Abstract
Anchote, a high antioxidant with great functional potential, is a multipurpose edible part and an underutilized endemic tuber in Ethiopia. Studies were carried out to determine the impact of pretreatment on phytochemical contents, antioxidant activity, and functional properties of tubers and seeds of anchote in order to explore their potential uses. The pretreatment methods adopted were boiling and sun drying for tubers and boiling, soaking, germination, and roasting for seeds of anchote. All the analyses were conducted using official standard methods and graded chemical reagents. Boiling significantly (P < 0.05) decreased the total flavonoid content of the tubers by 20.19% and increased the total phenolic content of the tubers by 4.80%. Soaking significantly (P < 0.05) decreased the total phenolic content of the seeds by 21.03%. Total flavonoid and total phenolic content was significantly (P < 0.05) increased due to germination by 15.12% and 17.32%, respectively. Roasting significantly (P < 0.05) increased total phenolic and total flavonoid content by 14.89% and 14.12%, respectively. Oil absorption and water capacity were increased by all pretreatment methods and decreased by emulsion stability and capacity, bulk density, and foam stability and capacity. In general, germination and roasting increased flavonoid content and total phenolic and enhanced DPPH and chelating effects. In addition, the flour of anchote seeds and tubers was initiated to reveal decent functional properties and can bargain a prodigious latent in many food systems.
1. Introduction
Anchote (Coccinia abyssinica) is an endemic crop to Ethiopia (Admassu, Citation2009; Beruk et al., Citation2015) and is a very delicious and cultural food to the western part of Ethiopian dishes (Fekadu et al., Citation2014). Particularly, anchote is a traditional food for the Oromo community (one of the largest ethnic groups in Ethiopia) and could be consumed as both tuber and vegetable. Anchote is a multipurpose cultural food in which the tuber part is rich in many essential nutrients and antioxidants, and the seeds are rich in minerals. The yield of anchote tubers per hectare is about 150–180 quintals.
The qualities of anchote that make it differ from the other tuber crops are its good keeping qualities. The tubers can be stored in an underground pit and retrieved when needed, providing food security in times of other crop failures. In addition, like other roots and tubers, anchote is a perishable and seasonal plant food that is subjected to postharvest losses. Therefore, traditionally, the tubers of anchote are sun-dried in order to extend their shelf life for consumers (Tsado et al., Citation2013). Flour made from sun-dried ground tubers also keeps well.
Most tubers including anchote are commonly cooked before being consumed (Adetuyi & Stella, Citation2012). In the Wollega region of Ethiopia, the dish Anchote is considered a distinctive and cultural food. The seeds of many mature food crops are reported to be applied by various treatments (Çalışır et al., Citation2005) and roasting is reported to improve flavor (Akingbala et al., Citation2003). Traditionally, some forms of processing methods such as boiling, sun drying, soaking, germination, and roasting are applied before consumption. This pretreatment has a harmful and valuable influence on the antioxidant and functional properties of food. The expected use of such processing is to make anchote more palatable and digestible. Even though such traditional pretreatment methods may result in improvements, some nutritional values of tubers, nutrients, and antioxidants may be lost (Oghbaei et al., Citation2016). In the local communities, it is expected that anchote contains essential nutrients and antioxidants (Ansari et al., Citation2005), which inhibit the reactions of free radicals and protect the body from damage (Yang et al., Citation2006).
On the other hand, an acquisition of an understanding of the functional properties of food and its processing effect may demonstrate its further utilization and potential uses in the food system. However, there is scanty information available on the impact of pretreatment on antioxidants and functional properties of Ethiopian endemic anchote tubers and seeds. Therefore, it is imperative to investigate the influence of pretreatment methods on antioxidants and functional properties of Ethiopian endemic anchote flour in order to explore their potential uses in various food systems.
2. Materials and methods
2.1. Sample collection
The tubers and seeds of raw (unprocessed) anchote were collected from famers' farms around Nekemte city, Ethiopia. In the previous study, nutritional and antioxidant properties of tubers and seeds of 15 accessions were evaluated, and it was observed that tubers and seeds of the anchote accession ATLR-3 contained relatively high proximate, mineral and antioxidant contents as compared to the rest of the anchote accessions. Based on this information, the tubers and seeds of ATLR-3 were selected and further evaluated for their effect on photochemical content, antioxidant, and functional properties. Tubers in this document refer to the tubers of ATLR-3, and the seeds refer to ATLR-3 of the seed accession. The selected tuber and seed are shown in Figure . The tubers and seeds of anchote accessions were packed and transported to Wollega University research laboratory of Food Science and Technology.
2.2. Sample preparation
2.2.1. Raw anchote
The tubers and seed accession were separated for raw and analysis. The washed tuber was sliced into 5 mm diameter using a knife. The seeds were manually sorted and sun-dried. The tubers and seeds of raw Anchote are shown in Figure .
2.2.2. Pretreatment methods
Anchote tubers were processed by boiling and sun drying. The seeds (Figure ) of Anchote were roasted, soaked, germinated, and boiled. The detailed procedures of these traditional processing methods were described as follows:
2.2.2.1. Boiling
The sliced tubers (800 g) and seeds (200 g) were boiled (100°C) separately in the traditional pot (locally known as Tuwe) in tubers/seeds-to-water ratio of 1:10 (w/v) for 10 and 20 minutes, respectively, until they became soft. After boiling, the excess water was discarded. Then, it was dried in an oven (Ovmbr stl, germen) for 72 hours at 45°C.
2.2.2.2. Soaking
Anchote seeds (200 g) were soaked for 8 hours (Nasar-Abbas et al., Citation2008). The soaked seeds were rinsed and dried using oven at 45°C for 72 hours.
2.2.2.3. Germination
About 200 g of anchote seeds were soaked (1:10, w/v) for 3 hours at 25°C. Water was discarded, and the seeds were spread on wet yarn angora on a tray and allowed to germinate for about 24 hours. Germinated seeds were removed from the cotton wool and dried in a drying oven at 45°C for 72 hours.
2.2.2.4. Roasting
About 200 g of seeds were roasted in a roasting plate (Genlab Widnes, England) at 160 °C for 10 minutes (Adebiyi et al., Citation2002) and were allowed to cool for about 10 minutes.
