Abstract
Problems of foodborne diseases relating to the microbiological quality/safety of food continue to be of big concern globally. The study was undertaken to assess the microbial safety of ready-to-eat (RTE) foods sold in retailers around Thohoyandou, Limpopo province, South Africa. A total of 96 RTE food products were purchased from four (4) different retailers and transported to a laboratory for analysis. Food handling practices, personal hygiene and pest control measures were observed and recorded. To enumerate Coliform, Salmonella species, Staphylococcus aureus, Bacillus cereus, Escherichia coli, total plate count, Yeasts and Moulds serial dilution of (10−1 − 10−5) and pour plate/spread method were employed. The checklist on the retailers revealed that they followed good manufacturing practice and good personal hygiene expect retailer 4. Microbial counts for all samples ranged from 1.10 to 3.95 log x cfu/g. Bacillus cereus and yeasts counts for all samples ranged from 1.71 to 3.95 and 1.43 to 3.73 log x cfu/g, respectively. S. aureus and coliforms mean counts ranged from 1.10 to 2.68 and 1.53 to 3.58 log x cfu/g. Total plate count ranged from 2.12 to 3.91 log x cfu/g for all the retailers. Moulds and Salmonella were not detected in all the samples tested. Indole test confirmed positive E. coli and citrate test confirmed positive for S. aureus and B. cereus. Mean counts of some microorganisms were above satisfactory microbial limits, and this may pose hazard to public health.
1. Introduction
Ready-to-eat (RTE) foods are known as fast foods, and they are purchased at an outlet featuring quick service, convenience, and at low cost (Osakue et al., Citation2016). These foods are easy to make and easily available from retailers, street roadside vendors and hawkers, hence people find them more interesting without questioning their safety (Sudad, Citation2018). The most popular RTE around Thohoyandou are sandwich, burger, pizza, french fries, fried fish, meat pies and potato chips. The traditional way of cooking is tedious hence RTE outlets are visible everywhere due to current lifestyle, which paved the way for the RTE industry globally (Darwish, Citation2018). Nutritional needs of the consumers are expected to be met through the consumption of RTE food. Moreover, ensuring that RTE foods are safe from contaminants and microorganisms is of utmost importance (Osakue et al., Citation2016; Bondoc, 2016b). The consumption of RTE is increasing in most parts of the world, and serious health problems have been associated with them (Food and Drug Administration act (FDA), 1997). High levels of bacterial counts in RTE foods indicate poor storage, food handling and it is likely the mode of disease transmission (Monday et al., Citation2014).
Bacterial counts in water and prepared food are the key factors in assessing the safety and quality of food. The level of hygiene practices adopted during food preparation by food handlers is also revealed by bacterial counts. Levels of Staphylococcus aureus, coliforms, Clostridium perfringens, Bacillus cereus are mainly associated with foodborne diseases (Bintsis, Citation2017). While faecal coliforms such as Escherichia coli are used as sanitary conditions indicators, detection of the latter may indicates the occurrence of other pathogenic microorganisms to man (Osakue et al., Citation2016). Symptoms of foodborne diseases often include aches, vomiting, fever, diarrhoea, and abdominal pains (Darwish, Citation2018; Mashak et al., Citation2015). Contaminated water used in the preparation of RTE foods has been associated with Salmonella spp which is a common cause of the foodborne disease called Salmonellosis (Sudad, Citation2018). Salmonella spp is particularly found in undercooked chicken and eggs and it is a cause of many outbreaks globally. Pathogenic microorganisms on raw vegetables, such as lettuce used in sandwiches may occur because of contaminated irrigation water leading to an increase of enteric pathogens incidence. The high prevalence of S. aureus in raw foods can cause a wide range of infections and is mainly from the environmental sources or food handlers (Osakue et al., Citation2016). Furthermore, the prevalence of Salmonella spp, Campylobacter spp, E. coli, Staphylococcus spp, Listeria and Yersinia spp. were reported on meat, vegetable ingredients, raw and cooked foods, chicken shawarmas, raw chicken, sandwiches, beef burger, commercial mayonnaise, RTE salad vegetables, frozen chicken, seafood, poultry products and lastly the hands of food handlers (Asiegbu et al., Citation2020; Carrasco et al., Citation2012; Farid et al., Citation2013; Öz et al., Citation2014).
Most fast-food handlers have no knowledge of causes of foodborne diseases (Adebukola et al., Citation2015). Foodborne illness may be a result of inadequate cooking, poor handling, contaminated equipment, poor personal hygiene and unclean working surfaces or environment. Therefore, the food handlers are the ones who play major roles in ensuring food safety throughout the processing steps (Asiegbu et al., Citation2020; Goubraim & Chakor, Citation2015). The Food and Drug Administration (FDA) recommends that food must be prepared with the slightest possible human contact, clean and suitable utensils and sanitised surfaces to prevent cross-contamination. Utensils and equipment may sometimes be contaminated with pathogens from rodents, insects, not portable water and human. Most of the contamination problems can be controlled by the food handlers in restaurants or processing plants. As suggested by Rane (Citation2011), food must be cooked to the correct temperature to kill pathogenic microorganisms. Studies revealed that food cooked at 70°C for at least 2 min is safe for consumption. Chilled RTE foods must be stored below 5°C before serving and hot foods must be stored above 60°C (Sudad, Citation2018). RTE foods must be assessed for microbiological quality as it reflects sanitary condition during its production and distribution. The aim of this study was to determine the microbiological quality of RTE foods sold by retailers in Thohoyandou, Limpopo province, South Africa.
