?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Recently, resource consumption and population growth have negatively impacted the environment. The use of algae as biofertilizers or biostimulants is one of the most innovative agronomic techniques as an alternative to chemicals. It holds promise for the development of sustainable agricultural systems. Accordingly, this study aimed to determine the effects of spirulina (a microalgae species) and brown seaweed (a macroalgae species) on the yield and quality of peppers. A total of 7 applications [seaweed (2000 ppm, 4000 ppm, and 6000 ppm), spirulina (2000 ppm, 4000 ppm, and 6000 ppm), and control] were carried out 3 times at 21-day intervals from the seedling planting. Parameters such as yield per plant, number of fruits, fruit weight, fruit length, fruit width, stem diameter, fruit flesh thickness, fruit colors, chlorophyll content, titratable acidity, vitamin C, total/reducing sugars, total phenolic, and ß-carotene were analyzed. The results showed that both spirulina and seaweed applications had a positive effect on the yield and quality parameters of pepper, however, spirulina applications provided better results in terms of yield parameters. The highest yield (g/plant) values were obtained in all spirulina treatments. While the highest fruit weight (26.50 g) and fruit width (23.52 mm) values were determined in the 4000 ppm spirulina application, the highest fruit length value (21.55 cm) was obtained in the 6000 ppm spirulina application. Fruit numbers ranging between 92.01 and 115.35 and chlorophyll (SPAD) values ranging between 70.89 and 78.97 were highest in 2000 ppm spirulina application.
1. Introduction
In order to supply the fast rising demand for food as a result of the world’s expanding population, crop production must be increased (Mandal et al., Citation2020). The use of the available resources in a sustainable way becomes essential because natural resources are limited. It is important that agricultural activities that provide food production, which is one of the most basic requirements for the continuation of human life, are carried out without harming the environment and natural resources by taking into account the needs of future generations while meeting today’s needs (Tuncer, Citation2022). So, it is becoming more and more important to use natural resources with biological and organic origins in agriculture as a substitute for chemicals to maintain both excellent output and sustainability (Chen et al., Citation2022).
Algae have been reported to be one of the resources that can help change our world’s food system by promoting healthy diets and minimizing environmental damage (Tagliapietra & Clerici, Citation2023). Algae can be utilized both as a natural raw material source and directly as a biofertilizer or enhancer in agricultural production due to their high carbon dioxide-binding ability and the nutrients they contain (Piwowar & Harasym, Citation2020). Algae is an environmentally friendly alternative to synthetic chemicals used in agriculture due to its many positive effects such as reducing pollution caused by chemical use by improving plant and soil structure (Abdel-Raouf et al., Citation2012), increasing soil fertility, and fixing nitrogen required for plants (Das et al., Citation2019). Algae are classified as macro and microalgae. Brown algae are the largest and one of the three types of macroalgae [red algae (Rhodophyceae), green algae (Chlorophyceae), and brown algae (Phaeophyceae)] (Pereira, Citation2021) that are the most widely used macroalgae species in agricultural production because they are richer than other macroalgae in macro and microelements, amino acids, and vitamins essential for plant growth and development (Hong et al., Citation2007). Spirulina is a Cyanophyceae-class microalga, but because of its prokaryotic structure, it is classified as a bacterium (Koru, Citation2012). According to Morais et al. (Citation2015), spirulina is a rich source of protein and contains significant amounts of essential polyunsaturated fatty acids and phenolic compounds and due to its active biological compounds and high nutritional value, spirulina is one of the most studied microalgae worldwide.
Pepper fruit is a valuable source of health-beneficial compounds (Darko et al., Citation2022) such as vitamin C, vitamin E, carotenoids, capsaicinoids (Wahyuni et al., Citation2013), and minerals such as iron, calcium, magnesium, sodium, phosphorus, copper, and zinc (Rubio et al., Citation2002). Capsaicin, primarily produced by the chili pepper fruits of the genus Capsicum, is a heterocyclic vanilloid compound (Brown et al., Citation2023). The main ingredient that gives the pepper its bitterness, has very positive health effects; it stimulates the cardiovascular and respiratory systems as well as its pain relieving effect and anti-tumor properties (Muchena, Citation2009).
As part of traditional medicine, pepper is used to treat coughs, rheumatism, sore throats, and gastrointestinal disorders (Wahyuni et al., Citation2013). Due to their strong antioxidant properties, phytochemicals such as carotenoids, capsaicinoids, and flavonoids found in fruits have positive effects such as anticancer and antimicrobial (Darko et al., Citation2022). Having wide varieties with a wide range of colors, sizes, shapes, and chemical compositions, being a valuable raw material source for the agriculture-based industry, and its suitability for consumption in different forms are pepper’s essential features (Kayak et al., Citation2022).
The present study aimed to determine the effects of brown seaweed and spirulina on the yield and fruit quality (fruit colors, chlorophyll content, titratable acidity, vitamin C, total/reducing sugars, total phenolic, and ß-carotene) of pepper.
2. Materials and methods
2.1. Materials
The study’s plant material was the Şehzade F1 pepper variety (Anamas Tohum Ltd. Gi., Antalya), which is suitable for fall, spring, greenhouse, and open field cultivation, tolerant to TSWV (Tomato Spotted Wilt Virus), and has smooth, green fruits. In this study, a commercial liquid seaweed material named “Blue Fresh” (Ouragro S. L. company) obtained from brown seaweed (Ascophyllum nodosum) was used (Table ).
Table 1. Seaweed properties identified by the Ouragro S. L. company
The spirulina material used in the study was supplied and produced by the Egert company (İzmir). Spirulina was provided as a powder, which was then dissolved in distilled water (2000, 4000, 6000 ppm) and sprayed until the plants were completely wet. Some properties related to the vitamin content of spirulina with 5% water and 60–70% protein ratio are given in Table .