2.2.2.5. Sun drying
The sliced tubers (800 g) were spread on polythene bags and covered with netted cloth to keep off insects and dust (Mepba et al., Citation2007). The tubers were placed in direct sunlight and dried for 5 days.
2.2.2.6. Flour preparation
The dried raw and processed tubers and seeds of anchote were milled into a powder using an electric grinder (Grinouer MbS, Auitus, China) until the flour passed through a 0.425 mm sieve size. Finally, the flour was packed. The packed flour was labeled and stored in a desiccator for further analysis.
3. Determination of phytochemical contents
3.1. Methanolic extraction
Methanolic extraction of the sample was conducted according to Woldegiorgis et al. (Citation2014). Ten grams of the sample was extracted with 100 ml of methanol at room temperature at 150 rpm for 24 hours. Then filtration was conducted, and 100 ml of methanol was added and repeated as described above. Then, evaporation was conducted at 40 °C using a rotary evaporator and stored at 4°C until analysis.
Total phenolic content (TPC) was determined according to the methods described by Ferreira et al. (Citation2007), in which gallic acid was used for the calibration curve standard. Total flavonoid content was evaluated according to the procedure described by Xu and Chang (Citation2007).
3.2. Evaluation of antioxidant activity
Evaluation of diphenylpicrylhydrazyl (DPPH) scavenging activity was conducted as described by Woldegiorgis et al. (Citation2014). The determination of ferric-reducing power was conducted as per the methods outlined by Ferreira et al. (Citation2007). The determination of the effects of metal chelating was evaluated according to the method described by Woldegiorgis et al. (Citation2014). Determination of Azino-Bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) activities was conducted according to the procedure described by Nishaa et al. (Citation2012).
3.3. Evaluation of functional properties
The bulk density of the flour was determined as described by Butt and Batool (Citation2010). The determination of the absorption capacities of oil and water was evaluated according to the procedure outlined by Aremu et al. (Citation2007). The emulsifying stability and capacity of the flour was conducted as per the procedure described by Aremu et al. (Citation2007). Evaluation of stability and capacity foam of the flours was conducted according to Aremu et al. (Citation2007).
4. Statistical analysis
Two replicates of the completely randomized experimental design were used. One-way analysis of variance and SPSS were employed to analyze data. Means were separated using Duncan’s and reported by the standard error. A statistically significant difference was considered at p-value of 0.05 or less value.
5. Results and discussion
5.1. Effect of pretreatment on phytochemical contents of anchote tuber and seed flour Effect of pretreatment on the total phenolic contents
The result of the total phenolic contents of the raw and processed tubers and seeds of anchote is presented in Table . The total phenolic contents of the raw, sun-dried, and boiled tubers were 85.21, 89.78, and 82.35 mg GAE/g, respectively, while 47.34, 47.99, 35.28, 57.27, and 55.88 mg GAE/g for raw, boiled, soaked, germinated, and roasted seeds, respectively.
Table 1. Effect of pretreatment on total flavonoid (mg CE/g) and phenolic (mg GAE/g) content of tubers and seeds of anchote
The total phenolic content of the boiled tuber was significantly (P < 0.05) increased by 4.80% compared to the raw tuber. This increase in total phenol could be attributed to the fact that some phytochemicals that are insoluble at room temperature get solubilized and extracted at increased temperature (Adeniyan et al., Citation2013). Thus, the consumption of boiled anchote dried tubers could be advantageous because it may lower the cellular aging process in the human body (Amić et al., Citation2003). Since there was no significant difference between the sun-dried tubers and the raw, the consumer can use the sun-dried tubers throughout the year without significant reduction of the phenolic content.
In the same trend, the total phenolic contents of germinated and roasted seeds significantly (P < 0.05) increased by 17.32% and 14.89%, respectively. However, the total phenolic content of soaked seeds significantly (P < 0.05) decreased by 21.03% compared to raw seeds. Pandey and Awasthi (Citation2015) reported the same trend of an increase in the total phenolic content of roasted sesame seeds. Many authors (Gallegos-Infante et al., Citation2010; Siddhuraju & Becker, Citation2001) also reported that the total phenolics content was increased by heat induced. The reason for increasing the total phenolic content during roasting could be due to heat-induced formation, as it is reported that the breakdown of phenolic acid will occur during thermal processing.
The total phenolic content increased by heat treatments of different food products (Boateng et al., Citation2008; Kim et al., Citation2006; Sultana et al., Citation2008). Maillard reaction induced by roasting (Manzocco et al., Citation2000) and the amounts of total phenolics were increased by the Maillard reaction (Yu et al., Citation2005).
The increase in total phenolic content during germination of anchote seeds might be due to mainly endogenous enzyme activation and the complex biochemical metabolism of seeds during the process (Duenas et al., Citation2009). Many authors (Duenas et al., Citation2009; Khattak et al., Citation2007) reported that total phenolic content increased during germination.
5.2. Effect of pretreatment on the total flavonoids
The result of total flavonoid content of anchote tubers and seeds is shown in Table . The content of total flavonoids of the raw, sun-dried, and boiled tuber were 7.09, 3.64 and 4.28 mg CE/g, respectively, and were 19.04, 16.92, 19.65, 23.43, and 23.14 mg CE/g for raw, boiled, soaked, germinated, and roasted seeds, respectively. The total flavonoid contents of the sun-dried tubers and soaked seeds were not significantly (P > 0.05) different from the raw tubers and seeds, respectively. However, the total flavonoid contents of the boiled tubers and seeds were significantly (P < 0.05) decreased by 20.19% and 7.30% when compared to the raw tubers and seeds, respectively. In contrast, the total phenolic contents of the germinated and roasted seeds were significantly increased by 15.12% and 14.12%, respectively, when compared to the raw seeds.
The increase in the total flavonoid content could result from the release of bound polyphenols or from Maillard reaction products forming during roasting, which then exhibited scavenging activity on the reactive oxygen species (Jannat et al., Citation2010; Jeong et al., Citation2004; Thidarat et al., Citation2016).
Flavonoids are polyphenolic compounds that are identified as important constituents and secondary metabolites to reduce the risk of chronic diseases, such as hyperlipidemia and cancer (Chen et al., Citation2014; Ebrahimzadeh et al., Citation2008; R. H. Liu, Citation2004; Scherer & Godoy, Citation2009).
