 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study is aimed at determining the physicochemical and microbiological quality of stingless bee honey (Meliponula beccarii) collected in potential areas in the Oromia region of Ethiopia. Thirty-nine honey samples from underground soils were collected. The findings of the investigation indicated that the mean values of physicochemical properties for stingless bee honey samples were: moisture content (30.69 ± 0.29%), pH value (3.30 ± 0.03), free acidity (92.39 ± 4.45 meq/kg), ash content (0.16 ± 0.01%), electrical conductivity (0.44 ± 0.2 mS/cm), HMF (6.58 ± 0.36 mg/kg), fructose (36.48 ± 0.54%), glucose (27.67 ± 0.43%), reducing sugar (64.15 ± 0.75%) sucrose (1.24 ± 0.18%) and maltose content (1.2 ± 0.18%). The honey sample studied from Meliponulla beccarii was mostly characterized as having a light amber color (50%). Honey produced by stingless bees is distinguished from Apis mellifera honey by having a lower level of sugar, a higher amount of moisture, and free acidity. There were 2.55 × 104 to 1.9 × 103 CFU/mL of Aerobic Mesophiles, 1.68 × 104 to 9 × 102 CFU/mL of yeast, and 1.8 × 103 to 2 × 102 CFU/mL of mold in the Meliponulla beccarii honey sample. Staphylococcus species could only be detected in a sample taken from the Guduru area; however, an aerobic spore-forming bacteria was undetectable in all stingless bee samples. This suggested that there may have been cross-contamination from primary and secondary sources during pre-harvesting and post-harvest handling. Specific quality specifications must be established to promote commercialization since the physicochemical and microbiological characteristics of stingless bee honey differ from those of A. mellifera.
PUBLIC INTEREST STATEMENT
Ethiopia has tremendous potential for the existence of different types of stingless bee species, which are renowned for producing stingless bee honey per annum. Meliponula beccarii is one of six stingless bee species identified in Ethiopia. Since ancient times, human beings have benefited from the nutritional and therapeutic benefits of stingless bee honey. The results of these study findings were intended to provide important data for the standardization and marketing of stingless bee honey in Ethiopia, particularly in the Oromia region. Additionally, the information generated from this study supports scientists to create stingless honey bee-based products that will benefit consumers, expand the stingless bee honey sector, and improve the economic standing of Ethiopian stingless bee honey. The results of the research will also be a guide for policymakers in the establishment of legislative standards and regulatory framework for stingless bee honey which will help to promote their usage and production.
1. Introduction
Honey is the most complex naturally occurring food; which is mostly composed of sugars and water. Honey also contains other components such as free amino acids, organic acids, enzymes, carotenoids, vitamins, essential minerals, and volatile compounds. Honey is a powerful source of polyphenols and flavonoids, which have a variety of biological effects and serve as strong antioxidants (Alqarni et al., Citation2014). The chemical composition of honey is determined by bee species, botanical origin, geographical origin, environmental condition, and, beekeeping practices (Gheldof et al., Citation2002).
The honey product obtained from the Apis mellifera species serves as the foundation for both global honey production and consumption. However, other smaller productions depend on the honey product produced by various species of bees, such as stingless bees (Temaru et al., Citation2007). Melipona and Trigona are the two main genera that contain the majority of the species and produce stingless bee honey worldwide (Michener, 2013). Pauly & Zewdu Ararso (2013) identified that the following stingless bee species have been discovered in different areas of Ethiopia, including Meliponula beccarii, Liotrigona bottegoi, Liotrigona baleensis, Hypotrigona gribodoi, Hypotrigona ruspolii, and Plebeina armata. Currently, only two major groups of stingless bees are known to produce honey and commercialize it, even though more stingless bee species are believed to exist in Ethiopia. The first is the Tazma or Damuu stingless bee (Meliponula beccarii), which has a considerably larger body size, and ”Tazma or Damuu ‘honey, which is stored underground in resinous cerumen pots by stingless wild honeybees. The second species of stingless bee is a little insect commonly known as Tingn or Boke. It may be a member of the Trigonini group, and it stores its honey in the trunks of trees to generate ’Tinign or Boke” honey. The production honey of produced by stingless bee species is still limited compared to Apis mellifera honey since it cannot reach industry, has a shorter shelf life, is not subject to quality standards, and is not widely known (Vit et al., Citation2004).