Table 2. Spirulina properties identified by the Egert company
The research was conducted in a greenhouse belonging to Anamas Seed Ltd. Şti. company (Antalya) in 2021. The research area, located between 36° 57’north latitude and 30° 57’ east longitude, is approximately 19 m above sea level. Throughout the study, the temperature (day: 21–26°C, night: 15–17°C) and air relative humidity (70–75%) required for optimum growth of pepper plants were maintained. The soil properties of the study area were analyzed by AgriOLabEN Food and Agricultural Laboratory and the results are given in Table . According to the soil analysis results, it was determined that the soil structure of the study area was clay-loamy (Table ).
Table 3. Physicochemical properties of the soil in experimental area
2.2. Methods
On 02.02.2021, seedlings with 4–5 leaves were planted in the greenhouse. The study was conducted according to the randomized plots experimental design with three replications and 15 plants in each replicate. The spacing between plants was planned as 40 cm, between narrow rows as 70 cm, and between wide rows as 110 cm. A total of seven applications, including three different doses of seaweed (2000 ppm, 4000 ppm, and 6000 ppm), three different doses of spirulina (2000 ppm, 4000 ppm, and 6000 ppm), and a control application (water spraying only), were carried out in the study.
Solutions were prepared containing 0.5% Tween 20 (Sigma) as surfactant and were applied as a foliar spray utilizing a hand-held sprayer. Applications were carried out three times until the plants were completely wet (seedlings with 4–5 leaves, seedlings with 14–15 leaves, and at the beginning of flowering) in total at 21-day intervals from the seedling planting. Pepper harvests were carried out on 27.04.2021, 05.05.2021, 11.05.2021, 18.05.2021, 27.05.2021, and 04.06.2021.
In the experiment, 54 kg potassium nitrate, 55 kg magnesium sulfate, 22 kg mono ammonium phosphate, 35 kg calcium nitrate, 32 kg mono potassium phosphate, 3.5 kg ammonium nitrate, 4 kg iron, and 4 kg micronutrients were given per decare with drip irrigation system during the vegetation period. During the period of the study, 200 g/L Fluopyram +200 g/L Tebuconazole and 125 g/L Fluopyram +375 g/L Pyrimethanil were used for powdery mildew and lead mildew diseases, respectively. Pesticides with active ingredients of 20% Acetamiprid, 100 g/L Imidacloprid +30 g/L Bifenthrin, 50 g/L Hexythiazox, 18 g/L Abamectin, 120 g/L Spinetoram, and 45 g/L Chlorantraniliprole +18 g/L Abamectin were used to control pests such as whitefly, thrips, and red spider.
The quality analysis of the pepper samples harvested at the end of the study was carried out in the laboratory of Isparta University of Applied Sciences, Faculty of Agriculture, Department of Horticulture.
2.2.1. Yield parameters
Yield per plant (g/plant) was determined by dividing the total fruit weight obtained from the plot by the number of plants. The number of fruits harvested from the plot was divided by the number of plants to determine the average number of fruits. The total fruit weight in each replicate of the plots was divided by the total number of fruits in the replicate and the average fruit weight was determined as g. For fruit length, 20 fruits were selected from each replicate and the averages were measured in cm from the end of the stem to the tip of the fruit using a ruler. For fruit width and flesh thickness, the averages of the measurements made with the help of calipers from the midpoint of 20 fruits selected from each replicate were determined in mm.
2.2.2. Stem diameter
The stem diameter of five randomly selected plants from each replicate was measured with the help of calipers about 5 cm above the soil, and the average was determined in mm.
2.2.3. Fruit colors
Color values were determined using CR 400 model Minolta colorimeter. L* (brightness), -a* (greenness), and b* (yellowness) were measured on both surfaces of the pepper fruits near the fruit stalk, and Chroma (C*) value and hue angle (h°) values were calculated using Equation (3.1) (McGuire, Citation1992).
2.2.4. Chlorophyll content
Chlorophyll SPAD 502 Plus chlorophyll meter was used to determine the chlorophyll values of pepper leaves selected from four different parts of four plants in replication, and the values obtained were given in SPAD.
2.2.5. Titratable acidity
According to Cemeroglu (Citation2013), 10 ml of juice was taken from the pepper samples and titrated with 0.1 N NaOH (Merck) solution until pH 8.1, and titratable acidity was expressed as % in citric acid.
2.2.6. Vitamin C content
The vitamin C content of pepper fruits was determined according to the 2% oxalic acid (Merck) method described by Cemeroglu (Citation2013). Using 2,6 dichlorophenolindophenol solution (Sigma), the data obtained as a result of the titration process were evaluated and the results were expressed in mg/100 g.
2.2.7. Total sugars/reducing sugars
First, the samples were homogenized in 80% ethanol (Sigma) to extract the sugars. Following the overnight incubation at −20°C, samples were centrifuged with an RCF (relative centrifugal force) of 2,000×g for 5 minutes. The supernatant was used to calculate the total soluble and reducing sugars after centrifugation. The amount of total soluble sugars was calculated using the phenol sulfuric acid method (Dubois et al., Citation1956), and the amount of reducing sugars was calculated in accordance with Honda et al. (Citation1980). For both assays, glucose (Merck) was employed as a standard at concentrations of 40, 80, 120, and 200 μg/ml. The data obtained were expressed in mg/g.
2.2.8. Total phenolic
In order to determine the total phenolic content of pepper fruits, the extraction process was carried out first. For this, 5 g pepper samples were homogenized in 10 ml of 95% ethanol. The samples were kept in a boiling water bath for 10 minutes and centrifuged at 8000 rpm. Then, 10 ml of 80% ethyl alcohol was added to the samples filtered through filter paper and kept in a boiling water bath again for 10 minutes. After this process, 100 ml of 80% ethyl alcohol was added to all samples. The readings of the samples obtained after the steps performed in accordance with the protocol using the Folin-Ciocalteu reagent (Sigma) were made at 760 nm wavelength in a spectrophotometer, and the results obtained were given in mg/100 g (Coseteng & Lee, Citation1987).
2.2.9. ß-carotene
The method of Nagata and Yamashita (Citation1992) was used to determine the ß-carotene content of pepper fruits. The homogenized samples were extracted with acetone: hexane (Sigma) mixture (4:6). The results of the samples, which were read in the spectrophotometer at 663, 645, 505, and 453 nm wavelengths, were calculated and expressed as µg/g.
