Scientific publications.
Asrat Arke
Asrat Arke is a researcher in South Western Agricultural Research Institute (SWARI), Bonga Agricultural Research Center. He has been conducting researches under Animal Health project and has published various research articles related with Animal Health in various peer reviewed scientific forums.
Nahom Belay
Nahom Belay is a researcher in South-Western Agricultural Research Institute (SWARI), Bonga Agricultural Research Center. His research is mainly related with animal health and has published various researches articles in various peer reviewed journals.
Dawit Habte Giorgis
Dawit Habte Giorgis is A researcher in South-Western Agricultural Research Institute (SWARI), Bonga Agricultural Research Center. His research is related with Apiculture and has published a number of research papers in various peer reviewed journals
References
- Abd El-Wahab, T. E., Shalaby, S. E., Al-Kahtani, S. N., Al Naggar, Y., Jamal, Z. A., & Masry, S. H. (2021). Mode of application of acaricides against the ectoparasitic mite (Varroa destructor) infesting honeybee colonies, determines their efficiencies and residues in honey and beeswax. Journal of King Saud University-Science, 33(1), 101236. https://doi.org/10.1016/j.jksus.2020.101236
- Allsopp, M. H., Govan, V., & Davison, S. (1997). Bee health report: Varroa in South Africa. Bee World, 78(4), 171–14. https://doi.org/10.1080/0005772X.1997.11099361
- A-Tai Truong, Mi-Sun Yoo, Bo-Ram Yun, Jeong Eun Kang, Jinhyeong Noh, Tae Jun Hwang, Soo Kyoung Seo, Soon-Seek Yoon & Yun Sang Cho. (2022). Prevalence and pathogen detection of Varroa and Tropilaelaps mites in Apis mellifera (Hymenoptera, Apidae) apiaries in South Korea, Journal of Apicultural Research. https://doi.org/10.1080/00218839.2021.2013425
- Aubert, M., Ball, B., Fries, I., Moritz, R., Milani, N., & Bernardinelli, I. (2008). Virology and the Honeybee (pp. 462). Publications Office of the European Union.
- Bahreini, R., Nasr, M., Docherty, C., de Herdt, O., Muirhead, S., & Feindel, D. (2020). Evaluation of potential miticide toxicity to Varroa destructor and honey bees, Apis mellifera, under laboratory conditions. Scientific Reports, 10(1), 1–14. https://doi.org/10.1038/s41598-020-78561-2
- Bahreini, R., Nasr, M., Docherty, C., Feindel, D., Muirhead, S., de Herdt, O., & Vontas, J. (2021). New bioassay cage methodology for in vitro studies on Varroa destructor and Apis mellifera. PLoS ONE, 16(4), e0250594. https://doi.org/10.1371/journal
- Ball, B. V. (1997). Secondary infections and diseases associated with Varroa jacobsoni. In Opt. Méditerr (Vol. 21 (pp. 49–58). http://om.ciheam.org/om/pdf/c21/97605907.pdf
- Beaurepaire, A., Piot, N., Doublet, V., Antunez, K., Campbell, E., Chantawannakul, P., Chejanovsky, N., Gajda, A., Heerman, M., Panziera, D., Smagghe, G., Yañez, O., de Miranda, J. R., & Dalmon, A. (2020). Diversity and global distribution of viruses of the Western Honey Bee, Apis mellifera. Insects, 11(4), 239. https://doi.org/10.3390/insects11040239
- Bernandi, S., & Venturino, E. (2016). Viral epidemiology of the adult Apis mellifera infested by the Varroa destructor mite. Heliyon, 2(2016), e00101. https://doi.org/10.1016/j.heliyon.2016.e00101
- Bordier, C., Pioz, M., Crauser, D., Le Conte, Y., & Alaux, C. (2017). Should I stay or should I go: Honeybee drifting behaviour as a function of parasitism. Apidologie, 48, 286–297. https://doi.org/10.1007/s13592-016-0475-1
- Cauia, E., & Cauia, D. (2022). Improving the Varroa (Varroa destructor) control strategy by brood treatment with formic acid-A pilot study on spring applications. Insects, 13(2), 149. https://doi.org/10.3390/insects13020149
- Chantawannakul, P., de Guzman, Li, J., Williams, G. R., & de Guzman, L. I. (2016). Parasites, pathogens, and pests of honey bees in Asia. Journal of Apidologie, 47(3), 301–324. https://doi.org/10.1007/s13592-015-0407-5
- Chemurot, M., Akol, C., de Graaf, D. C., de Graaf, D. C., de Graaf, D. C., & de Graaf, D. C. (2016). Factors influencing the prevalence and infestation levels of Varroa destructor in honeybee colonies in two highland agro-ecological zones of Uganda. Experimental & Applied Acarology, 68(4), 497–508. https://doi.org/10.1007/s10493-016-0013-x
- Conte, Y. L., Meixner, M. D., Brandt, A., Carreck, N. L., Costa, C., Mondet, F., & Buchler, R. (2020). Review on geographical distribution and selection of European honey bees resistant to Varroa destructor. Journal of Insects (MDPI), 11(12), 873. https://doi.org/10.3390/insects11120873
- Curie. (2008). Economic threshold for Varroa on the Canadian prairies. In Canadian Association of Professional Apiculturists (pp. 2N2). University of Manitoba, Department of Entomology.