2. Methods and materials
2.1. Sample collection
A total of 96 RTE food samples such as fried chicken, meat pie, fried Russian, chicken stew, sandwich, beef stew, fried chips and fried chicken were purchased in intervals for bacteriological analysis. They were randomly bought in their original packaging materials at Thohoyandou retailers and were put in ice box during transportation at 4°C to the Department of Food science and Technology, Food microbiology laboratory, University of Venda, South Africa.
2.2. Questionnaire development
A total of four (4) retailers were observed for their food handling practices based on the following categories: Food handling practice, personal hygiene and pest control measures (mostly cockroach, rodents and other insects) as they are the most dominate food parasites that can transmit diseases to the consumers. The observations were conducted during the sampling of food from different retailers. The questionnaires were developed from observation as indicated in Table .
Table 1. Checklist for food hygiene practices at different retailer shops
2.3. Microbial analyses
2.3.1. Enumeration of S. aureus
The method by Öz et al. (Citation2014) was employed to enumerate S. aureus. Approximately ten (10) g of each sample was weighed and put in a stomach bag containing 90 mL Buffered Peptone Water (BPW) (Neogen Culture Media, United Kingdom). The solution was homogenized for 2 min. A serial dilution of 10−1 to 10−5 was made on sterile test tubes using sterile pipettes. About 0.1 mL of the appropriate sample test dilutions was transferred in triplicate onto solidified Baird Parker agar (BPA) (Neogen Culture Media, United Kingdom) plates and use a sterile spreader to evenly distribute the sample (EN ISO 6888–1:2022). The plates were then incubated at 37°C for 24 ± 2 h. Colonies appearing black or grey surrounded by a clear zone but partially opaque zone after incubation were counted as presumptive S. aureus. The colony count was represented as colony forming unit per gram (CFU/g).
2.3.2. Enumeration of coliform bacteria and E. coli
E. coli was isolated using method described by Ramashia et al. (Citation2020). About ten (10) g of each sample was weighed and put in a stomacher bag containing 90 mL BPW (Neogen Culture Media, United Kingdom). The sample was homogenized for 2 min. A serial dilution of the homogenised solution was performed on sterile test tubes using sterile pipette from 10−1 to 10−5. One mL of each dilution was transferred from the test tubes into the petri dishes and Chromogenic coliform agar (Neogen Culture Media, United Kingdom) was added, then rotated clockwise and anticlockwise to mix; this was done in triplicates for each dilution (ISO 16,649–2:Citation2004). Blue-green colonies for E. coli and pink-red colonies for coliform were counted after 24 h of incubation at 37°C. The number of colony-forming unit per gram (CFU/g) was calculated using the colony count technique of presumptive E. coli/coliform per gram of sample.
2.3.3. Enumeration of salmonella species
Method described by Sente et al. (Citation2019) was employed to enumerate salmonella species. Approximately 10 g of each food sample was weighed and placed into stomacher bag, which contains 90 mL of BPW (Neogen Culture Media, United Kingdom). The stomacher bag was homogenized for 2 min. A serial dilution of 10−1 to 10−5 was made on sterile test tubes and incubated at 37°C for 18 h. Then 0.1 mL of the inoculum were transferred to pre—enrichment broth (Rappaport-Vassiliads broth) (Neogen Culture Media, United Kingdom) and then incubated at 41°C for 48 h. Then each dilution from test tubes was streaked to solidified Xylose lysine deoxycholate agar (XLD) (Neogen Culture Media, United Kingdom) in triplicates (ISO 6579–1: Citation2017). The Plates were incubated at 37°C for 48 h. After incubation, transparent red halo and a black-centre colonies were counted to establish microbial population (CFU/g) of Salmonella species.
2.3.4. Enumeration of total plate count
Method explained by Mafune et al. (Citation2016) was followed to enumerate total plate count. Approximately 10 g of the sample was weighed and put into stomacher bag containing 90 mL BPW (Neogen Culture Media, United Kingdom), then homogenised for 2 min. Subsequent 10-fold dilutions were made to 10−5. About one mL of all dilutions was pipetted into petri dishes and pour plate method was employed using Plate Count Agar (PCA) (Neogen Culture Media, United Kingdom), in triplicates (ISO 4833–1: Citation2013). The plates were incubated at 30°C for 72 h. All colonies were counted to determine the overall microbial population. The results were recorded as CFU/g.