2.3. Statistical analysis
The data obtained from the study were subjected to a one-way analysis of variance using Minitab (17) inc. package program. Differences between significant means were determined using the Tukey test and indicated by different letters.
3. Results and discussion
3.1. Yield
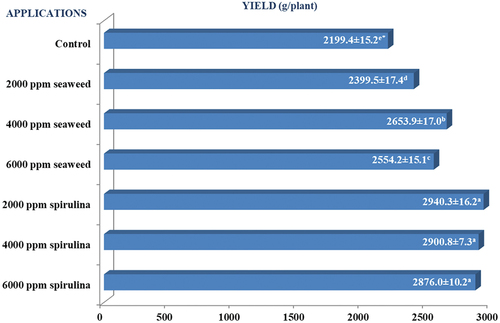
The effectiveness of the applications on pepper yield was found to be significant (P < 0.05) (Figure ). According to the applications, it was determined that the yield values of pepper varied between 2199.4 and 2940.3 g/plant. The lowest value was found in the control application (2199.4 g/plant), while the highest yield values were obtained in 2000 ppm spirulina (2940.3 g/plant), 4000 ppm spirulina (2900.8 g/plant), and 6000 ppm spirulina (2876.0 g/plant) applications.
Shedeed et al. (Citation2022) found that the foliar application of spirulina increased yield. They suggested that this may be related to the active compounds produced by spirulina, similar to phytohormones, and may be related to its participation in signaling pathways that support yield. They also reported that microalgae contain a wide range of beneficial compounds such as vitamins, amino acids, and exopolysaccharides and thus may have played a role in promoting yield. Wuang et al. (Citation2016) reported that spirulina had similar effects compared to chemical fertilizers (15:15:15). They reported that this is due to the fact that spirulina contains less nitrogen, phosphorus, and potassium, but more calcium, iron, manganese, and zinc than chemical fertilizers and discussed the roles of these nutrients. Iron is a structural component of several essential enzymes and manganese acts as an enzyme activator for nitrogen assimilation and is an essential element for chlorophyll. Zinc is involved in the synthesis of growth substances, enzyme systems, and root tips. Calcium is important in maintaining the elasticity and expansion of cell walls, which prevents growth points from becoming stiff and brittle. Jufri et al. (Citation2016) found that foliar spirulina applications provided higher yields than the control treatment in their study on hot pepper. They reported that this was due to the fact that spirulina contains macro and microelements and helps plants to provide the necessary elements directly through the leaves. According to Mógor et al. (Citation2018), spirulina treatments increased the amount of protein, sugar, total free amino acids, and chlorophyll in leaves, which promoted the yield. They also stated that spirulina can be considered as a source of biofertilizer/biostimulant that supports sustainable yield increase. Aly and Esawy (Citation2008) reported that foliar applications of spirulina to peppers increased yields due to some growth-promoting substances, high free amino acids, and macro and microelements. All these reports explain the reason for the 33.67% (2000 ppm spirulina), 31.89% (4000 ppm spirulina), and 30.76% (6000 ppm spirulina) increase in yield compared to the control group in our study. In fact, studies conducted by different researchers reported that spirulina applications significantly increased yield values in pepper (Jufri et al., Citation2016), arugula (Hassan et al., Citation2017), artichoke (Sayed et al., Citation2018), red beet (Mógor et al., Citation2018), lettuce (Siringi et al., Citation2022), and pea (Ismaiel et al., Citation2022). Moreover, spirulina applications were reported to cause yield increases of about 75% in eggplant (Dias et al., Citation2016), 42.34% in spinach (Abo-Basha et al., Citation2019), 21.4% in okra (Uddin et al., Citation2019), 52.19% (Acun, Citation2019) and 12.5% (Lerer et al., Citation2021) in lettuce. These results support our study.
3.2. Fruit number
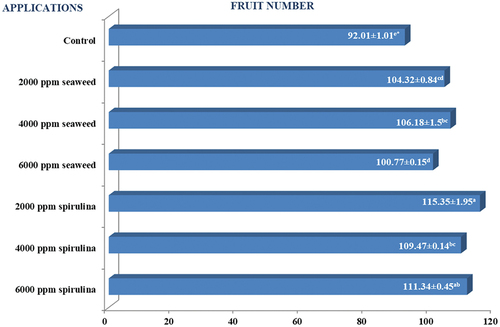
Figure shows that the effect of the applications on fruit number was found to be significant (P < 0.05). The control application had the lowest fruit number value (92.01), whereas the 2000 ppm spirulina application had the highest fruit number value (115.35).
It was determined that spirulina-treated pepper plants positively affected the fruit number values by 25.4% compared to the plants in the control treatment. Different researchers have used higher doses of spirulina (20–45 g/L) than in our study and found that it caused an increase in fruit number by approximately 43% in tomato (Aghofack et al., Citation2015) and 178% in eggplant (Dias et al., Citation2016). Different researchers explained the increase in the number of fruits with the positive effect of spirulina on flowering. Indeed, Dias et al. (Citation2016) reported that spirulina applications increased the number of buds by 46%. Aghofack et al. (Citation2015), in their study on tomatoes, found that spirulina applications caused earlier flowering and prevented bud drop by about 46% and flower drop by 96%. In addition, El-Eslamboly et al. (Citation2019) reported that spirulina applications in cucumber increased fruit formation by 19–35%.
3.3. Average fruit weight
The effect of applications on average fruit weight was found to be significant (P < 0.05). The study’s results showed that the average weight of pepper fruits varied between 23.00 and 26.50 g according to the applications. The lowest value was found in the 2000 ppm seaweed application, while the highest average fruit weight value was obtained from the 4000 ppm spirulina application (Table ).