5.3. Effect of pretreatment on the ratio of flavonoid to phenolic
The ratio of flavonoids to phenolics is used for prediction of a number of flavonoids present in phenolics. The result of flavonoid-to-phenolic ratio is shown in Table . The flavonoid-to-phenolic ratios of the raw, sun-dried, and boiled tuber were 0.18, 0.14, and 0.16, respectively, and were 0.51, 0.47, 0.65, 0.50, and 0.50 for raw, boiled, soaked, germinated, and roasted seeds, respectively. As compared to the raw seeds, the ratio of flavonoid to phenolic contents of the soaked seeds was increased significantly (P < 0.05). This indicates that soaked seed extracts have high flavonoid content. However, the ratios of flavonoid to phenolic contents of the boiled, germinated, and roasted seeds were significantly (P < 0.05) reduced when compared to raw seeds.
5.4. Effect of pretreatment on the antioxidant activity of anchote seed and tuber flour Effect of pretreatment on DPPH activity
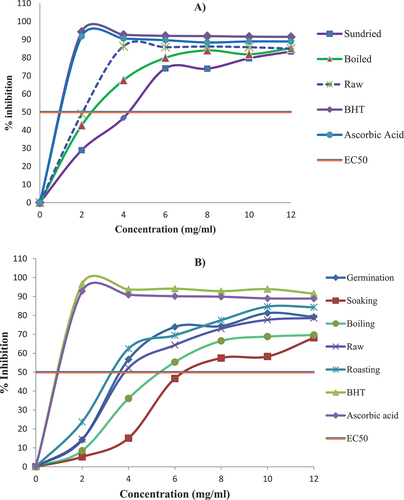
In addition to the raw anchote tubers and seeds, synthetic antioxidants were also used at the same concentrations as the control. The inhibition percentage of DPPH activity of processed anchote tubers and seeds was evaluated at concentrations of 2–12 mg/ml. The present study revealed that when the concentration of the processed tuber and seed increased, the DPPH activity was also increased.
The DPPH scavenging activity of the boiled and sun-dried tuber extracts was lower than that of the raw tubers at each evaluated concentration level (Figure ). In a similar trend, the DPPH scavenging activity of the boiled and soaked seed extracts was lower than that of the raw anchote seeds at each concentration measured. However, the DPPH scavenging activities of the germinated and roasted seeds were relatively higher than those of raw seeds at each concentration (Figure ). The activity of radical-scavenging increase during roasting seed extract is in line with the earlier studies conducted by many authors (Adelakun et al., Citation2009; Dewanto et al., Citation2002; Şensoy et al., Citation2006).
On the other hand, the increase in the DPPH activity of the roasted anchote seed extracts might be due to the formation of Maillard reaction during thermal treatments (Dewanto et al., Citation2002). The study revealed that the extracts of roasted and germinated seeds exhibit proton-donating ability and could serve as free radical inhibitors or scavengers.
5.5. Effect of pretreatment on the metal chelating
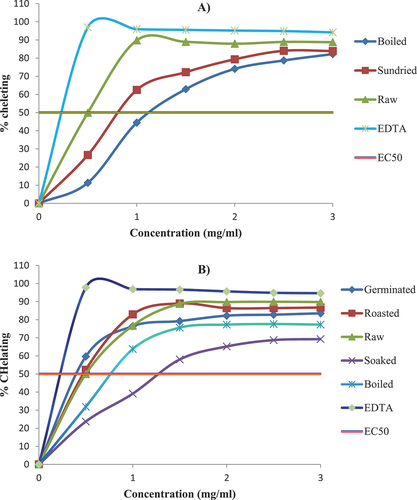
The value of the chelating effect of the processed tubers and seeds was compared with the value of raw tubers and seeds, respectively. In addition, it was compared with synthetic antioxidants (EDTA). The chelating effect percentage of the processed tubers and seeds was evaluated at concentrations of 0.5–3 mg/ml (Figure ). Similar to the DPPH scavenging activity, it was observed that there was an increase in the chelating effect through increasing the processed tubers and seeds extract concentration. The chelating effects of the boiled and sun-dried tubers were lower than those of the raw anchote tubers and synthetic antioxidants (Figure ).
The boiled and soaked seeds were lower than the chelating effect of the raw seeds. On the other hand, at the initial concentration, the chelating effects of germinated and roasted seeds were higher than those of the raw seeds; however, when the concentration increases, the chelating effect of the germinated and roasted seeds decreased compared to the raw seeds (Figure ). During thermal processing, metal chelating increased due to the alteration of phenolic compounds and the formation of melanoid (Randhir et al., Citation2008).
5.6. Effect of pretreatment on effective concentration values of the tuber and seed flour Effect of pretreatment on the effective concentration values
The effective concentration (EC50) is the amount of antioxidant required to decrease the antioxidant concentration by 50% (S. C. Liu et al., Citation2009). A higher effective concentration indicates a lower antioxidant activity (Do et al., Citation2014). Phenolics were the dominant antioxidant components, and it is directly proportional to antioxidant activity (S. C. Liu et al., Citation2009). The effective concentration (EC50) values for DDPH scavenging of the raw, sun-dried, and boiled tubers were 2.05, 2.40, and 4.20 mg/ml, respectively, and were 3.85, 5.30, 6.30, 3.40, and 3.20 mg/ml for raw, boiled, soaked, germinated, and roasted seeds, respectively. The result of this study revealed that boiled anchote tubers and germinated and roasted seeds had lower EC50 values of 2.40, 3.40, and 3.20 mg/ml, respectively, compared to raw tubers (2.10) and seed (3.9) (Table ).
Table 2. Effect of processing on effective concentration (EC50) values (mg/ml) of the raw andprocessed anchote tubers and seeds
The EC50 values for the metal chelating effect of the raw, sun-dried, and boiled tubers were 0.50, 1.10, and 0.80 mg/ml, respectively, and were 0.50, 0.78, 1.30, 0.40, and 0.47 mg/ml for raw, boiled, soaked, germinated, and roasted seed, respectively (Table ). In this study, it was observed that the EC50 values of the germinated and roasted seeds were lower than the raw seeds, which implies that germination and roasting exhibit superior performance and better antioxidant activities (Finkel & Holbrook, Citation2000).