Currently, honey produced by stingless bee species is gaining more attention on an international level since it has been discovered to have therapeutic characteristics. These characteristics indicated that it might help in the healing of wounds, treat eye conditions, and serve as an anti-inflammatory agent (Rao et al., Citation2016).Stingless bees have long been known in Ethiopia and are referred to as “Tazma or Damuu” locally. It is widely utilized in the country for its strong antibacterial, immune-stimulatory, anti-inflammatory, antioxidant, and wound-healing effects (Ashenafi, Citation1994; Berhanu, Citation2014; Yalemwork et al.,Citation2013). This honey is also widely used in both traditional and modern medicine (Temaru et al., Citation2007). On the other side, stingless bee (M. beccarii) honey is highly valued in Ethiopia for fresh consumption due to its therapeutic benefits as well as its more acidic taste, higher fluidity, and lower viscosity. In tropical Africa, the selling price of stingless bee honey can reach up to 10 times more than the price of honey produced by Apis mellifera, making the products highly valuable (Vit et al., Citation1998). Similarly, Kebede et al. (Citation2012) reported stingless bee honey prices in Ethiopia were three times higher than those for honey produced by Apis mellifera due to the perception that stingless bee honey had higher medicinal and healing properties. A range of 2–4 liters of honey can be collected from the Meliponula species in Ethiopia (Shenkute et al., Citation2012). Furthermore, Melipinoculture and the production of honey from stingless bee species have a significant impact on socio-economic development in Ethiopia and can contribute to up to 50% of household income (Shenkute et al.,Citation2012). Due to this, honey produced by stingless bees can be found in local markets and is considerably more expensive than A. mellifera honey.
Many studies have been conducted on the physicochemical characteristics of stingless bee honey in different areas of the world, including Venezuela (Vit et al., Citation1994), Thailand (Chuttong et al., Citation2016a), and Mexico (Moo-Huchin et al., Citation2015). So far, the majority of Ethiopian study has concentrated on the physicochemical characterization of A. mellifera honey, as well as its botanical and geographical characteristics (Alemu et al., Citation2010; Belay et al., Citation2017; Gobessa et al., Citation2012; Kinati et al., Citation2011; Mulugeta & Tadese, Citation2017; Tesfaye et al., Citation2016; Yadata, Citation2014). Despite the fact that a substantial amount of stingless bee honey is produced and there is a large market demand, only a few studies have been performed on the physicochemical and microbiological qualities of stingless bee honey produced in Ethiopia. The study by Gela et al. (Citation2021) is the only finding reported on stingless bee honey, and it only focuses on the physicochemical characteristics of honey produced by stingless bees (M. beccarii) from the West Shoa area of Oromia. At present it is challenging to develop an official quality control standard reference due to a lack of understanding regarding the characteristics of honey produced by stingless bees in Ethiopia. The quality requirements and indicators currently established by Ethiopian legislation are focused on Apis mellifera species honey parameters (ESA, Citation2005), which contradict those present in Meliponini honey. In addition, honey produced by stingless bee species is not recognized by international regulatory standards of honey (Codex, Citation2001; EU, Citation2002). Therefore, characterizing stingless bee honey in regard to its physicochemical composition and microbiological quality is crucial for the certification procedures that prove its quality and origin (geographical and botanical) as well as facilitate the inspection procedure. Also, the result of this study helps to develop specific standard quality specifications for stingless bee honey to assist its commercialization by comparing the physicochemical requirements of stingless bee honey with the Codex standard (2019). Therefore, the present study aimed to characterize the physicochemical and microbiological properties of honey produced by a stingless bee (M. beccarii) collected from different agro ecology of the Oromia region in Ethiopia.