Table 4. Effects of applications on average fruit weight, fruit width, fruit length, stem diameter, flesh thickness, and chlorophyll content of pepper
Table demonstrates that spirulina applications increased the average fruit weight by 10.83% (4000 ppm spirulina), 8.03% (6000 ppm spirulina), and 6.65% (2000 ppm spirulina) compared to the control application. In support of these results, it was reported that spirulina applications increased the average fruit weight in melon (Bayram, Citation2014), persimmon (Hussien, Citation2017), artichoke (Sayed et al., Citation2018), and okra (Uddin et al., Citation2019). Moreover, according to some studies, spirulina resembles (Mógor et al., Citation2018) or even contains (Sayed et al., Citation2018) the hormones cytokinin, auxin, and gibberellin. It is thought that spirulina application has a positive effect on fruit weight because cytokinins stimulate cell division and promote flower, fruit, and seed formation (Vankova, Citation2014), auxins stimulate cell division and chemical activity (Kırdar & Allahverdiev, Citation2020), and gibberellins play a role in fruit formation and development (Yavas & İ̇lker, Citation2020) and increase flowering (Kırdar & Allahverdiev, Citation2020).
3.4. Fruit width
Table shows that the effects of applications on the fruit width of pepper were statistically significant (P < 0.05). It was determined that the fruit width values of pepper changed between 21.37 and 23.52 mm according to the applications. The 2000 ppm seaweed (21.37 mm) and control (21.88 mm) applications had the lowest fruit width values. The highest fruit width value was obtained in the 4000 ppm spirulina application (23.52 mm), followed by 2000 ppm spirulina (23.20 mm), 6000 ppm spirulina (22.76 mm), and 6000 ppm seaweed (22.68 mm) applications.
Spirulina contains molecules such as polyamines that promote plant growth (Mógor et al., Citation2018). Polyamines affect important cellular processes such as cell division, differentiation, DNA replication, protein synthesis, and cell viability (Sahin & Orgec, Citation2022). Dias et al. (Citation2016) determined that spirulina applications (10, 15, 25, and 35 g/L) increased the fruit width value compared to the control application in their study on eggplant. This finding supports our study.
3.5. Fruit length
According to Table , the effects of applications on the pepper fruit length were statistically significant (P < 0.05). Fruit length values were found to vary between 19.63 and 21.55 cm according to the applications. The 6000 ppm spirulina application had the highest fruit length value, while the control and 2000 ppm seaweed applications had the lowest fruit length values.
Sayed et al. (Citation2018), in their study on artichoke, stated that the stimulating effects of spirulina on growth parameters may be due to plant growth promoting substances such as cytokines, auxins, gibberellins, amino acids, and vitamins. They explained this situation with spirulina’s positive effect on growth characteristics by promoting cell division and differentiation, many enzyme activities, leaf growth, and photosynthesis capacity.
3.6. Stem diameter
The effect of the applications, which were found to be significant on the stem diameter of the pepper plant is given in Table . In the study, the highest stem diameter values of 19.08 mm and 18.94 mm were obtained from 2000 ppm seaweed and control applications, respectively, while the lowest values were obtained from 2000 ppm spirulina, 4000 ppm seaweed, 6000 ppm spirulina, and 4000 ppm spirulina applications.
Youssef et al. (Citation2019) in cowpea and Al-Bayati et al. (Citation2020) in eggplant reported that seaweed applications increased plant stem diameter. In their research on eggplant plants, Dias et al. (Citation2016) found that the effect of spirulina application on stem diameter was insignificant when compared to the control application. In this study, stem diameter values in spirulina applications were found to be lower than in the other applications.
3.7. Fruit flesh thickness
When Table is examined, it is seen that the effects of the applications on fruit flesh thickness in pepper are insignificant (P < 0.05). It was determined that fruit flesh thickness values varied between 2.35 (6000 ppm seaweed application) and 2.92 mm (control) according to the applications.
3.8. Chlorophyll content
The effect of the applications on chlorophyll concentration was determined to be significant, as shown in Table Chlorophyll SPAD values varied between 70.89 and 78.97 according to the applications. While the lowest chlorophyll SPAD value was obtained in the control application (70.89), the highest chlorophyll SPAD value was determined in the 2000 ppm spirulina application (78.97).
In this study, it was determined that pepper plants treated with spirulina had improved chlorophyll SPAD values compared to the control plants. Similarly, Hassan et al. (Citation2017) in arugula, Sayed et al. (Citation2018) in artichoke, Uddin et al. (Citation2019) in okra, Abo-Basha et al. (Citation2019) in spinach, and Quelal et al. (Citation2022) in lettuce reported that spirulina applications increased chlorophyll content. Yigenoglu (Citation2015) evaluated the effects of spirulina applications in biological control against tomato bacterial wilt disease. As a result of the study, it was reported that the highest chlorophyll values were detected in spirulina-treated plants despite the presence of the disease. This indicates that spirulina had a positive contribution to plant development by suppressing the disease. Yassen et al. (Citation2019) found that spirulina application increased chlorophyll content in lettuce in their study. They stated that this might be because spirulina contains compounds such as cytokines that increase photosynthetic activity and positively affect growth characteristics. Furthermore, Abd El-Aleem et al. (Citation2021) found that spirulina applications to parsley plants increased oil yield and chlorophyll content. They stated that this increase may be due to the presence of high levels of macro and microelements, free amino acids, carotene-xanthophyll phytopigments, and various plant hormones such as auxins and cytokinins in spirulina.
3.9. Titratable acidity
The effect of applications on titratable acidity value in pepper was found significant (Table ). According to the applications, titratable acidity values ranged from 0.07% to 0.09%. The 4 and 6000 ppm spirulina applications had the lowest titratable acid values, while the control application had the highest titratable acid value. Hussien (Citation2017) reported that 1% spirulina treatment decreased titratable acidity values from 0.300 to 0.211 in 2015 and from 0.305 to 0.219 in 2016 compared to the control treatment in a study conducted in persimmon. This report supports our findings.