2.3.5. Enumeration of yeast and moulds
Approximately, 10 g of each food sample was weighed and placed into stomacher bag, which contained 90 mL of BPW (Neogen Culture Media, United Kingdom). The stomach bag was homogenized for 2 min. Serial dilution of 10−1 to 10−5 was conducted on sterile test tubes. One mL of each dilution was transferred from test tubes to petri dishes in triplicates. Potato Dextrose Agar (PDA) (Neogen Culture Media, United Kingdom) was poured in each petri dish and mixed by rotating clockwise and anti- clockwise (ISO 21,527–1: 2008). Plates were allowed to cool down and then incubated at 25°C for 5 days (Gilbert et al., 2000). Creamy and white colonies were counted as yeasts while moulds were velvety and had different colours, i.e. black, green or orange. The results were recorded as CFU/g.
2.3.6. Enumeration of bacillus cereus
About 10 g of each food sample was used and put into a stomacher bag that contains 90 mL of BPW (Neogen Culture Media, United Kingdom); the sample was homogenized from the stomacher bag for 2 min. A subsequent dilution of 10−1 up to 10−5 was made on the sterile test tubes. One mL of each sample was transferred from the test tubes into the petri dishes. Bacillus Cereus agar (BCA) (Neogen Culture Media, United Kingdom) was poured in each petri dish and mixed as above (ISO 7932:2005/A1:2020); this was done in triplicates for each food sample. The plates were allowed to cool and then incubated at 37°C for 24 h (Agwa et al., Citation2012). The results were recorded as CFU/g.
2.4. Biochemical tests
2.4.1. Indole test
A method described by MacWilliams (Citation2012) was employed. Sterilized test tubes containing 4 mL of tryptophan broth (Neogen Culture Media, United Kingdom) were prepared; then inoculated aseptically by taking the pure colonies from 18 to 24 h culture. The test tube was incubated at 35°C for 24 h. Kovac’s reagent (Pro Lab diagnostics, United Kingdom) was added to the broth culture and the presence or absence of the ring was observed.
2.4.2. Citrate utilisation test
Saline preparations of the organisms were done, diluents were inoculated into the Simmons citrate medium (Neogen Culture Media, United Kingdom) and undergone incubation for 24 h at 37°C. The colour change in the medium was observed; the development of blue colour indicates the alkylation process (Chukwuma et al., Citation2020).
2.5. Statistical analysis
All mean values of triplicates determination were reported with their standard deviations and the averages of plate counts were converted to log x CFU/g. The data was subjected to the analysis of variance (ANOVA) using the IBM Statistical Package for Social Sciences (SPSS) version 26.0. Statistical significance was tested at p < 0.05. Means of the microbial load were separated using Duncan’s multiple range test.
3. Results and discussion
3.1. Observation questionnaire
Observation of food hygiene practice from four (4) different retailers revealed that good hygiene was practiced in three (3) retailers (Table ). As shown in Table , all retailers used separate utensils and equipment for preparation of foods. All retailers in exception of Retailer 4, performed well in terms of food handling practice, personal hygiene and pest control. However, poor food hygiene practice observed in Retailer 4 may be due to lack of knowledge in food safety. Kumie and Zeru (Citation2007) indicate that food handlers should always keep their hands and protective clothes clean, cut short fingernails as well as covering hair with nets to ensure safety of RTE foods. Protective clothes are important in protecting both the food handlers and RTE foods from cross-contamination. In this study, food handlers in retail four did not wear protective clothes. In this regard, food handlers in Retailer 4 may require awareness/training or education on food safety, personal hygiene, and good manufacturing practice. The training may improve their performance in terms of understanding and following food hygiene principles and save the public from health risks (Ndaramu et al., Citation2020).
Table 2. Food hygiene practices observation
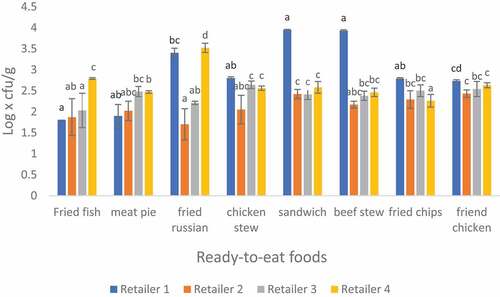
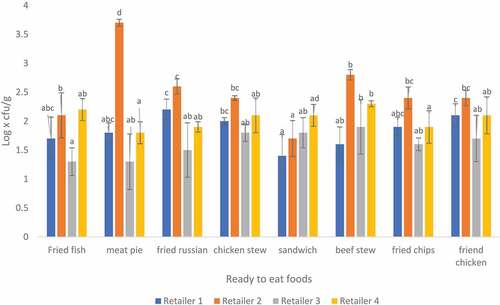
3.2. Microbial analysis of Bacillus spp in RTE foods sampled from four different retailers in Thohoyandou
Bacillus spp mean count for all RTE foods is presented in Figure . The counts ranged from 1.71 to 3.93 log x cfu/g. The lowest count was observed on fried Russian from retailer 2 while the highest was observed on sandwich prepared from retailer 1. Overall, the majority (62.5%) of the RTE food samples had borderline (102 to 103) Bacillus spp counts, 25% were satisfactory (<102) and 12.5% had unsatisfactory (103 − 104) Bacillus spp counts (Eglezos et al., Citation2010; Jessberger et al., Citation2020).