2. Materials and methods
2.1. Description of the study area
The was conducted in the Oromia Regional state of Ethiopia. For the harvesting of stingless bee honey, the potential areas for stingless bee honey production were purposively selected. Samples of stingless bee honey were obtained from 14 districts in Eight (8) Zones of the Oromia Region (West Shewa, Horo Guduru Welega, East Welega, West Welega, Buno Bedele, Illubabor, Jimma, and Guji).
2.2. Honey harvesting and collection
The Meliponula beccarii stingless bee (Gribodo, 1879) utilized in this experiment is a species that Pauly and Hora (Citation2013) identified. Thirty-nine (39) honey samples were collected by carefully digging into the underground nest until reaching the nest chamber containing both pollen and honey store. Using sterile sticks and sterile syringes, pure and fresh honey samples of stingless bees (Meliponula beccarii) were aseptically taken directly from sealed honey pots. The harvested honey samples were placed in sterilized glass or plastic containers, packed in thermal boxes with ice, and transported to the Holeta Bee Research and other respected laboratories. The honey sample was further strained through a fine-mesh filter into a plastic container to remove the impurities. The plastic container and other equipment for honey harvesting were washed with hot, soapy water and warmed in a preheated oven at 160–180°C for around 15 minutes to sterilize the container. Finally, distilled water is used to rinse the plastic container. The sample was kept refrigerated (−4°C) and analyzed as quickly as possible. Following the collection of samples, physicochemical and microbiological properties analyses were conducted for one month.
2.3. Analyzing the botanical origin of the Meliponula beccarii honey sample
The botanical origin analysis was done under the light microscope (Swift Instrument International, Japan, high power 40×) linked to a computer using the methods suggested by Louveaux et al. (Citation1978). Ten grams of honey samples were dissolved in 20 mL of distilled water. The sediment of the solution was then concentrated by repeatedly centrifuging at 3800 rpm for 10 minutes, and the supernatant was decanted. To completely dissolve the residual sugar crystals, 20 mL of distilled water was added once more. The supernatant was removed, after centrifuging at 3800 rpm for additional 5 minutes. On a microscope slide, the sediment was evenly spread by a sterile micro spatula, and the sample was allowed to dry for some minutes. After that, one drop of glycerin jelly was put into the coverslip, and the pollen type was identified using a pollen atlas (Adgaba, Citation2002). Based on the total number of different pollen grain types that were counted in each sample, the percentage of pollen types was calculated. Honey with a pollen count of greater than 45% is classified as monofloral honey, while honey with less than 45% pollen count is classified as multifloral honey (Louveaux et al., Citation1978)
2.4. Determination of physicochemical properties of Meliponula beccarii honey sample
2.4.1. Determination of color
The colors of the samples were evaluated with the aid of Pfund Color Grader (Koehler Instrument, New York). The scale of Pfund runs from 0 mm (colorless) to 140 mm (black) (Fell, 1978). The sample (which is placed in a glass wedge-shaped trough) is compared to the amber glass wedge at a position along a calibrated amber glass wedge that serves as the scale. Approximately 100 g of honey was prepared to measure color by heating them to 50°C and centrifuging them for 5 minutes at 3500 rpm to eliminate air bubbles. The sample was placed into the sample holder of the Pfund grader in liquid form for reading.
2.4.2. Determination of moisture content
The moisture content was measured by an Abbe refractometer (ABBE- 5 Bellingham Stanley. Ltd, UK) at room temperature (20°C) and is calibrated with distilled water regularly, according to International Honey Commission(IHC) (Citation2009). To dissolve all of the sugar crystals, homogenized honey samples were placed in a water bath at 50 °C for 20 minutes. Following homogenization, the honey sample was placed over the prism of the refractometer, and the refractive index was obtained from the equipment. Using a standard table recommended by the IHC, the moisture content value of the honey was determined from the measured refractive index (IHC, Citation2009).