- Dereje, T., Melkam, A., Gezahegn, M., Ararsa, B., Amsalu, B., Esayas, M., & Yildiz, F. (2020). Improved beekeeping technology in Southwestern Ethiopia: Focus on beekeepers’ perception, adoption rate, and adoption determinants. Cogent Food and Agriculture, 6(1), 1814070. https://doi.org/10.1080/23311932.2020.1814070
- De Souza, A. P. F., Rodrigues, N. R., Fernández-Alba, A. R., & Reyes, F. G. R. (2022). Occurrence of pesticide residues in Brazilian Apis mellifera beeswax by gas chromatography-tandem mass spectrometry and pesticide hazard evaluation. Journal of Apicultural Research, 1–7. https://doi.org/10.1080/00218839.2022.2043228
- Dessalegn, B., Alemayehu, G., Taye, N., & Amssalu, B. (2016). Identifying the species, effects and seasonal dynamics of honeybee varroa mites: A newly emerging parasite to Ethiopian honeybee. International Journal of Toxicology and Environmental Science, 1(1), 4. https://doi.org/10.15226/2572-3162/2/1/00102
- Dessalegne, B. (2015). Occurrences and distributions of honeybee (Apis mellifera Jemenetica) varroa mite (Varroa destructor) in Tigray Region, Ethiopia. Journal of Fishery and Livestock. Production, 2(3). https://doi.org/10.4172/2332-2608.1000126
- Dietemann, V., Beaurepaire, A., Page, P., Yañez, O., Buawangpong, N., Chantawannakul, P., & Neumann, P. (2019). Population genetics of ectoparasitic mites Varroa spp. in Eastern and Western honey bees. Parasitology, 146(11), 1429–1439. https://doi.org/10.1017/S003118201900091X
- Dietemann, V., Nazzi, F., Martin, S. J., Anderson, D. L., Locke, B., Delaplane, K. S., Wauquiez, Q., Tannahil, C., Frey, E., Ziegelmann, B., Rosenkranz, P., & Ellis, J. L. (2013). Standard methods for varroa research. Journal Apiculture Research, 52(1), 1–54. https://doi.org/10.3896/IBRA.1.52.1.09
- El-Niweiri, M. A. A., & El- Sarrag, M. S. A. (2006). Detection of the parasitic mite (Varroa jacobsoni) of honey bees (Apis mellifera) in Sudan. Albuhuth, 10(1), 61–76.