The results obtained are concerning because most of the samples were at the maximum acceptable limits. This is an indicator of poor hygiene and handling practices. The results from this study are similar to a study conducted by Osakue et al. (Citation2016) in which the B. cereus counts ranged from 2. 46 to 3.94 log x cfu/g. B. cereus represents a significant cause of food poisoning in various foods and is also responsible for food spoilage (Aruwa & Akinyosoye, Citation2015).
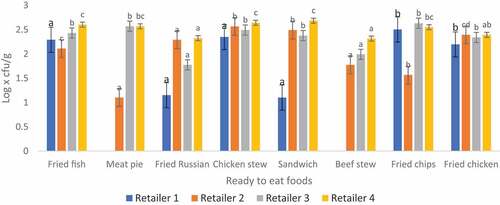
3.3. Total staphylococcus aureuscount of ready-to-eat foods sampled from four different retailers in thohoyandou
Presumptive staphylococcus aureus counts obtained from this study ranged from 1.10 to 2.68 log x cfu/g (Figure ). A study conducted by Mafune et al. (Citation2016) observed 2,48 log x cfu/g S. aureus in potato chips, and their results are in line with the results of the current study. Another study by Miko et al. (Citation2013) revealed that S. aureus was not detected in street vended food which is similar to the finding of this study. S. aureus was not detected in meat pie and beef stew prepared in retailer 1. In summary, majority (75%) of the RTE foods had borderline (102-103) presumptive S. aureus counts while 25% (<102) are satisfactory and none were unsatisfactory according to Wealth Health Organization World Health Organization (Citation2017); New Zealand and Australia guidelines for the microbiological examination of RTE foods (2001). The 25% of the samples were within acceptable microbial quality indicating that there was no contamination during food preparation, and this is considered beneficial for public health consumption. The 75% indicate that retailers need to improve on good manufacturing practice and personal hygiene of food handlers (Miko et al., Citation2013). Improper handling and poor hygiene practice may result in food contamination leading to foodborne diseases. S. aureus can produce enterotoxins and upon ingestion can cause diarrhoea and vomiting (Thompson, Citation2018)
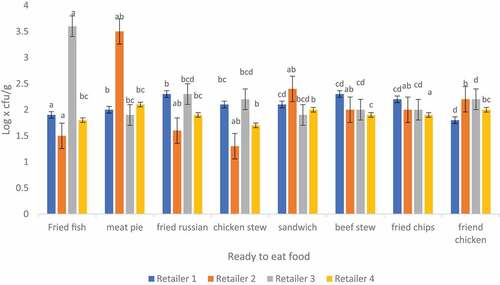
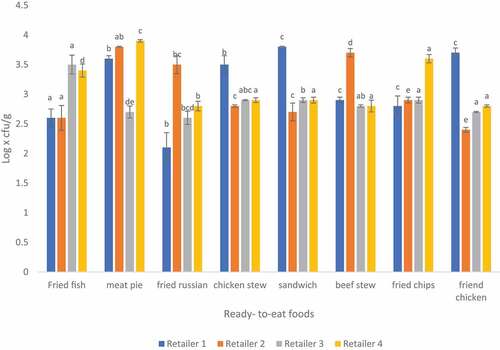
3.4. Total coliform count in ready-to-eat foods sampled from four different retailers in Thohoyandou
The mean counts of coliform obtained in this study ranged from 1.53 to 3.58 log x cfu/g in all samples (Figure ). Highest coliform counts were found in retailer 3 and lowest counts in retailer 2. Mafune et al. (Citation2016) found<2. 18 log x cfu/g in fried potato chips of street vended food samples. The study revealed that 59.4% (<102) of RTE food samples were on acceptable microbial quality while 40.6% were on the borderline (102-104) (Food Standards Australia New Zealand, Citation2001; World Health Organization, Citation2017). Coliforms are commonly found in faecal matter, soil and water and are widely distributed in vegetation. Coliforms are among the most common microorganisms that causes diseases. The presence of coliforms in RTE food indicates poor hygiene and poor sanitary practices during preparation (Gaafar et al., Citation2019).