2.4.3. Determination of pH and free acidity
The pH and free acidity were performed as described by IHC (Citation2009). Ten (10) grams of honey were dissolved in 75 mL of distilled water in a 250 mL beaker and stirred with a magnetic stirrer. The pH of the honey was measured when the pH meter electrode was immersed in the honey solution. To determine the free acidity of a honey sample, the solution of honey was further adjusted to pH 8.30 by adding 0.1 M sodium hydroxide (NaOH) solution. Using a 10 ml burette, the reading was recorded precisely to the nearest 0.2 ml. Free acidity is presented in millimoles of acid per kg of honey, which is equal to mL of 0.1 M NaOH multiplied by 10. According to the IHC (Citation2009) procedure, the result is presented in one decimal place.
Free acidity = 10 V, Where: V = Volume of NaOH(0.1 Normality)
2.4.4. Determination of ash content
The ash concentration was determined using the methodology described by the IHC (Citation2009) in a Muffle furnace (BioBase JKKZ.5.12GJ, Shandong, China). The ash crucible was heated first in a Hot plate to an ashing temperature, then cooled to ambient temperature in a Desiccator, and weighed to 0.001 g (M2). A platinum crucible was then used to weigh 5 g (M0) honey sample to the nearest 0.001 g, and 2–3 drops of olive oil were added to prevent foaming. The moisture was removed and began to ash on a Hot plate at 350 to 400 °C. Following the preliminary ashing the crucible was heated for at least 4–6 hours in the Muffle furnace. After cooling in the desiccators, the ash crucible was weighed. The ashing process was carried out repeatedly until the weight (M1) stayed consistent. The following calculation was employed to get the percentage of the weight of ash in g/100 g of honey.
Where
M0= Weight of measured honey
M1= Weight of crucible and ash
M2= Weight of empty crucible
2.4.5. Determination of electrical conductivity
The determination of electrical conductivity was evaluated with Electrical Conductivity Meter (BANTE, Instrument-520 conductive and temperature control, China). A 0.745 g of potassium chloride (KCl), was dried at 130°C, dissolved in freshly distilled water in a 100 ml volumetric flask, and filled with distilled water. Forty milliliters (40) of KCl solution were transferred to a beaker. The conductivity cell was connected to the conductivity meter and thoroughly rinsed with KCl solution, and it was submerged in the solution. The electrical conductance of the solution was then measured in mill siemen after the temperature of the instrument had stabilized at 20°C with the guidelines recommended by IHC (Citation2009). The following formula was used for the calculation of cell constant(K).
Where:
K= The cell constant in cm−1
G= The electrical conductance in mill siemen.
11.691= The sum mean value of the electrical conductivity of freshly distilled water in mS.cm−1 and the electrical conductivity of a 0.1 M KCL solution at 20°C.
2.4.6. Determination of hydroxyl methyl furfural (HMF)
The determination of Hydroxymethylfurfural was evaluated based on the spectrophotometric method recommended by IHC (Citation2009). A five (5) gram honey sample was weighed in a beaker, dissolved in 25 mL of distilled water, and then transferred to a 50 mL volumetric flask (IHC, Citation2009). The solution of honey was mixed with 0.5 mL of carrez solution I (15 g K4Fe (CN)6. 3H20/100 mL distilled water), and then with 0.5 mL of carrez solution II (30 g Zn acetate/100 mL distilled water). The honey solution was thoroughly mixed with both types of carrez solutions before being filtered by Whatman filter paper and the first filtrate (10 mL) was discarded. A five (5) milliliter of the filtrate was added to two test tubes, followed by 5 mL distilled water in the first (sample solution) and 5 mL sodium bisulfite solution (0.20% of 0.20 g NaHSO3/100 mL distilled water) in the other (reference solution). The solution in each test tube was thoroughly mixed on a Vortex mixer. Finally, the absorbance was measured by comparing the absorbance of the reference solution (the same honey solution treated with sodium bisulfite) at 336 nm to the absorbance measured at 284 nm in the honey sample solution. The results were calculated and expressed in accordance with IHC standards (IHC, Citation2009).