- FAO (Food and Agriculture Organization). (2018). Good Beekeeping practices. Food and Agriculture Organization of the United Nations. http://teca.fao.org
- Fazier, M., Muli, E., Conklin, T., Schmehl, D., Torto, B., Frazier, J., Tumlinson, J., Evans, J. D., & Raina, S. (2010). A scientific note on Varroa destructor found in East Africa; threat or opportunity? Apidology, 41(4), 463–465. https://doi.org/10.1051/apido/2009073
- Floris, I., Pusceddu, M., & Satta, A. (2020). How the infestation level of Varroa destructor affects the distribution pattern of multi-infested cells in worker brood of Apis mellifera. Journal of Veterinary Science, 7(3), 136. https://doi.org/10.3390/vetsci7030136
- Galindo-Cardona, A., Scannapieco, A. C., Russo, R., Escalante, K., Geria, M., Lepori, N., Monmany-Garzia, A. C., Muntaabski, I., Liendo, M. C., Landi, L., Giray, T., & Monmany-Garzia, A. C. (2020). Varroa destructor parasitism and genetic variability at honey bee (Apis mellifera) drone congregation areas and their associations with environmental variables in Argentina. Frontiers in Ecology and Evolution, 8, 590345. https://doi.org/10.3389/fevo.2020.590345
- Giacobino, A., Cagnolo, N. B., Merke, J., Orellano, E., Bertozzi, E., Masciangelo, G., Pietronave, H., Salto, C., & Signorini, M. (2014). Risk factors associated with the presence of Varroa destructor in honey bee colonies from east-central Argentina. Journal of Preventive Veterinary Medicine, 115(3–4), 280–287. http://dx.doi.org/10.1016/j.prevetmed.2014.04.002
- Gliński, Z., & Jarosz, J. (1992). Varroa jacobsoni as a carrier of bacterial infections to a recipient bee host. Apidologie, 23(1), 25–31. https://doi.org/10.1051/apido:19920103
- Gregorc, A., & Sampson, B. (2019). A review on: diagnosis of Varroa mite (Varroa destructor) andSustainable Control in Honey Bee (Apis mellifera) Colonies. Journal of Diversity, 11(12), 243. https://doi.org/10.3390/11120243
- Guichard, M., Dietemann, V., Neuditschko, M., & Dainat, B. (2020). A review on: Advances and perspectives in selecting resistance traits against the parasitic mite Varroa destructor in honey bees. Journal of Genetics Selection Evolution (GSE), 52, 71. https://doi.org/10.1186/s12711-020-00591-1
- Haftom, G., Amssalu, B., Smet, L. D., De Graaf, D. C., & Lihoreau, M. (2019). Factors restraining the population growth of Varroa destructor in Ethiopian honey bees (Apis mellifera simensis). PLoS ONE, 14(9), e0223236. pone.0223236. https://doi.org/10.1371/journal
- Highfield, A. C., Nagar, A. E., Mackinder, L. C. M., Noe, L. M., Hall, M. J., Martin, S. J., & Schroeder, D. C. (2009). Deformed wing virus implicated in Overwintering Honey bee Colony Losses. Applied and Environmental Microbiology, 75(22), 7212–7220. https://doi.org/10.1128/AEM.02227-09
- Hillayova, M.K., Korený, L., and Skvarenina, J.(2022). The local environmental factors impact the infestation of bee colonies by mite Varroa destructor. Journal oof Ecological Indicators, 141,109104. https://doi.org/10.1016/j.ecolind.2022.109104
- Hristov, P., Shumkova, R., Palova, N., & Neov, B. (2020). Factors associated with honey bee colony losses: A mini-review. Journal of Veterinary Science, 7(4), 166. https://doi.org/10.3390/vetsci7040166
- Hubert, J., Bicianova, M., Ledvinka, O., Kamler, M., Lester, P. J., Nesvorna, M., Erban, T., & Erban, T. (2017). Changes in the bacteriome of honey bees associated with the parasite Varroa destructor, and pathogens Nosema and Lotmaria passim. Journal of Microbial Ecology, 73(3), 685–698. https://doi.org/10.1007/s00248-016-0869-7
- Jack, C. J., & Ellis, J. D. (2021). Integrated pest management control of Varroa destructor (Acari: Varroidae), the most damaging pest of (Apis mellifera L. (Hymenoptera: Apidae)) Colonies. Journal of Insect Science, 21(5), 6, 1–32. https://doi.org/10.1093/jisesa/ieab058
- Locke, B. (2016). Natural Varroa mite-surviving Apis mellifera honey bee populations. Apidologie, 47(3), 467–482. https://doi.org/10.1007/s13592-015-0412-8
- Locke, B., Thaduri, S.,Stephan, J.G., Low, M.,Blacquière, T., Dahle, B., Le Conte, Y., Neumann, P., and de Miranda, J.R.(2021). Adapted tolerance to virus infections in four geographically distinct Varroa destructor resistant honeybee populations. Scientific Reports, 11(1), 1–12.