5.7. Pretreatment effect on the functional properties of the tubers and seeds flour
5.7.1. Pretreatment effect on the bulk density
Bulk densities of the raw and processed anchote tubers and seeds are shown in Table . The bulk densities of the flour of raw tubers and seeds were 0.55 and 0.68 g/ml, respectively. This indicates that the bulk density of the raw seed was higher than the raw anchote tubers. This may be related to the fact that the flour of raw seeds contains high protein than the flour of raw tubers.
Table 3. Functional properties of the flour of raw and processed anchote tubers and seeds
The obtained bulk density value for the flour of raw anchote tubers (0.55 g/ml) was relatively similar to those from African breadfruit kernel (0.54 g/ml). However, it was lower than that of tiger nut (0.62 g/ml) and wheat flour (0.71 g/ml) (Oladele & Aina, Citation2007). These results were also lower than those of raw and fermented wheat flours, with respective values of 0.80 and 0.86 g/ml (Steve, Citation2011). Indeed, the lower bulk density implies less quantity of the food samples, which could be packaged in a constant volume, ensuring an economical packaging (Osundahunsi & Aworh, Citation2002). Thus, the flour of anchote tubers could be a good candidate for complementary food formulation for infant and young children.
On the other hand, the bulk density of the flour of raw anchote seeds (0.68 g/ml) was almost comparable with the value reported by Bryant et al. (Citation1988) for the whole anchote seed (0.68 g/ml) and higher than the value reported by Adelakun et al. (Citation2017) for five anchote seed varieties (0.12–0.216 g/ml). Products with a high bulk density are known to exhibit better packaging properties than those with a low bulk density. Arinola et al. (Citation2016) reported that high bulk density is desirable in that it offers a greater packaging advantage as greater quantity may be packaged within a constant volume. A higher bulk density is desirable for greater ease of dispersibility and a reduction of paste thickness, and therefore, the high bulk density of the raw anchote seeds indicates that they would serve as good thickeners in food products.
The bulk densities of the flour of boiled and sun-dried tubers were 0.45 and 0.38 g/ml, respectively, and were 0.53, 0.63, 0.63, and 0.46 g/ml for the flour of boiled, soaked, germinated, and roasted seeds, respectively. Bulk densities of the flour of boiled and sun-dried tubers were significantly (P < 0.05) decreased by 17.54% and 29.82%, respectively, when compared to the raw tubers. The bulk densities of the flour of boiled and roasted seeds were also significantly (P < 0.05) decreased by 21.43% and 31.43%, respectively, compared to the raw tubers. The trends of this result were in agreement with the finding of Enujiugha et al. (Citation2003), who stated that bulk density is reduced due to heat application. Drying also decreases the bulk density of flour. The high bulk density of flour indicates that it would serve as a good thickener in food products. In contrast, low bulk density would be an advantage in the formulation of complementary foods. Therefore, the high bulk density of the flour of seeds suggests their suitability to be used as thickener in food products. However, the exhibition of low bulk density value by the flour of anchote tubers could be an advantage in the formulation of complementary foods where high nutrient density and low bulk density are desired.
5.8. Effect of pretreatment on the water absorption capacity
Table shows the result of water absorption capacity of the flour of raw and processed anchote tubers and seeds. Water absorption capacities of the flour of raw tubers and seeds of anchote were 2.03 and 2.53 ml/g, respectively. The water absorption capacity of the raw anchote seeds (0.68 g/ml) was lower than the value reported by Adelakun et al. (Citation2017) for five anchote seed varieties (1.93–3.23 g/cm). From this result, it was observed that the water absorption capacity of the raw tubers of anchote (2.03 ml/g) was lower than the seeds (2.53 ml/g). This indicates that the protein content of raw seeds of anchote is higher than that of the flour of anchote tubers since the amount of protein in the sample is the most determinant for the capacity of water absorption.
Water absorption capacities of the flour of boiled and sun-dried tubers were 3.78 and 4.43 ml/g, respectively, and were 3.78, 3.03, 4.33, and 4.28 ml/g for the flour of boiled, soaked, germinated, and roasted seeds, respectively. Water absorption capacity of the soaked anchote seeds was not significantly (P > 0.05) different from that of the flour of raw anchote seeds. However, the water absorption capacity of the flour of boiled, germinated, and roasted seeds was significantly (P < 0.05) increased by 49.02%, 71.52%, and 68.63%. The water absorption capacities of the flour of boiled and sun-dried tubers were also significantly (P < 0.05) increased by 85.37% and 117.07%, respectively.
The increase in water absorption capacity during germination may be due to increased levels of protein and quality of protein, which enhance interactions with water (Chauhan & Singh, Citation2013). The increase in water absorption capacity during roasting could be attributed to increased level of damaged starch, which was induced by gelatinization of starch during roasting (P. Sharma & Gujral, Citation2013). The formation of a porous structure that imbibes and holds water by capillary action is also a reason for the increase in water absorption capacity (V. SHARMA et al., Citation2016). Abbey and Ibeh (Citation1988) also reported that the increase in the water absorption capacity has always been associated with an increase in the amylose leaching and solubility and loss of starch crystalline structure.
High water absorption capacity of the flours suggests that they can be used in the formulation of some foods such as sausage, dough, processed cheese, and bakery products (Butt & Batool, Citation2010). Water absorption capacity of 1.25 ml/g and above is an indication of good bakery property (Giami & Alu, Citation1994). Therefore, the flour of all the raw and processed anchote tubers and seeds analyzed in this work would be good functional ingredients in bakery products. This study suggests that the boiled, germinated, and roasted seeds and boiled and sun-dried tubers of anchote flour can serve as a thickener in the food system.
5.9. Effect of pretreatment on the oil absorption capacity
The result of oil absorption capacity of the flour of raw and processed anchote tubers and seeds is presented in Table . The oil absorption capacities of the flour of raw anchote tubers and seeds were 1.68 and 1.93 ml/g, respectively. The oil absorption capacity of the raw anchote seeds (1.93 ml/g) was in the range of the value reported by Adelakun et al. (Citation2017) for five mung seed varieties (1.80–2.94 ml/g). A high oil absorption capacity is valuable in ground meat formulations, meat replacers and extenders, doughnuts, pancakes, and baked foods (Amandikwa & Chinyere, Citation2012).