Hydroxyl methyl furfural/100 g of honey = (A284- A336) *149.7 × 5*D/W sample
Whereas A284= Absorbance at 284 nm, A336 = Absorbance at 336 nm, 149.7= Constant, 5= Theoretical nominal sample weight and W= Mass of honey sample taken, and D =Dilution factor
2.4.7. Determination of sugar concentration
The quantification of sugar concentration was done according to the methodology described by IHC (Citation2009) by High-performance liquid chromatography (Agilent, Germany, HPLC-1260 Infinity Series). Five (5) grams of honey were dissolved in 40 mL of distilled water. Into a 100 mL volumetric flask, 25 mL of acetonitrile was pipetted. The honey solution was then transferred to a volumetric flask and distilled water was added till the desired level was reached. Before chromatographic analysis, the solution of the honey sample was finally filtered through a syringe filter (0.45 µm). The sugar profile of honey samples was separated on an analytical stainless steel column (25 × 4.6, 5–7 μm,) that contained amine-modified silica gel. The mobile phase composition was composed of Acetonitrile: Water (80:20, v/v), and the flow rate was 1.3 mL/min. The injection volume of the sample was 10 µL. The sugar concentration was detected with a Refractive Index Detector thermostated at 30°C and a temperature-controlled column oven set at 30°C. By comparing the retention times of the sugar content of honey to the standard sugars, the sugar profile of honey was determined (IHC, Citation2009). The standard sugars produced in Germany by Sigma Aldrich and had purity levels of > 99.5% for glucose, >90% for sucrose, >90% for maltose, and > 99.5% for fructose were used in this experiment. Five series serial dilutions of the standards weighing a mixture of 2, 1.5, 1, 0.5, and 0.15 g of fructose, glucose, sucrose, and maltose were weighed and dissolved in 40 mL of distilled water and 25 mL of acetonitrile to create a calibration curve following the IHC procedures (IHC, Citation2009).
2.4.8. Characterization of microbiological quality of Meliponula beccariihoney samples
The honey sample was weighed out at 20 g, and 180 mL distilled which is sterilized was added. The solution was then homogenized by an orbital shaker at 110 rpm for 5–10 minutes. This stock solution was then used as the basis for subsequent serial dilutions. Then 0.1 mL of a 10−1 serial dilution was pipetted onto the center of the corresponding sterilized plates. Thus, 20–25 mL of the appropriate sterilized media were placed onto the plate with the aliquots and thoroughly mixed. The inoculation plates were incubated for 24–96 hours at the proper temperatures. From the countable plates, the colonies were counted.
2.4.9. Aerobic mesophilic counts
The aerobic mesophilic count was performed by plate counting. 0.1 mL of aliquots was taken from the stock and serially diluted in 9 mL sterilized normal saline (0.85% NaCl). A 0.1 mL dilution was taken from a 1:10 dilution and placed in duplicate onto sterilized plate count agar plates, which were then incubated at 32°C for 48 hours. Using a colony counter, viable cells were counted and the result was reported as CFU/mL of honey (Muleta & Ashenafi, Citation2001).
2.4.10. Staphylococci counts
The determination of Staphylococci was carried out using Mannitol Salt Agar methods.Aliquots of one (1) mL were taken from the stock and serially diluted in 9 mL of sterilized normal saline solution (0.85% NaCl). A 0.1 mL was prepared from a 1:10 dilution and placed in duplicate onto plates of sterilized Mannitol Salt Agar, which were then incubated at 32°C for 36 hours. Following incubation, staphylococci were counted as yellow colonies with yellow zones and colorless or red colonies with red zones. A colony count was performed using a colony counter, and the result was presented as CFU/mL of honey (Muleta & Ashenafi, Citation2001).
2.4.11. Aerobic bacterial spore formers
To determine the number of aerobic bacterial spores present, 0.1 mL of the serial dilutions were heated in a water bath at 80°C for 10 minutes to destroy vegetative cells and quickly cooled in tap water. Nutrient Agar (Oxoid) plates were covered with an aliquot of 0.1 mL. After being incubated at 32°C for 48 hours, the growing colonies were counted as aerobic spore-forming bacteria (Muleta & Ashenafi, Citation2001).