- Maggi, M., Antúnez, K., Invernizzi, C., Aldea, P., Vargas, M., Negri, P., Brasesco, C., De Jong, D., Message, D., Weinstein, E., Principal, J., Barrios, C., Ruffinengo, S., Da Silva, R. R., & Eguara, M. (2016). Honey bee health in South America. Apidologie, 47(6), 835–854. https://doi.org/10.1007/s13592-016-0445-7
- Mancuso, T., Croce, L., & Vercelli, M. (2020). Total brood removal and other biotechniques for the sustainable control of varroa mites in honey bee colonies: Economic impact in beekeeping farm case studies in Northwestern Italy. Journal of Sustainability, 12(6), 2302. https://doi.org/10.3390/su12062302
- Marin, K., & Zlatko, P. (2020). Prediction of total number of Varroa destructor mites in the honey bee (Apis mellifera) colony in late summer. The 13th International scientific/professional conference, Faculty of Agro-biotechnical Sciences Osijek, Josipa Juraj Strossmayer University of Osijek,Osijek, Croatia. https://www.researchgate.net/publication/354544607
- Martin, S. J., & Brettell, L. E. (2019). Deformed wing virus in honey bees and other insects: Annual review of virology. 2019(6), 49–69. https://doi.org/10.1146/annurev-virology-092818-015700
- Masaquiza, D., Vargas, J., Ortíz, N., Salazar, R., Curbelo, L., Pérez, A., & Arenal, A. (2021). Hygienic,Behavior of Apis mellifera and Its Relationship with Varroa destructor. Infestation and Honey Production in the Central Highlands of Ecuador. Insects, 12, 966. https://doi.org/10.3390/insects12110966
- Mendoza, Y., Tomasco, I. H., Antúnez, K., Castelli, L., Branchiccela, B., Santos, E., & Invernizzi, C. (2020). Unraveling honey bee–Varroa destructor interaction: Multiple factors involved in differential resistance between two Uruguayan populations. Journal of Veterinary Science, 7, 116. https://doi.org/10.3390/vetsci7030116
- Mengistu, S., Kebede, Y., & Begna, D. (2016). Major honeybee health problem with particular emphasis to Anti-Varroa Investigation of Propolis in Toke-Kutaye District, Ethiopia. American-Eurasian Journal of Scientific Research, 11(5), 320–331. https://doi.org/10.5829/idosi.aejsr.2016.11.5.10418
- Mezgabu, E., Hirpa, E., Begna, D., Yimer, L., Bayan, A., & Chali, M. (2016). Occurrence and distribution of Varroa mite and antivarroa effect of propolis in Walmara District of Oromia special zone around Finfine, Ethiopia. Journal of Veterinary Science & Technology, 7(5), 370. https://doi.org/10.4172/2157-7579.1000370
- Mondet, F., de Miranda, J. R., Kretzschmar, A., Le Conte, Y., Mercer, A. R., & Schneider, D. S. (2014). On the front line: Quantitative virus dynamics in honeybee (Apis mellifera L.) colonies along a new expansion front of the parasite Varroa destructor. PLoS Pathogens, 10(8), e1004323. https://doi.org/10.1371/journal.ppat.1004323
- Muli, E., Patch, H., Frazier, M., Frazier, J., Torto, B., Baumgarten, T., Kilonzo, J., Kimani, J. N., Mumoki, F., Masiga, D., Tumlinson, J., & Grozinger, C. (2014). Evaluation of the distribution and impacts of parasites, pathogens, and pesticides on honey bee (Apis mellifera) populations in East Africa. PLoS ONE, 9(4), e94459. https://doi.org/10.1371/journal.pone.0094459
- Namayanja, D., Akol, A. M., & Kugonza, D. R. (2016). Prevalence of varroa mite infestations among honey bee colonies in Uganda (MSc. Thesis). Department of Zoology, Entomology and Fisheries Sciences, College of Natural Sciences, Makerere University.