The oil absorption capacities of the flour of boiled and sun-dried tubers were 3.13 and 2.53 ml/g, respectively, and were 2.93, 2.33, 2.38, and 3.73 ml/g for the flour of boiled, soaked, germinated, and roasted seeds, respectively. Oil absorption capacities of the boiled and sun-dried tubers were significantly (P < 0.05) increased by 85.29% and 50.00%, respectively, compared to the raw anchote tubers. The increased oil absorption capacity of heat processed sample may be due to the denaturation and dissociation of their constituent proteins that occur upon heating, which unmasks the non-polar residues from the interior of the protein molecule (Taffesse et al., Citation2012).
In the same trends, the oil absorption capacities of the boiled and roasted seeds were significantly (P < 0.05) increased by 51.28% and 92.31%, respectively, compared to the flour of raw anchote seeds. The increase in oil absorption capacity upon roasting may be attributed to the denaturation and dissociation of the constituent protein. The oil absorption capacity is also effected by different factors such as the starch–protein–lipid bindings, the sequence of polypeptides, different conformational features of macromolecules, and the amount of nonpolar amino acids. This study suggests that the flour of boiled and sun-dried tubers and boiled and roasted seeds of anchote could give better palatability and better flour retention. The flours in the present study are potentially useful in structural interaction in food especially in flavor retention, improvement of palatability, and extension of shelf life, particularly in the bakery or meat products where oil absorption is desired (Aremu et al., Citation2007).
5.10. Effect of pretreatment on the emulsifying capacity
The emulsifying capacities of the flour of raw anchote tubers and seeds were 25.01% and 45.06%, respectively (Table ). The emulsifying capacity of the raw anchote seeds (45.06%) was higher than the value reported by Adelakun et al. (Citation2017) for five pumpkin seed varieties (2.92–3.44%). Emulsifying capacities of the flour of boiled and sun-dried tubers were 21.60% and 22.43%, respectively, and were 38.00%, 41.51%, 43.41%, and 36.06% for the flour of boiled, soaked, germinated, and roasted seeds, respectively. Emulsifying capacities of the flour of boiled and sun-dried tubers were significantly (P < 0.05) decreased by 13.59% and 10.27%, respectively, compared to the flour of raw tubers. The emulsifying capacities of the flour of boiled, soaked, and roasted seeds were also significantly (P < 0.05) decreased by 15.66%, 7.87%, and 19.96%, respectively, compared to raw seed flour. Reduction in emulsion capacities of the samples may be probably effected by their respective oil contents (Ihemeje et al., Citation2015). Another possible cause of the reduction could be the thermal denaturation of the protein caused by heating (Chandra et al., Citation2015). The flour of all the raw and processed tubers and seeds showed relatively good emulsion capacities.
5.11. Effect of pretreatment on the emulsion stability
Table shows the emulsion stability of the flour of raw and processed anchote tubers and seeds. The result of emulsion stability of the flour of anchote tubers and seeds were 31.78% and 42.19%, respectively. The emulsion stability of the raw anchote seeds (42.19%) was higher than the value reported by Adelakun et al. (Citation2017) for five seed varieties (1.33–1.81%). Emulsion stability of the flour of boiled and sun-dried tubers was 30.24% and 31.19%, respectively, and it was 40.44%, 41.94%, 41.50%, and 38.13% for the flour of boiled, soaked, germinated, and roasted seeds, respectively (Table ). Emulsion stability of the flour of all the processed tubers and seeds of anchote were not significantly (P > 0.05) different from that of the flour of raw tubers and seeds of anchote, respectively. Emulsion stability can be greatly increased when highly cohesive films are formed by the absorption of rigid globular protein molecules that are more resistant to mechanical deformation (Chandra et al., Citation2015). Increasing emulsion stability and fat binding during processing are the primary functional properties of protein foods such as comminuted meat products, salad dressing, frozen desserts, and mayonnaise. In line with this, the raw and processed tubers and seeds of anchote showed relatively good emulsion stability.
5.12. Effect of pretreatment on the foaming capacity
Table shows the result of the foaming capacities of the flour of raw and processed anchote tubers and seeds. The result of foaming capacities of the flour of raw anchote tubers and seeds were 53.47% and 61.49%, respectively. The foaming capacity of the raw anchote seeds (61.49%) was higher than the value reported by Adelakun et al. (Citation2017) for five seed varieties (73–6.44%). Flours with high foaming ability could form large air bubbles surrounded by thinner less flexible protein film. This air bubbles might be easier to collapse and, consequently, lower the foam stability (Jitngarmkusol et al., Citation2008). Foaming capacities of the flour of boiled and sun-dried tubers were 52.03% and 51.28%, respectively, and were 57.81%, 58.02%, 60.11%, and 54.72% for the flour of boiled, soaked, germinated, and roasted seeds, respectively. Foaming capacities of the flour of boiled and sun-dried tubers were not significantly (P > 0.05) different from that of the raw tubers. Foaming capacities of the flour of boiled, soaked, and roasted seeds were significantly (P < 0.05) decreased by 8.28%, 8.70%, and 17.32%. The decrease observed in foaming capacity might have been as a result of denaturation of protein molecules during boiling, soaking, and roasting processes. The effect of heat processing on foam capacity and stability of winged bean flour has been reported (Ihemeje et al., Citation2015).
Kouakou also reported that native protein provides higher foam capacity than denatured protein. Since proteins are heat labile, the reduced foaming capacity and stability of heat processed flours can be explained on the basis of protein denaturation, the flour of raw seeds gave a higher foam capacity than the processed one. The reduction in foaming capacity could be as a result of a fat reduction in processed seeds, and this suggests its utilization as a supplement in infant feed formulations where aeration is not necessary.
5.13. Effect of pretreatment on the foam stability
The foam stability of the flour of raw anchote tubers and seeds was 33.57% and 27.11%, respectively (Table ). The foam stability of the raw anchote seeds (27.11%) was higher than the value reported by Adelakun et al. (Citation2017) for five seed varieties (3.78–4.50%). Foam stability of the flour of boiled and sun-dried tubers was 31.16% and 31.63%, respectively, and t was 21.52%, 24.75%, 25.94%, and 22.41% for the flour of boiled, soaked, germinated, and roasted seeds, respectively. The foaming stability of the flour of boiled and sun-dried anchote tubers was not significantly (P > 0.05) different from that of raw tubers flour. In addition, the foaming stability of the flour of soaked and germinated seeds was also not significantly (P > 0.05) different from that of the raw seeds. However, the foam stability of the flour of boiled and roasted seeds was significantly (P < 0.05) decreased by 20.60% and 17.32% compared to the raw seeds. Foam stability has been suggested to be related to the amount of native protein, which is considerably low in heat-treated proteins. A similar effect of heat processing on foam capacity and stability of cowpea flour has been reported by Giami (Citation2003). The ability to form stable foam is an important property in whipped toppings, frozen desserts, and sponge cakes (Adelakun et al., Citation2012); thus, the flour of roasted anchote seeds cannot be used in these formulations.