Table 5. Effects of applications on titratable acidity, total phenolic, B-Carotene, total soluble and reducing sugar, and vitamin C content of pepper
3.10. Total phenolic
The effect of applications on the total phenolic content in pepper was found to be significant (P < 0.05). Total phenolic values varied between 27.23 and 34.91 mg/100 g according to the applications. While the highest total phenolic values were determined in 4000 ppm seaweed and control applications, the lowest total phenolic value was obtained from the 2000 ppm spirulina application (Table ). According to studies conducted by Colla et al. (Citation2017) in tomato, Elsharkawy et al. (Citation2021) in artichoke, Ashour et al. (Citation2021) in Capsicum annuum, and El-Shenody et al. (Citation2023) in Eruca vesicaria, total phenolic values increased when seaweed was applied to the plant by 9.5%, 14.4%, 48.79%, and 141%, respectively. Ashour et al. (Citation2023), also reported that total phenolic content increased between 42% and 92% as a result of seaweed liquid extract (True-Algae-Max) applied to strawberries at 50% and 100% concentrations. Using the similar seaweed liquid extract, Hassan et al. (Citation2021), stated that the maximum phenolic content in Eruca vesicaria was obtained with a concentration of 10%. Samuels et al. (Citation2022), determined that seaweed applications to grapevines affected phenolic compounds in grapes differently depending on the season.
3.11. ß-carotene
Table shows that the effects of applications on the ß-carotene content of pepper were significant (P < 0.05). According to the applications, ß-carotene values ranged from 2.68 to 3.90 g/g. The lowest ß-carotene value was found in 2000 ppm spirulina application (2.68 µg/g), while the highest values were obtained from control (3.90 µg/g) and 4000 ppm seaweed (3.68 µg/g) applications. In support of our findings, Abu et al. (Citation2022) investigated the effects of seaweed applications on tomatoes and found that seaweed increased the ß-carotene value.
3.12. Total soluble sugars/reducing sugars
The effects of the applications on total sugar values were determined to be significant (Table ). The lowest values among the applications were found in 6000 ppm seaweed (9.44 mg/g) and 2000 ppm spirulina (9.49 mg/g) applications, while the highest total sugar value was obtained from 2000 ppm seaweed (12.09 mg/g), control (12.06 mg/g), and 6000 ppm spirulina (11.85 mg/g) applications.
In studies conducted by different researchers, it was found that seaweed applications increased the total sugar content in watermelon (Abdel-Mawgoud et al., Citation2010) and celery (Shehata et al., Citation2011). Sayed et al. (Citation2018) applied different concentrations of spirulina to artichoke plants for two seasons. They found that spirulina applications increased total sugar content at increasing concentrations compared to control plants in both seasons. These reports support our findings.
Table shows that the effects of seaweed and spirulina applications on reducing sugar values were insignificant (P < 0.05). As a result of seaweed and spirulina applications, it was determined that the reducing sugar values of pepper varied between 7.43 and 8.76 mg/g. Although the effects of seaweed and spirulina applications on the reducing sugar value were found to be insignificant, it was observed that 2000 ppm seaweed, 4000 ppm seaweed, and 4000 ppm spirulina applications increased the reducing sugar values when compared to the control application.
3.13. Vitamin C
When Table is examined, it was seen that the effects of the treatments on vitamin C values were significant. It was determined that vitamin C values of pepper varied between 86.47 and 96.13 mg/100 g according to the applications. The lowest vitamin C values were found in the control (86.47 mg/100 g), 6000 ppm seaweed (86.70 mg/100 g), and 2000 ppm seaweed (86.94 mg/100 g) applications. The highest vitamin C value was determined in 4000 ppm seaweed application.
As a result of our study, it was determined that 4000 ppm seaweed application increased the vitamin C value in pepper by 11.17% compared to the control application. Similar to our results, Miceli et al. (Citation2021), La Bella et al. (Citation2021), Abu et al. (Citation2022), and Jalali et al. (Citation2022) observed that seaweed applications increased vitamin C value in lettuce, spinach, and tomato, respectively.
3.14. Color values
The effects of the applications on color values were determined to be insignificant, as shown in Table . The results showed that the seaweed application at 4000 ppm had the lowest L* value (43.02), whereas the spirulina application at 2000 ppm had the highest L* value (44.78). In our study, it was determined that -a* values varied between −20.14 and −20.79, and b* values varied between 33.48 and 34.48. Among the applications, the lowest C* value was obtained from spirulina 6000 ppm application (39.25), and the highest value was obtained from 2000 ppm seaweed (40.17). Hue (h°) angle value was found to vary between 120.35 (4000 ppm seaweed) and 121.49 (6000 ppm spirulina).
Table 6. Effects of applications on color values of pepper
4. Conclusions
An evaluation of the literature shows that there are insufficient studies comparing the effects of both macroalgae and microalgae on plant cultivation. For this purpose, in this study, the effects of different doses of spirulina and seaweed on the yield and quality characteristics of the pepper were investigated.
According to the results, it was determined that 2000 ppm, 4000 ppm, and 6000 ppm spirulina applications increased the yield values by 33.67%, 31.89%, and 30.76%, respectively, compared to the control treatment. It was observed that both seaweed and spirulina applications positively affected fruit number and chlorophyll values, and the highest values were found in the 2000 ppm spirulina application. In the 4000 ppm spirulina application, the highest average fruit weight (g) and fruit width (mm) values were determined, while in the 6000 ppm spirulina application, the highest fruit length (cm) value was obtained. The highest vitamin C value was found in 4000 ppm seaweed application.
As a result, when all parameters in the study were evaluated, it was determined that spirulina applications provided better results in pepper production, especially in terms of yield parameters.
Acknowledgments
This study is derived from a master’s thesis and was financially supported by the Isparta University of Applied Sciences Scientific Research Projects Coordination Unit (Project No: 2021-YL1-0148).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Emine Seğmen
Emine Seğmen, holds a master's degree in the Department of Horticulture from the Institute of Graduate Education, Isparta University of Applied Sciences.
Halime Özdamar Ünlü
Halime Özdamar Ünlü, is an associate professor at Isparta University of Applied Sciences, Faculty of Agriculture, Department of Horticulture. Her academic studies cover the fields of vegetable cultivation and breeding.
References
- Abd El-Aleem, W. H., Hamed, E. S., & Toaima, W. I. M. (2021). Effect of blue green algae extract on three different curly parsley varieties under Sinai conditions. Bulgarian Journal of Agricultural Science, 27(5), 887–16.