- Nganso, B. T., Fombong, A. T., Yusuf, A. A., Pirk, C. W. W., Stuhl, C., Torto, B., & Blenau, W. (2017). Hygienic and grooming behaviors in African and European honeybees—New damage categories in Varroa destructor. PLoS ONE, 12(6), e0179329. https://doi.org/10.1371/journal
- Nuru, A., Awraris, G., Ahmed, A. A., Amenay, A., Mohammad, J. A., Brian, T., & Sarah, R. (2014). Crematogaster chiarinii ants as a potential biological control agent for protecting honeybee colonies from attack by Dorylus quadratus driver ants in Ethiopia (Hymenoptera: Formicidae). Agricultural and Forestry Entomology, 16(3), 302–313. https://doi.org/10.1111/afe.12060
- Paray, M., & Gupta, S. (2017). Economic threshold of Varroa destructor (Anderson and Trueman) infesting Apis mellifera in Kashmir. Indian Journal of Entomology, 79(1), 27–31. https://doi.org/10.5958/0974-8172.2017.00007.4
- Pirk, C. W. W., Strauss, U., Yusuf, A. A., Demares, F., & Human, H. (2016). A review on: Honeybee health in Africa. Apidologie, 47(3), 276–300. https://doi.org/10.1007/s13592-015-0406-6
- Qadir, Z. A., Idrees, A., Mahmood, R., Sarwar, G., Bakar, M. A., Ahmad, S., Raza, M. M., & Li, J. (2021). Effectiveness of different soft acaricides against honey bee ectoparasitic mite Varroa destructor (Acari: Varroidae). Insects, 12(11), 1032. https://doi.org/10.3390/insects12111032
- Ramsey, S. D., Ochoa, R., Bauchan, G., Gulbronson, C., Mowery, J. D., Cohen, A., vanEngelsdorp, D., Joklik, J., Cicero, J. M., Ellis, J. D., Hawthorne, D., & vanEngelsdorp, D. (2019). Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proceedings of the National Academy of Sciences, 116(5), 1792–1801. https://doi.org/10.1073/pnas.1818371116
- Rondeau, S., Giovenazzo, P., & Fournier, V. (2019). Risk assessment and predation potential ofStratiolaelaps scimitus (Acari: Laelapidae) to control Varroa destructor (Acari: Varroidae) in honey bees. Risk assessment and predation potential of Stratiolaelaps scimitus (Acari: Laelapidae) to control Varroa destructor (Acari: Varroidae) in honey bees. PLoS ONE, 13(12), e0208812. https://doi.org/10.1371/journal.pone.0208812
- Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103(2010), S96–S119. https://doi.org/10.1016/j.jip.2009.07.016
- Roth, M. A., Wilson, J. M., Tignor, K. R., Gross, A. D., & Messenger, M. (2020). Biology and management of Varroa destructor (Mesostigmata: Varroidae) in Apis mellifera (Hymenoptera: Apidae) Colonies. Journal of Integrated Pest Management, 11(1), 1; 1–8. https://doi.org/10.1093/jipm/pmz036
- Spivak, M., & Reuter, G. S. (2016). Honeybee diseases and pests: A companion to Beekeeping in Northern Climate. University of Minnesota Extension, Department of Entomology and Minnesota Extension Service, St. Paul.
- Tesfu, S., & Dawit, H. G. (2021). Identification, characterization and evaluation of honeybee floras in Kafa, Sheka and Benchi Maji zones of Southern Nations, Nationalities and Peoples Region (SNNPR), Ethiopia. Journal of Agricultural Science and Food Technology, 7(3), 310–326. https://doi.org/10.17352/2455-815X.000125
- Thrusfield, M. (2005). Veterinary Epidemiology (3rd) ed., pp. 232–242). Blackwell Science Ltd., London.
- Tsuruda, J. M., Harris, J. W., Bourgeois, L., Danka, R. G., Hunt, G. J., & Amdam, G. V. (2012). High-Resolution linkage analyses to identify genes that influence varroa sensitive hygiene behavior in honey bees. PLoS ONE, 7(11),e48276. pone.0048276. https://doi.org/10.1371/journal
- Underwood, R., & Currie, R. (2007). Effects of release pattern and room ventilation on survival of varroa mites and queens during indoor winter fumigation of honeybee colonies with formic acid. Journal of Canadian Entomology, 139(6), 881–893. https://doi.org/10.4039/N06-085
- Underwood, R., & Lopez-Uribe, M. (2022). Methods to control varroa mites: An integrated pest management approach. Penn State Extension (PSE), Pennsylvanian State University: ART-5874. Extension.psu.edu.
- Van der Zee, R., Gray, A., Pisa, L., & De Rijk, T. (2015). An observational study of honey bee colony winter losses and their association with varroa destructor. Neonicotinoids and Other Risk Factors. PLoS ONE, 10(7),e0131611. pone.0131611. https://doi.org/10.1371/journal