3.5. Total Escherichia coli count in ready-to-eat foods sampled from four different retailers in thohoyandou
Figure shows the mean counts obtained for E. coli which ranged from.53 to 2.26 log x cfu/g. Rita et al. (Citation2020) observed zero E. coli in RTE foods. The obtained results are alarming because even the lowest range is out of the acceptable ranges for E. coli (Food Standards Australia New Zealand, Citation2001; World Health Organization, Citation2017). According to these standards, acceptable range for E. coli is <3 while the borderline is 3–100 colonies. A study carried out by Rockliff (Citation2014) observed <3 log x cfu/g of E. coli and met the satisfactory criterion, nine (9) samples were in the marginal category (3–100), this contrasts with our findings. E. coli indicates faecal contamination in food and suggests poor personal hygiene, improper storage and poor handling (Agarwal, Citation2014; Rockliff, Citation2014). Moreover, the personnel working in these different retailers should be trained in good hygiene especially after using the bathrooms. This implies that environmental health official that audits these retailers should determine the reasons of the above counts and come up with a corrective action to minimise the counts to levels that are satisfactory.
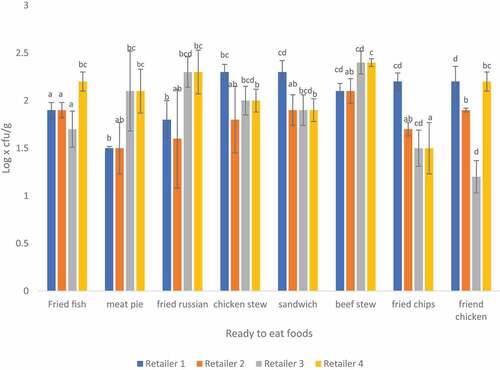
3.6. Total yeasts of ready-to-eat food sampled from four different retailers in Thohoyandou
The results of analysis obtained for the mean counts of yeasts are presented in Figure and ranged from 1.26 to 3.73 log10 cfu/g in all retailers. Rita et al. (Citation2020) recorded 2.21 log x cfu/g overall counts of yeast in pastry and RTE foods. Marinelli et al. (Citation2012) observed a range of 3. 30 to 7.76 log x cfu/g of yeast in mixed salads. The presence of yeast in food often signals spoilage in the food and should be addressed immediately. Poor handling of RTE foods, including poor hygiene and sanitary practices could explain the presence of yeast in the food.
3.7. Total plate of ready-to-eat foods sampled from four different retailers atThohoyandou
Total plate mean counts ranged from 2.12 to 3.91 log x cfu/g for all the RTE foods sold in Thohoyandou retailers (). The results of the study are within the acceptable microbial limits for the samples. World Health Organization (Citation2017) and New Zealand and Australia guidelines for the microbiological examination of RTE foods (2001) indicated that the satisfactory level for total plate count is <104. The study conducted by Rita et al. (Citation2020) recorded the value of 4.70 log x cfu/g in RTE meat products which contracts with our findings. High total plate count in food is an indicator that the food was prepared and served under unhygienic conditions of cross-contamination of utensils (Agarwal, Citation2014).
3.8. Total moulds and salmonella in ready-to-eat foods sampled from four different retailers at thohoyandou
Mould and Salmonella were not detected among the food product sampled from all the different retailers. This could be due to the proper storage of RTE foods which varies depending on the type of food, thus restricting the growth of mould in food. Moulds produces poisonous substance called mycotoxins which causes the respiratory and allergic reactions (Hope, Citation2013). The absence of Salmonella is a good indication that food samples are at low risk of causing foodborne diseases, this means that all four different retailers were serving RTE within Salmonella acceptable limits. These results may be due to Salmonella been destroyed during preparation or cooking at temperature above 65° C. These results align with findings by Khelissa et al. (Citation2017) that indicated that salmonella was not detected in RTE foods. The results align with WHO (2017); New Zealand and Australia guidelines for the microbiological examination of RTE foods (2001) standards which states that Salmonella should not be detected in 25 g of the sample.
3.9. Biochemical tests results
Two biochemical tests were conducted to differentiate as well as to identify the biochemical activities of pathogenic bacteria that affect RTE food. Table shows biochemical tests conducted such as indole and citrate utilization tests. The results obtained show that E. coli culture tested positive for the presence of indole as indicated by a pink colour. While S. aureus and B. cereus tested negative, indicated by a yellow colour. Ahmed et al. (Citation2014) observed a positive indole indicated by a pink colour. According to their study, E. coli tested indole positive because of the enzyme tryptophanase, which can convert the amino acid tryptophan to indole. Indole reacts with added Kovac’s reagent to generate rosindole dye forming a red in colour (indole +). S. aureus and B. cereus lack intracellular enzymes such as tryptophanase that degrade the tryptophan into indole. Hence, the indole test was negative for such bacteria (Moushumi et al., Citation2018). Indole test is performed on bacterial species to determine its ability to convert tryptophan into indole.