- Abdel-Mawgoud, A. M. R., Tantaway, A. S., Hafez, M. M., & Habib, H. A. (2010). Seaweed extract improves growth, yield and quality of different watermelon hybrids. Research Journal of Agriculture and Biological Sciences, 6(2), 161–168.
- Abdel-Raouf, N., Al-Homaidan, A. A., & Ibraheem, I. B. M. (2012). Agricultural importance of algae. African Journal of Biotechnology, 11(54), 11648–11658. https://doi.org/10.5897/AJB11.3983
- Abo-Basha, D. M., Afify, R. R., & Abdel-Kader, H. H. (2019). Response of spinach (Spinacia olerasea L.) to algae extract under different nitrogen rates. Middle East Journal, 8(1), 47–55.
- Abu, N. J., Bujang, J. S., Zakaria, M. H., Zulkifly, S., & Ali, S. (2022). Use of Ulva reticulata as a growth supplement for tomato (Solanum lycopersicum). PLoS One, 17(6), 1–19. https://doi.org/10.1371/journal.pone.0270604
- Acun, M. (2019). The effects of microalgae application on yield quality and biochemical composition of salad and lettuce. Master’s Thesis, Institute of Science and Technology, Ege University
- Aghofack, J., Schinzoumka, P. A., & Tatchago, V. (2015). Effets des extraits ou de la poudre de Spirulina platensis et Jatropha curcas sur la croissance et le développement de la tomate. Journal of Applied Biosciences, 90(1), 8413–8420. https://doi.org/10.4314/jab.v90i1.2
- Al-Bayati, A. S., Jaafar, H. S., & Alhasnawi, N. J. R. (2020). Evaluation of eggplant via different drip irrigation intervals and foliar sprays with seaweed extract biostimulant. International Journal of Agricultural and Statistical Sciences, 16(2), 633–639. https://www.researchgate.net/publication/347388198_Evaluation_of_Eggplant_via_Different_Drip_Irrigation_Inter
- Aly, M. S., & Esawy, M. A. (2008). Evaluation of Spirulina platensis as bio stimulator for organic farming systems. Journal of Genetic Engineering & Biotechnology, 6(2), 1–7.
- Ashour, M., Al-Souti, A. S., Hassan, S. M., Ammar, G. A., Goda, A., El-Shenody, R., Abomohra, A. E. F., El-Haroun, E., & Elshobary, M. E. (2023). Commercial seaweed liquid extract as strawberry biostimulants and bioethanol production. Life, 13(1), 85. https://doi.org/10.3390/life13010085
- Ashour, M., Hassan, S. M., Elshobary, M. E., Ammar, G. A., Gaber, A., Alsanie, W. F., Mansour, A. T., & El-Shenody, R. (2021). Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on growth and antioxidant activities of hot pepper (Capsicum annuum). Plants, 10(6), 1045. https://doi.org/10.3390/plants10061045
- Bayram, C. A. (2014). Effects of plant activators on yield, quality, plant growth and soil fertility on melon cultivars (Galia c8 and kirkağaç 637) in Adıyaman conditions Ph.D Thesis, Institute of Science and Technology, Çukurova University
- Brown, K. C., Modi, K. J., Light, R. S., Cox, A. J., Long, T. E., Gadepalli, R. S., Rimoldi, J. M., Miles, S. L., Rankin, G., Valentovic, M., Denning, K. L., Tirona, M. T., Finch, P. T., Hess, J. A., & Dasgupta, P. (2023). Anticancer activity of region B capsaicin analogs. Journal of Medicinal Chemistry, 66(7), 4294–4323. https://doi.org/10.1021/acs.jmedchem.2c01594
- Cemeroglu, B. (2013). Basic analysis methods in fruit and vegetable processing industry. Biltav publications.
- Chen, Y., Fu, X., & Liu, Y. (2022). Effect of farmland scale on farmers’ application behavior with organic fertilizer. International Journal of Environmental Research and Public Health, 19(9), 4967. https://doi.org/10.3390/ijerph19094967
- Colla, G., Cardarelli, M., Bonini, P., & Rouphael, Y. (2017). Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience, 52(9), 1214–1220. https://doi.org/10.21273/HORTSCI12200-17
- Coseteng, M. Y., & Lee, C. Y. (1987). Changes in apple polyphenoloxidase and polyphenol concentrations in relation to degree of browning. Journal of Food Science, 52(4), 985–989. https://doi.org/10.1111/j.1365-2621.1987.tb14257.x
- Darko, E., Hamow, K. A., Marček, T., Dernovics, M., Ahres, M., & Galiba, G. (2022). Modulated light dependence of growth, flowering, and the accumulation of secondary metabolites in Chilli. Frontiers in Plant Science, 13, 1–15. https://doi.org/10.3389/fpls.2022.801656
- Das, P., Khan, S., Chaudhary, A. K., AbdulQuadir, M., Thaher, M. I., & Al-Jabri, H. (2019). Potential applications of algae-based bio-fertilizer. Biofertilizers for Sustainable Agriculture and Environment, 41–65. https://doi.org/10.1007/978-3-030-18933-4
- Dias, G. A., Rocha, R. H. C., Araújo, J. L., de Lima, J. F., & Guedes, W. A. (2016). Growth, yield, and postharvest quality in eggplant produced under different foliar fertilizer (Spirulina platensis) treatments. Semina: Ciências Agrárias, 37(6), 3893–3901. https://doi.org/10.5433/1679-0359.2016v37n6p3893
- Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356. https://doi.org/10.1021/ac60111a017
- El-Eslamboly, A. A. S. A., Abd El-Wanis, M. M., & Amin, A. W. (2019). Algal application as a biological control method of root-knot nematode Meloidogyne incognita on cucumber under protected culture conditions and its impact on yield and fruit quality. Egyptian Journal of Biological Pest Control, 29(1), 1–9. https://doi.org/10.1186/s41938-019-0122-z