6. Conclusion
The study revealed that the various pretreatment methods significantly affect phytochemical contents, antioxidant activity, and functional properties of tubers and seeds of anchote. The study showed that germination and roasting is a good way to increase total phenolic and flavonoid content and enhance DPPH scavenging and chelating effect. All the processing methods resulted in an increase in the water and oil absorption capacities and decrease in the bulk density, emulsion capacity and stability, and foaming capacity and stability. In general, the functional properties of the flour of raw and processed tubers and seeds of anchote revealed their uniqueness to each parameter measured, and these results may probably assist in determining the behavior and application of these flours in various food formulations.
Acknowledgments
I would like to express my sincere gratitude to Wollega University for funding the project.
Disclosure statement
No potential conflict of interest was reported by the author.
Data availability statement
All the data generated from this study have been presented in the manuscript.
Additional information
Notes on contributors

Habtamu Fekadu Gemede
Habtamu Fekadu Gemede (PhD) is an Asociate Professor and researcher at department of Food Technology and Process Engineering, Wollega University, Ethiopia. His research focus is on the characterization of food compositions, food quality and safety underutilized indigenous food crops of Ethiopia. He has particularly focused on the nutritional quality, Nutritional analysis, Food Microbiology, Food chemistry and phytochemical properties food related findings. He has published a number of research papers.
References
- Abbey, B. W., & Ibeh, G. O. (1988). Functional properties of raw and heat processed cowpea (Vigna unguiculata, Walp) flour. Journal of Food Science, 53(6), 1775–15. https://doi.org/10.1111/j.1365-2621.1988.tb07840.x
- Adebiyi, A. P., Adeyemi, I. A., & Olorunda, A. O. (2002). Effects of processing conditions and packaging material on the quality attributes of dry‐roasted peanuts. Journal of the Science of Food and Agriculture, 82(13), 1465–1471. https://doi.org/10.1002/jsfa.1192
- Adelakun, O. E., Ade Omowaye, B. I. O., Adeyemi, I. A., & Van de Venter, M. (2012). Mineral composition and the functional attributes of Nigerian okra seed (Abelmoschus esculentus -Moench) flour. Food Research International, 47(2), 348–352. https://doi.org/10.1016/j.foodres.2011.08.003
- Adelakun, O. E., Olanipekun, B. F., Akingbaso, O., & Adhikar, B. M. (2017). Effect of fermentation and variety on quality attributes of okra seed (Abelmoschus esculentus (L) Moench) flour. Donnish Journal of Food Science and Technology, 3(1), 12–34.
- Adelakun, O. E., Oyelade, O. J., Ade Omowaye, B. I. O., Adeyemi, I. A., & Van de Venter, M. (2009). Chemical composition and the antioxidative properties of Nigerian okra seed (Abelmoschus esculentus Moench) flour. Food and Chemical Toxicology, 47(6), 1123–1126. https://doi.org/10.1016/j.fct.2009.01.036
- Adeniyan, O. O., Ibukun, E. O., Ogunbolude, Y., & Eseigbe, M. I. (2013). Effect of boiling on the nutritional composition and antioxidant properties of beniseed (Sesamum indicum L.). Food Science and Quality Management, 11, 123–132.
- Adetuyi, O. F., & Stella, B. O. (2012). Effects of different traditional cooking methods on nutrients and mineral bioavailability of okra (Abelmoschus esculentus). Journal of Agricultural Research and Development, 11(1), 51–59.
- Admassu, S. (2009). Potential health benefits and problems associated with phytochemicals in food legumes. East African Journal of Sciences, 3(2), 116–133. https://doi.org/10.4314/eajsci.v3i2.53210
- Akingbala, J. O., Akinwande, B. A., & Uzo-Peters, P. I. (2003). Effects of color and flavor changes on acceptability of ogi supplemented with okra seed meals. Plant Foods for Human Nutrition, 58(3), 1–9. https://doi.org/10.1023/B:QUAL.0000040354.96979.f2
- Amandikwa, C., & Chinyere, E. (2012). Proximate and functional properties of open air, solar and oven dried cocoyam flour. International Journal of Agriculture and Rural Development, 15, 988–994. https://doi.org/10.12691/jfs-7-2-3
- Amić, D., Davidović-Amić, D., Bešlo, D., & Trinajstić, N. (2003). Structure-radical scavenging activity relationships of flavonoids. Croatica Chemica Acta, 76(1), 55–61.
- Ansari, N. M., Houlihan, L., Hussain, B., & Pieroni, A. (2005). Antioxidant activity of five vegetables traditionally consumed by South-Asian migrants in Bradford, Yorkshire, UK. Phytotherapy Research, 19(10), 907–911. https://doi.org/10.1002/ptr.1756
- Aremu, M. O., Olaofe, O., & Akintayo, E. T. (2007). Functional properties of some Nigerian varieties of legume seed flours and flour concentration effect on foaming and gelation properties. Journal of Food Technology, 5(2), 109–113.
- Arinola, S. O., Ogunbusola, E. M., & Adebayo, S. F. (2016). Effect of drying methods on the chemical, pasting and functional properties of unripe plantain (Musa paradisiaca) flour. British Journal of Applied Science & Technology, 14(3), 1–7. https://doi.org/10.9734/BJAST/2016/22936
- Beruk, B. D., Fikre, T. T., & Dereje, H. (2015). Physical and proximate characterization of anchote (Coccinia abyssinica) accessions grown under hawassa and wondo genet conditions, Southern Ethiopia. Food Science and Quality Management, 42, 62–74.