Table 3. Biochemical test results
S. aureus and B. cereus tested positive for citrate utilisation test as indicated by Green—blue colour. The change of colour to blue in the tubes is a positive indicator of citrate utilisation (Akkari et al., Citation2017). E. coli tested negative for citrate utilisation test with no colour change. The growth of E. coli was inhibited, and the colour of the medium remains green. However, some microorganisms are capable of growth on citrate and do not produce colour change. Growth is considered a positive citrate utilisation test even in the absence of a colour change. Such test with equivocal results should be repeated (Akkari et al., Citation2017). Citrate test is conducted to assess the ability of an organism to utilise citrate as a sole source of energy. It is performed to differentiate members of Enterobacteriaceae. The test is also used for the differentiation between faecal coli and members of the aerogenes groups on the basis of citrate utilisation. Other microorganisms that test positive for citrate utilisation tests include Klebsiella pneumoniae.
4. Conclusion
In this study, most of the RTE samples analysed had borderline microbial limits for S. aureus and Bacillus spp according to the national and international standards. According to the same standards, most samples showed that coliform is within acceptable limits, while E. coli had limits that are not suitable for human consumption. Therefore, RTE foods may cause serious health problems especially for people who are immune compromised. It is necessary for environmental health officials to create awareness and provide training/educate food handlers and premises cleaners. These parameters will ensure that food safety and hygiene practices are practiced in retailers to ensure that people who depend on RTE for convenience are protected.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Bono Nethathe
Dr Nethathe B works in the Department of Food Science and Technology, University of Venda (South Africa). She is currently a Food microbiology chief laboratory technician and specializes in Microbiology and Molecular biology
Phato Avheani Matsheketsheke
Ms Matshekhetshekhe P.A was a honours student in the Department of Food Science and Technology specialiazing in Food microbiology
Mpho Edward Mashau
Mr Mashau M.E also works in the Department of Food Science and Technology, University of Venda as a lecturer
Shonisani Eugenia Ramashia
Dr Ramashia S.E works in the Department of Food Science and Technology, University of Venda (South Africa) as a seniour lecturer. Her area of interest is Food microbiology
References
- Adebukola, O. C., Opeyemi, A. O., & Ayodeji, A. I. (2015). Knowledge of food borne infection and food safety practices among local food handlers in Ijebu-Ode Local Government Area of Ogun State. Journal of Public Health and Epidemiology, 7(9), 268–13. https://doi.org/10.5897/JPHE2015.0758
- Agarwal, M., (2014). Prevalence of pathogens and indicators in foods ordered from online vendors. Doctoral dissertation, Rutgers University-Graduate School-New Brunswick, Prevalence of pathogens and indicators in foods ordered from online vendors ProQuest.
- Agwa, O. K., Uzoigwe, C. I., & Wokoma, E. C. (2012). Incidence and antibiotic sensitivity of Bacillus cereus isolated from ready to eat foods sold in some markets in Portharcourt, Rivers state, Nigeria. Asian Journal of Microbiology, Biotechnology and Environmental Science, 14(1), 13–18. envirobiotechjournals.com.
- Ahmed, S., Tasnim, U. T., Pervin, S., & Islam, M. T. (2014). An assessment of bacteriological quality of some fast-food items available in Jessore city and antibiotic susceptibility of isolated Klebsiella spp. International Journal of Biosciences, 5, 125–130. https://doi.org/10.12692/ijb/5.9.125-8
- Akkari, L. L., Couvert, O., Lepage, J. F., Mouro, C. R., Desriac, N., Mathot, A. G., Leguérinel, I., Coroller, L., & Decourcelle, N. (2017). Proceedings of the Effect of constituents of a model emulsion on the germination and growth of bacterial spores. In Microbial spoilers in Food. Quimper, France. Effect of constituents of a model emulsion on the germination and growth of bacterial spores - INRAE - Institut national de recherche pour l’agriculture, l’alimentation et l’environnement.
- Aruwa, C. E., & Akinyosoye, F. A. (2015). Microbiological assessment of ready-to-eat foods (RTEs) for the presence Bacillus species. Journal of Advanced Biology and Biotechnology, 3(4), 145–152. https://doi.org/10.9734/JABB/2015/17407
- Asiegbu, C. V., Lebelo, S. L., & Tabit, F. T. (2020). Microbial quality of ready-to-eat street vended food groups sold in the Johannesburg Metropolis, South Africa. Journal of Food Quality and Hazards Control, 7(1), 18–26. https://doi.org/10.18502/jfqhc.7.1.2448
- Bintsis, T. (2017). Foodborne pathogens. AIMS Microbiology, 3(3), 529–563. https://doi.org/10.3934/microbiol.2017.3.529
- Bondoc, I. (2016). European Regulation in the veterinary sanitary and food safety area, a component of the European policies on the safety of food products and the protection of consumer interests: a 2007 retrospective Part Two: Regulations. Universul Juridic, Supliment. https://nam04.safelinks.protection.outlook.com/?url=http%3A%2F%2Frevista.universuljuridic.ro%2Fsupliment%2Feuropean-regulation-veterinary-sanitary-food-safety-area-component-european-policies-safety-food-products-protection-consumer-interests-2007-retrospective-2%2F&data=05%7C01%7Cbono.nethathe%40univen.ac.za%7C0d5e51879e7f49b2b6e008dab55e4bd3%7Cf38ba9d8554c48a2ae4213b1e7f3c797%7C0%7C0%7C638021713891981229%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMDAiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C3000%7C%7C%7C&sdata=h2zmCFFBfDfIRGUiUvAfxe91xcCv8rMlPvcDBPydM%2BU%3D&reserved=0)
- Carrasco, E., Morales-Rueda, A., & García-Gimeno, R. M. (2012). Cross-contamination and recontamination by Salmonella in foods: A review. Food Research International, 45(2), 545–556. https://doi.org/10.1016/j.foodres.2011.11.004
- Chukwuma, O. U., Precious, N. I., & Maxwell, O. (2020). Microbial assessment of different formulations of “kunu”: an indigenous non-alcoholic beverage, vended in awka, Anambra state, Nigeria. American Journal of Food Science and Health, 6(2), 74–79. 70160153. researchgate.net.