- Elsharkawy, G. A., Ibrahim, H. A. H., Salah, A. H., Akrami, M., Ali, H. M., & Abd-Elkader, D. Y. (2021). Early and total yield enhancement of the globe artichoke using an ecofriendly seaweed extract-based biostimulant and PK fertilizer. Agronomy, 11(9), 1819. https://doi.org/10.3390/agronomy11091819
- El-Shenody, R. A., Elshobary, M. E., Ragab, G. A., Huo, S., & Essa, D. (2023). Towards biorefinery: Exploring the potential of seaweed-derived biodiesel and its residual biomass in improving the traits of Eruca vesicaria (L.) cav. South African Journal of Botany, 155, 361–371. https://doi.org/10.1016/j.sajb.2023.02.029
- Hassan, S. M., Ashour, M., & Soliman, A. A. F. (2017). Anticancer activity, antioxidant activity, mineral contents, vegetative and yield of eruca sativa using foliar application of autoclaved cellular extract of spirulina platensis extract, comparing to npk fertilizers. Journal of Plant Production, 8(4), 529–536. https://doi.org/10.21608/jpp.2017.40056
- Hassan, S. M., Ashour, M., Soliman, A. A., Hassanien, H. A., Alsanie, W. F., Gaber, A., & Elshobary, M. E. (2021). The potential of a new commercial seaweed extract in stimulating morpho-agronomic and bioactive properties of Eruca vesicaria (L.) Cav. Sustainability, 13(8), 4485. https://doi.org/10.3390/su13084485
- Honda, S., Takeda, K., & Kakehi, K. (1980). Studies of the structures of the carbohydrate components in plant oligosaccharide glycosides by the dithioacetol method. Carbohydrate Research, 73(1), 135–143. https://doi.org/10.1016/S0008-6215(00)85482-8
- Hong, D. D., Hien, H. M., & Son, P. N. (2007). Seaweeds from Vietnam used for functional food, medicine and biofertilizer. Journal of Applied Phycology, 19(6), 817–826. https://doi.org/10.1007/s10811-007-9228-x
- Hussien, M. A. (2017). Productive performance of sewy date palms in relation to spraying spirulina platensis algae, plant compost tea, salicylic acid and tocopherol. New York Science Journal, 10(7), 126–135. https://doi.org/10.7537/marsnys100717.17
- Ismaiel, S. A. R., Khedr, F. G., Metwally, A. G., & Soror, A. F. S. (2022). Effect of biostimulants on soil characteristics, plant growth and yield of Pea (Pisum sativum L.) under field conditions. Plant Science Today, 9(3), 650–657. https://doi.org/10.14719/pst.1748
- Jalali, P., Roosta, H. R., Khodadadi, M., Torkashvand, A. M., Jahromi, M. G., & Naveed, M. (2022). Effects of brown seaweed extract, silicon, and selenium on fruit quality and yield of tomato under different substrates. PLoS One, 17(12), e0277923. https://doi.org/10.1371/journal.pone.0277923
- Jufri, A. F., Sulistyono, E., & Sulistyono, A. E. (2016). Effects of dry spirulina platensis and antitranspirant on growth and yield of chili pepper (Capsicum annuum L.). Indonesian Journal of Agronomy, 44(2), 170–175. https://doi.org/10.24831/jai.v44i2.13486
- Kayak, N., Arı, B. Ç., Dal, Y., Kal, Ü., Seymen, M., & Türkmen, Ö. (2022). Konya ekolojik koşullarında farklı dolmalık hibrit biber çeşidi adaylarının verim, kalite ve bazı morfolojik özelliklerinin belirlenmesi. Ereğli Tarım Bilimleri Dergisi, 2(1), 41–47. https://doi.org/10.54498/etbd.2022.10
- Kırdar, E., & Allahverdiev, S. (2020). Büyüme Düzenleyiciler ve Etkileri. In Fidan Standardizasyonu: Standart Fidan Yetiştirmenin Biyolojik ve Teknik Esasları (pp. 245–250).
- Koru, E. 2012. Earth food spirulina (Arthrospira): Production and quality standards. Food additive IntechOpen: Food additive, books.google.com. https://doi.org/10.5772/31848.
- La Bella, S., Consentino, B. B., Rouphael, Y., Ntatsi, G., De Pasquale, C., Iapichino, G., & Sabatino, L. (2021). Impact of ecklonia maxima seaweed extract and mo foliar treatments on biofortification, spinach yield, quality and NUE. Plants, 10(6), 1139. https://doi.org/10.3390/plants10061139
- Lerer, L., Varia, J., Kamaleson, C., Lerer, B., & Lerer, L. (2021). Psychedelic mushrooms in the USA: Knowledge, patterns of use, and association with health outcomes. Frontiers in Psychiatry, 12. Preprints, 2020110354. https://doi.org/10.3389/fpsyt.2021.780696
- Mandal, A., Sarkar, B., Mandal, S., Vithanage, M., Patra, A. K., & Manna, M. C. (2020). Impact of agrochemicals on soil health. In Agrochemicals Detection, Treatment and Remediation, 161–187. https://doi.org/10.1016/B978-0-08-103017-2.00007-6
- McGuire, R. G. (1992). Reporting of objective color measurements. HortScience, 27(12), 1254–1255. https://doi.org/10.21273/HORTSCI.27.12.1254
- Miceli, A., Vetrano, F., & Moncada, A. (2021). Influence of Ecklonia maxima extracts on growth, yield, and postharvest quality of hydroponic leaf lettuce. Horticulturae, 7(11), 440. https://doi.org/10.3390/horticulturae7110440
- Mógor, Á. F., de Oliveira Amatussi, J., Mógor, G., & de Lara, G. B. (2018). Bioactivity of cyanobacterial biomass related to amino acids induces growth and metabolic changes on seedlings and yield gains of organic red beet. American Journal of Plant Sciences, 9(5), 966. https://doi.org/10.4236/ajps.2018.95074
- Morais, M. G., da Vaz, B. S., de Morais, E. G., & Costa, J. A. V. (2015). Biologically active metabolites synthesized by microalgae. BioMed Research International, 2015, 1–15. https://doi.org/10.1155/2015/835761