- Boateng, J., Verghese, M., Walker, L. T., & Ogutu, S. (2008). Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.). Lwt-Food Science and Technology, 41(9), 1541–1547. https://doi.org/10.1016/j.lwt.2007.11.025
- Bryant, L. A., Montecalvo, J., Morey, K. S., & Loy, B. (1988). Processing, functional, and nutritional properties of okra seed products. Journal of Food Science, 53(3), 810–816. https://doi.org/10.1111/j.1365-2621.1988.tb08960.x
- Butt, M. S., & Batool, R. (2010). Nutritional and functional properties of some promising legumes protein isolates. Pakistan Journal of Nutrition, 9(4), 373–379. https://doi.org/10.3923/pjn.2010.373.379
- Çalışır, S., Özcan, M., Hacıseferoğulları, H., & Yıldız, M. U. (2005). A study on some physico-chemical properties of Turkey okra (Hibiscus esculenta L.) seeds. Journal of Food Engineering, 68(1), 73–78. https://doi.org/10.1016/j.jfoodeng.2004.05.023
- Chandra, S., Singh, S., & Kumari, D. (2015). Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. Journal of Food Science and Technology, 52(6), 3681–3688. https://doi.org/10.1007/s13197-014-1427-2
- Chauhan, A., & Singh, S. (2013). Influence of germination on physico–chemical properties of amaranth (Amaranthus Spp.) flour. International Journal of Agriculture, Food Science and Technology, 4, 215–220.
- Chen, G., Wang, H., Zhang, X., & Yang, S. T. (2014). Nutraceuticals and functional foods in the management of hyperlipidemia. Critical Reviews in Food Science and Nutrition, 54(9), 1180–1201. https://doi.org/10.1080/10408398.2011.629354
- Dewanto, V., Wu, X., Adom, K. K., & Liu, R. H. (2002). Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry, 50(10), 3010–3014. https://doi.org/10.1021/jf0115589
- Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S., & Ju, Y. H. (2014). Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. Journal of Food and Drug Analysis, 22(3), 296–302. https://doi.org/10.1016/j.jfda.2013.11.001
- Duenas, M., Hernandez, T., Estrella, I., & Fernandez, D. (2009). Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chemistry, 117(4), 599–607. https://doi.org/10.1016/j.foodchem.2009.04.051
- Ebrahimzadeh, M. A., Pourmorad, F., & Hafezi, S. (2008). Antioxidant activities of Iranian corn silk. Turkish Journal of Biology, 32(1), 43–49. https://journals.tubitak.gov.tr/biology/vol32/iss1/7
- Enujiugha, V. N., Badejo, A. A., Iyiola, S. O., & Oluwamukomi, M. O. (2003). Effect of germination on the nutritional and functional properties of African oil bean (Pentaclethra macrophylla Benth) seed flour. Journal of Food Agriculture and Environment, 1, 72–75.
- Fekadu, H., Beyene, F., & Desse, G. (2014). Evaluation of bioavailability and sensory preference of processed Anchote (Cocciniaabyssinica) tubers in Eastern Wollega, Ethiopia. Journal of Food and Nutrition Sciences, 2(1), 1–12. https://doi.org/10.11648/j.jfns.20140201.11
- Ferreira, I. C., Baptista, P., Vilas-Boas, M., & Barros, L. (2007). Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chemistry, 100(4), 1511–1516. https://doi.org/10.1016/j.foodchem.2005.11.043
- Finkel, T., & Holbrook, N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature, 408(6809), 239–247. https://doi.org/10.1038/35041687
- Gallegos-Infante, J. A., Rocha-Guzman, N. E., Gonzalez Laredo, R. F., & Pulido-Alonso, J. (2010). Effect of processing on the antioxidant properties of extracts from Mexican barley (Hordeum vulgare) cultivar. Food Chemistry, 119(3), 903–906. https://doi.org/10.1016/j.foodchem.2009.07.044
- Giami, S. Y. (2003). Effect of processing on the proximate composition and functional properties of cowpea (Vigna ungniculate) flour. Food Chemistry, 47, 153–158. https://doi.org/10.1016/0308-8146(93)90237-A
- Giami, S. Y., & Alu, D. A. (1994). Changes in composition and certain functional properties of ripening plantain (Musa spp., AAB group) pulp. Food Chemistry, 50(2), 137–140. https://doi.org/10.1016/0308-81469490110-4
- Ihemeje, A., Ukauwa, O., & Ekwe, C. C. (2015). Effects of cooking and germination on physiochemical properties and sensory attributes of African walnut (Tetracarpidium conophorum). International Journal of Pharmacology, Phytochemistry and Ethnomedicine, 1, 93–102. https://doi.org/10.18052/www.scipress.com/IJPPE.1.93
- Jannat, B., Oveisi, M. R., Sadeghi, N., Hajimahmoodi, M., Behzad, M., Choopankari, E., & Behfar, A. A. (2010). Effects of roasting temperature and time on healthy nutraceuticals of antioxidants and total phenolic content in Iranian sesame seeds (Sesamum indicum L.). Iranian Journal of Environmental Health Science and Engineering, 7, 97–102.
- Jeong, S. M., Kim, S. Y., Kim, D. R., Nam, K. C., Ahn, D. U., & Lee, S. C. (2004). Effect of seed roasting conditions on the antioxidant activity of defatted sesame meal extracts. Journal of Food Science Chicago, 69, 377–381. https://doi.org/10.1111/j.1365-2621.2004.tb10701.x
- Jitngarmkusol, S., Hongsuwankul, J., & Tananuwong, K. (2008). Chemical compositions, functional properties, and microstructure of defatted macadamia flours. Food Chemistry, 110(1), 23–30. https://doi.org/10.1016/j.foodchem.2008.01.050
- Khattak, G. S. S., Ashraf, M., Zamir, R., & Saeed, I. (2007). High yielding DESI chickpea (Cicer arietinum L.) variety. Pakistan Journal of Botany, 39(1), 93.