- Darwish, A. Z. M. (2018). Foodborne pathogens of fast food and ready-to-eat foods in Tabuk city and evaluating hazard for food quality. International Journal of Healthcare and Biomedical Research, 6(02), 149–158. Microsoft Word - 20. soudhi paper (ijhbr.com).
- Eglezos, S., Huang, B., Dykes, G. A., & Fegan, N. (2010). The prevalence and concentration of Bacillus cereus in retail food products in Brisbane, Australia. Foodborne Pathogens and Disease, 7(7), 867–870. https://doi.org/10.1089/fpd.2009.0469
- Farid, E. M., Salim, D. A., & Dapgh, A. N. (2013). Occurrence of Enterotoxigenic S. aureus in half-cooked chicken products. Zagazig Veterinary Journal, 41(2), 140–151. https://doi.org/10.21608/zvjz.2013.95689
- Food Standards Australia New Zealand. (2001). Guidelines for the microbiological examination of ready-to-eat foods.
- Gaafar, R., Hassanin, F. S., Shaltout, F., & Zaghloul, M. (2019). Hygienic profile of some ready to eat meat product sandwiches sold in Benha city, qalyubia governorate, Egypt. Benha Veterinary Medical Journal, 37(1), 16–21. https://doi.org/10.21608/bvmj.2019.15642.1064
- Gilbert, P., & Lappin-Scott, H. (2000). Biofilms: United they stand, divided they fall. Microbiology Today, (27), 136–139–160.
- Goubraim, N., & Chakor, A. (2015). Impact of fast food on the socio-economic behavior of the Moroccan consumer: A study of the influencing factors. IOSR Journal of Business and ManagementVer III, 17(6), 2319–7668.
- Hope, J. (2013). A review of the mechanism of injury and treatment approaches for illness resulting from exposure to water-damaged buildings, mold, and mycotoxins. Scientific World JournalArticle, ID, 767482. https://doi.org/10.1155/2013/767482
- International Organization for Standardisation. (2013). Microbiology of food and animal feeding stuffs—horizontal method for the enumeration of microorganisms. Part 1: Colony count at 30 °C by the pour plate technique. In-ternational Organisation for Standardisation. ISO 4833-1:2013.
- International Standards Organisation (ISO) 16649-2: 2004. Microbiology of food and animal feeding stuffs - horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli - part 2: colony-count technique at 44 degrees c using 5-bromo-4-chloro-3-indolyl beta-d-glucuronid
- International Organization for Standardisation ISO 7932: 2004+A1: 2020. Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of presumptive Bacillus cereus. Colony-count technique at 30 °C.
- International Standard Organization (ISO). 215271:. 215271:2008. Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of yeasts and moulds — Part 1: Colony count technique in products with water activity greater than 0. 21527-1(95).
- International Standards Organisation ISO 6579-1:2017. Microbiology of the food chain — Horizontal method for the detection, enumeration and serotyping of Salmonella — Part 1: Detection of Salmonella spp.
- Jessberger, N., Dietrich, R., Granum, P. E., & Märtlbauer, E. (2020). The Bacillus cereus food infection as multifactorial process. Toxins, 12(11), 701. https://doi.org/10.3390/toxins12110701
- Khelissa, S. O., Abdallah, M., Jama, C., Faille, C., & Chihib, N. E. (2017). Bacterial contamination and biofilm formation on abiotic surfaces and strategies to overcome their persistence. Journal of Material and Environmental Science, 8(9), 3326–3346. jmaterenvironsci.com.
- Kumie, A., & Zeru, K. (2007). Sanitary conditions of food establishments in Mekelle town, Tigray, North Ethiopia. Ethiopian Journal of Health Development, 21(1), 3–11. https://doi.org/10.4314/ejhd.v21i1.10025
- Law, P. (2013). Food and Drug Administration Modernization Act of 1997. Public Law, 105–115.
- MacWilliams, M. P. (2012). Indole test protocol. Society for Microbiology. American.