- Muchena, J. K. (2009). Studies of capsaicinoids contents of locally grown and commercial chilies using reversed-phase High Performance Liquid Chromatography. (Doctoral dissertation, East Tennessee State University)
- Nagata, M., & Yamashita, I. (1992). Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Journal of the Japanese Society for Food Science and Technology, 39(10), 925–928. https://doi.org/10.3136/nskkk1962.39.925
- Pereira, L. (2021). Macroalgae. Encyclopedia, 1(1), 177–188. https://doi.org/10.3390/encyclopedia1010017
- Piwowar, A., & Harasym, J. (2020). The importance and prospects of the use of algae in agribusiness. Sustainability, 12(14), 5669. https://doi.org/10.3390/su12145669
- Quelal, C., Nicolas, A., & Salazar, U. (2022). Comparison of the Effect of Three Biofertilizers: Biol, Seaweed and Spirulina (Arthrospira platensis), on the Organic Production of Lettuce (Lactuca sativa). Retrieved December 20, 2022, from https://www.researchgate.net/profile/Alex-Coronel/publication/366961611_Comparison_of_the_effect_of_three_biofertilizers_Biol_Seaweed_and_Spirulina_Arthrospira_platensis_on_the_organic_production_of_lettuce_Lactuca_sativa/links/63bc98c1097c7832caa203d2/
- Rubio, C., Hardisson, A., Martín, R., Báez, A., Martín, M., & Álvarez, R. (2002). Mineral composition of the red and green pepper (Capsicum annuum) from Tenerife Island. European Food Research and Technology, 214(6), 501–504. https://doi.org/10.1007/s00217-002-0534-x
- Sahin, G., & Orgec, M. (2022). Güncel bir bakış açısıyla poliaminlerin bitki büyüme ve gelişimi üzerine etkileri. Türkiye Tarımsal Araştırmalar Dergisi, 9(2), 255–264. https://doi.org/10.19159/tutad.1088744
- Samuels, L. J., Setati, M. E., & Blancquaert, E. H. (2022). Towards a better understanding of the potential benefits of seaweed based biostimulants in Vitis vinifera L. cultivars. Plants, 11(3), 348. https://doi.org/10.3390/plants11030348
- Sayed, S. M., Abd El-Dayem, H. M., El-Desouky, S. A., Khedr, Z. M., & Samy, M. M. (2018). Effect of silicon and algae extract foliar application on growth and early yield of globe artichoke plants. Annals of Agricultural Sciences, 56(4), 207–214. https://doi.org/10.21608/assjm.2018.65139
- Shedeed, Z. A., Gheda, S., Elsanadily, S., Alharbi, K., & Osman, M. E. (2022). Spirulina platensis biofertilization for enhancing growth, photosynthetic capacity and yield of Lupinus luteus. Agriculture, 12(6), 781. https://doi.org/10.3390/agriculture12060781
- Shehata, S. M., Abdel-Azem, H. S., Abou El-Yazied, A., & El-Gizawy, A. M. (2011). Effect of foliar spraying with amino acids and seaweed extract on growth chemical constitutes, yield and its quality of celeriac plant. European Journal of Scientific Research, 58(2), 257–265.
- Siringi, J. O., Turoop, L., & Njonge, F. (2022). Biostimulant effect of spirulina (Arthrospira platensis) on lettuce (Lactuca sativa) cultivated under aquaponic system. SCIREA Journal of Biology, 7(1). https://doi.org/10.54647/biology18204
- Tagliapietra, B. L., & Clerici, M. T. P. S. (2023). Brown algae and their multiple applications as functional ingredient in food production. Food Research International, 167, 112655. https://doi.org/10.1016/j.foodres.2023.112655
- Tuncer, M. K. (2022). Definition of farmers’ knowledge, attitudes and practices on sustainable agriculture in Kızılırmak delta of Bafra district M.Sc. thesis, Institute of Postgraduate Education Ondokuz Mayıs University
- Uddin, A. F. M., Rakibuzzaman, M., Wasin, E. W., Husna, M. A., & Mahato, A. K. (2019). Foliar application of Spirulina and Oscillatoria on growth and yield of okra as bio-fertilizer. Journal of Bioscience and Agriculture Research, 22(2), 1840–1844. https://doi.org/10.18801/jbar.220219.227
- Vankova, R. (2014). Cytokinin regulation of plant growth and stress responses. Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications, 55–79. https://doi.org/10.1007/978-1-4939-0491-4_3
- Wahyuni, Y., Ballester, A. R., Sudarmonowati, E., Bino, R. J., & Bovy, A. G. (2013). Secondary metabolites of Capsicum species and their importance in the human diet. Journal of Natural Products, 76(4), 783–793. https://doi.org/10.1021/np300898z
- Wuang, S. C., Khin, M. C., Chua, P. Q. D., & Luo, Y. D. (2016). Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Research, 15, 59–64. https://doi.org/10.1016/j.algal.2016.02.009
- Yassen, A. A., Essa, E. M., & Zaghloul, S. M. (2019). The role of vermicompost and foliar spray of spirulina platensis extract on vegetative growth, yield and nutrition status of lettuce plant under sandy soil. Research Journal of Agriculture and Biological Sciences, 14, 1–7. https://doi.org/10.22587/rjabs.2019.14.1.1
- Yavas, İ., & İ̇lker, E. (2020). Çevresel stres koşullarına maruz kalan bitkilerde fotosentez ve fitohormon seviyelerindeki değişiklikler. Bahri Dağdaş Bitkisel Araştırma Dergisi, 9(2), 295–311.
- Yigenoglu, C. Y. (2015). Potential use of spirulina platensis on biological control of bacterial wilt’s of tomato. Master’s Thesis, Institute of Science and Technology, Çukurova University
- Youssef, F. A., El-Segai, M. U., Abou-Taleb, S. M., & Massoud, K. W. (2019). Response of cowpea (Vigna unguiculata L.) plant to seaweed and yeast extracts. Plant Archives, 19(2), 2363–2370.