- Kim, S. Y., Jeong, S. M., Park, W. P., Nam, K. C., Ahn, D. U., & Lee, S. C. (2006). Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chemistry, 97(3), 472–479. https://doi.org/10.1016/j.foodchem.2005.05.027
- Liu, R. H. (2004). Potential synergy of phytochemicals in cancer prevention: Mechanism of action. The Journal of Nutrition, 134(12), 3479S–3485S. https://doi.org/10.1093/jn/134.12.3479S
- Liu, S. C., Lin, J. T., Wang, C. K., Chen, H. Y., & Yang, D. J. (2009). Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chemistry, 114(2), 577–581. https://doi.org/10.1016/j.foodchem.2008.09.088
- Manzocco, L., Calligaris, S., Mastrocola, D., Nicoli, M. C., & Lerici, C. R. (2000). Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends in Food Science & Technology, 11(9), 340–346. https://doi.org/10.1016/S0924-2244(01)00014-0
- Mepba, H. D., Eboh, L., & Banigo, D. E. B. (2007). Effects of processing treatments on the nutritive composition and consumer acceptance of some Nigerian edible leafy vegetables. African Journal of Food Agriculture Nutrition & Development, 7(1), 1–16.
- Nasar-Abbas, S. M., Plummer, J. A., Siddique, K. H., White, P., Harris, D., & Dods, K. (2008). Cooking quality of faba bean after storage at high temperature and the role of lignins and other phenolics in bean hardening. LWT-Food Science and Technology, 41(7), 1260–1267. https://doi.org/10.1016/j.lwt.2007.07.017
- Nishaa, S., Vishnupriya, M., Sasikumar, J. M., Hephzibah, P. C., & Gopalakrishnan, V. K. (2012). Antioxidant activity of ethanolic extract of Maranta arundinacea L. tuberous rhizomes. Asian Journal of Pharmaceutical and Clinical Research, 5(4), 85–88.
- Oghbaei, M., Prakash, J., & Yildiz, F. (2016). Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent Food & Agriculture, 2(1), 1136015. https://doi.org/10.1080/23311932.2015.1136015
- Oladele, A. K., & Aina, J. O. (2007). Chemical composition and functional properties of flour produced from two varieties of tigernut (Cyperus esculentus). African Journal of Biotechnology, 6(21), 2473–2476. https://doi.org/10.5897/AJB2007.000-2391
- Osundahunsi, O. F., & Aworh, A. C. (2002). A preliminary study on the use of tempe-based formula as a weaning diet in Nigeria. Plant Foods for Human Nutrition, 57(3–4), 365–376. https://doi.org/10.1023/A:1021805117084
- Pandey, H., & Awasthi, P. (2015). Effect of processing techniques on nutritional composition and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed flour. Journal of Food Science and Technology, 52(2), 1054–1060. https://doi.org/10.1007/s13197-013-1057-0
- Randhir, R., Kwon, Y. I., & Shetty, K. (2008). Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innovative Food Science & Emerging Technologies, 9(3), 355–364. https://doi.org/10.1016/j.ifset.2007.10.004
- Scherer, R., & Godoy, H. T. (2009). Antioxidant activity index (AAI) by the 2, 2-diphenyl-1-picrylhydrazyl method. Food Chemistry, 112(3), 654–658. https://doi.org/10.1016/j.foodchem.2008.06.026
- Şensoy, Í., Rosen, R. T., Ho, C. T., & Karwe, M. V. (2006). Effect of processing on buckwheat phenolics and antioxidant activity. Food Chemistry, 99(2), 388–393. https://doi.org/10.1016/j.foodchem.2005.08.007
- Sharma, P., & Gujral, H. S. (2013). Extrusion of hulled barley affecting β-glucan and properties of extrudates. Food and Bioprocess Technology, 6(6), 1374–1389. https://doi.org/10.1007/s11947-011-0777-2
- SHARMA, V., Khan, A., & Shukla, R. K. (2016). Growth and yield attribute of Okra (Abelmoschus esculentus L.) under the application of bio and chemical fertilizers either alone or in combination. International Journal of Agricultural Science and Research (IJASR), 1(1), 189–198. https://doi.org/10.46492/IJAI/2016.1.1.17
- Siddhuraju, P., & Becker, K. (2001). Effect of various domestic processing methods on antinutrients and in vitro protein and starch digestibility of two indigenous varieties of Indian tribal pulse, Mucuna pruriens var. utilis. Journal of Agricultural and Food Chemistry, 49(6), 3058–3067. https://doi.org/10.1021/jf001453q
- Steve, I. O. (2011). Influence of germination and fermentation on chemical composition, protein quality and physical properties of wheat flour (Triticum aestivum). Journal of Cereals and Oilseeds, 3(3), 35–47.
- Sultana, B., Anwar, F., & Iqbal, S. (2008). Effect of different cooking methods on the antioxidant activity of some vegetables from Pakistan. International Journal of Food Science & Technology, 43(3), 560–567. https://doi.org/10.1111/j.1365-2621.2006.01504.x
- Taffesse, A. S., Dorosh, P., & Asrat, S. (2012). Crop production in Ethiopia: regional patterns and trends summary of ESSP II Working Paper, crop production in Ethiopia. Regional Patterns and Trends, 74(3), 53.
- Thidarat, S., Udomsak, M., Jindawan, W., Namphung, D., Suneerat, Y., Sawan, T., & Pisamai, T. (2016). Effect of roasting on phytochemical properties of Thai soybeans. International Food Research Journal, 23(2).
- Tsado, A. N., Lawa, B., Santali, E. S., Shaba, A. M., Chirama, D. N., & Balarabe, M. M. (2013). Effect of different processing methods on nutritional composition of bitter leaf (Vernonia amygdalina). IOSR Journal of Pharmacy and Biological Sciences, 5(6), 08–14.
- Woldegiorgis, A. Z., Abate, D., Haki, G. D., & Ziegler, G. R. (2014). Antioxidant property of edible mushrooms collected from Ethiopia. Food Chemistry, 157, 30–36. https://doi.org/10.1016/j.foodchem.2014.02.014
- Xu, B. J., & Chang, S. K. C. (2007). A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. Journal of Food Science, 72(2), S159–S166. https://doi.org/10.1111/j.1750-3841.2006.00260.x
- Yang, R., Tsou, S., Lee, T., Wu, W., Hanson, P. M., Kuo, G., Engle, L. M., & Lai, P. (2006). Distribution of 127 edible plant species for antioxidant activities by two assays. Journal of the Science of Food and Agriculture, 86(14), 2395–2403. https://doi.org/10.1002/jsfa.2630
- Yu, J., Ahmedna, M., & Goktepe, I. (2005). Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chemistry, 90(1), 199–206. https://doi.org/10.1016/j.foodchem.2004.03.048