- Mafune, T. S., Takalani, T. K., Anyasi, T. A., & Ramashia, S. E. (2016). Microbial safety of street vended foods sold in Thohoyandou, South Africa. Journal of Human Ecology, 53(3), 205–212. https://doi.org/10.1080/09709274.2016.11906973
- Marinelli, L., Maggi, O., Aurigemma, C., Tufi, D., & De Giusti, M. (2012). Fresh vegetables and ready-to eat salads: Phenotypic characterization of moulds and molecular characterization of yeasts. Annali di Igiene: Medicina Preventiva e di Comunità, 24(4), 301–309. PMID: 22913173.
- Mashak, Z., Langroodi, A. M., Ehsani, A., Ilkhanipoor, A., & Fathabad, A. E. (2015). Microbiological quality of ready-to-eat foods of Tehran province. African Journal of Food Science, 9(5), 257–261. https://doi.org/10.5897/AJFS2015.1260
- Miko, B. A., Hafer, C. A., Lee, C. J., Sullivan, S. B., Hackel, M. A., Johnson, B. M., Whittier, S., Della Latta, P., Uhlemann, A. C., & Lowy, F. D. (2013). Molecular characterization of methicillin-susceptible Staphylococcus aureus clinical isolates in the United States, 2004 to 2010. Journal of Clinical Microbiology, 51(3), 874–879. https://doi.org/10.1128/JCM.00923-12
- Monday, I. E., Francis, J. I., & Mohammad, S. U. (2014). Microbiological quality of ready-to-eat foods (rice and Moimoi) sold by food vendors in Federal Polytechnic Bali, Taraba State Nigeia. Journal of Environmental Science, Toxicology and Food Technology, 8(2), 145–149. https://doi.org/10.9790/2402-0824145149
- Moushumi, P., Rahman, S., Elahi, A. M. E., Roy, S., Hussain, T., & Lucky, N. S. (2018). Determination of microbiological (Staphylococci) quality of fast food sold in the different restaurant in Sylhet sadar. Research in Agriculture Livestock and Fisheries, 5(3), 327–333. https://doi.org/10.3329/ralf.v5i3.39581
- Ndaramu, O., Makopondo, R., Mary Njeri, M., & Muchiri, M. (2020). Effects of hazard analysis and critical control points principles on food safety at national youth service in Nakuru County, Kenya. International Journal of Research in Business and Social Science, 9(4), 314–322. https://doi.org/10.20525/ijrbs.v9i4.751
- Osakue, O. P., Igene, J. O., Ebabhamiegbebho, P. A., & Evivie, S. E. (2016). Proximate analysis and microbial quality of ready-to-eat fried Chicken part. Journal of Food and Industrial Microbiology, 2(1), 1–8.
- Öz, V., Karadayi, S., Çakan, H., Karadayi, B., & Çevik, F. E.(2014). Assessment of microbiological quality of ready-to-eat foods in Istanbul, Turkey. Journal of Food, Agriculture and Environment, 12(3&4), 56–60.
- Ramashia, S. E., Tangulani, T., Mashau, M. E., & Nethathe, B. (2020). Microbiological quality of different dried insects sold at Thohoyandou open market, South Africa. Food Research, 4(6), 2247–2255. https://doi.org/10.26656/fr.2017.4(6).233
- Rane, S. (2011). Street vended food in developing world: Hazard analyses. Indian Journal of Microbiology, 51(1), 100–106. https://doi.org/10.1007/s12088-011-0154-x
- Rita, W. S., Swantara, I. M. D., Asih, I. A. R. A., & Puspawati, N. M. (2020). Antibacterial activity and antioxidant capacity of selected local banana peel (Musa sp.) methanol extracts cultivated in Bali. International Journal of Agriculture, Environment and Bioresearch, 5(03), 242–251. https://doi.org/10.35410/IJAEB.2020.5519
- Rockliff, S. (2014). Microbiological quality of seed sprouts October–December 2014. Act Health Protection Service. In N. Waters, R. Krsteski, & S. Rockliff (Eds.), Microbiological Quality of Seed Sprouts.
- Sente, C., Khaitsa, M., Tomusange, J., Onyuth, H., Kahwa, D., & Bailey, H. (2019). Evaluation of the efficiency and quality of six surfaces in drying Haplochromis sp (enkejje) at Rubare fish landing site in Uganda. Cogent Food & Agriculture, 5(1), 1685444. https://doi.org/10.1080/23311932.2019.1685444
- Sudad, J. M. (2018). Quality and quantity microbial assessment of the mobile restaurants (caravans) in Baghdad. Journal of Pharmaceutical Sciences and Research, 10(9), 2354–2355.
- Thompson, L. J. (2018). Enterotoxins. In Veterinary Toxicology (pp. 759–761). Academic Press. https://doi.org/10.1016/B978-0-12-811410-0.00056-8
- World Health Organization. (2017). Interim practical manual: Supporting national implementation of the WHO guidelines on core components of infection prevention and control programmes (No. WHO/HIS/SDS/2017.8).