2.5. Yeasts and Molds
The mold and yeast count was performed by transferring an aliquot of 0.1 mL onto Potato Dextrose Agar (Oxoid) plates, which contained 200 g of potato infusion, 20 g of dextrose, 15 g of agar, 0.1 g of chloramphenicol, and 1000 mL of distilled water with a pH 5.6 ± 0.2. These plates were incubated at 25–28°C for 3 to 5 days. After incubation, smooth (non-hairy) colonies without extension were counted as yeasts, while hairy colonies with extension at the periphery were counted as molds (Muleta & Ashenafi, Citation2001). The results were expressed in Colony Forming Units (CFU/mL).
2.6. Data analysis
The Statistical analyses were performed using SPSS version 20 software. Data were analyzed by one-way ANOVA and presented as mean and standard error. The mean values were significant at values of P < 0.05. The Pearson correlation coefficient (r) was used to establish correlations between physicochemical parameters at a 99% (p < 0.001) level of significance.
3. Results and Discussions
3.1. The botanical origin of the honey sample
Pollen analysis (melissopalynology) is one of the most important procedures for determining and confirming the botanical and geographical origin of honey. In this study finding, the botanical origin analysis of samples indicated that the stingless bee honey mainly originated from twelve (12) nectar source plant species including Eucalyptus spp, Schefflera abyssinica, Coffea arabica, Vernonia amygdalina, Guizotia scabra, Croton macrostachyus, Syzygium guineense, Pterolobium spp, Tephrosia vogelii spp, Plantago lanceolata, Trifolium rueppellianum and Ilex mitis spp (Table and Figure ). These plant species are additionally known as main and minor honey plants in the study areas for Apis mellifera honey production.
Table 1. The relative frequency of nectar source plant species in each sample of the studied areas (%)
Figure 1. Photographs of major pollen types observed in honey samples of stingless honey collected Oromia region. A, Coffea Arabica, B, Schefflera abyssinica, C, Eucalyptus spp, D, Guizotia scabra.
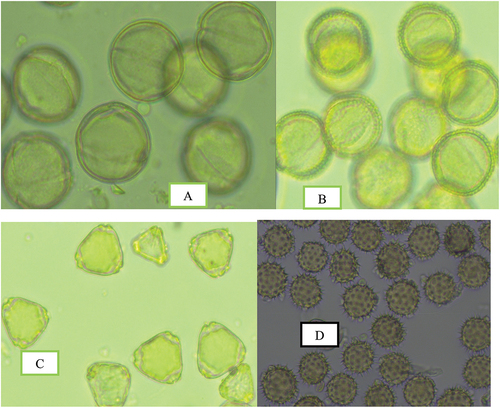
In addition to predominant frequency, the abundance of specific pollen types in nectar, such as secondary, tertiary, and quaternary enrichment, is critical for the botanical origin description of honey (Von Der Ohe et al., Citation2004). The relative pollen distribution enriching honey sample revealed that S. abyssinica was the most predominant honey plant in the samples collected from the Gechi area of Buno Bedele with a total percentage frequency of 61.7%. Schefflera abyssinica was a secondary honey plant source in the Gera district and the Didu district with percentage frequency of 42.38% and 42.38% respectively. This indicated that Schefflera abyssinica is an abundant honey source tree in the Southwestern and Southeastern highlands of Ethiopia, where it produces unifloral honey (Bareke & Addi, Citation2019). Similarly, a study conducted by Degaga (Citation2017) also suggested that S. abyssinica is a major unifloral honey source in the Gera surrounding of Jimma zone.
Pollen dominance for Guizotia scabra was identified in honey samples collected from the Horo Guduru Welega district of Guduru and Jimma Genete, Diga and Sibu sire districts of East Welega, and Ale district of the Illubabor Zone. Arega et al. (Citation2020) observed a similar finding, that Guizotia scabra is the predominant bee forage that produces monofloral honey in Sibu Sire and Jimma Genete of East Welega and Horo Guduru Welega Zone, respectively. Furthermore, the majority of Ethiopian highlands are covered with golden-yellow flowers of Guizotia spp (Fichtl & Adi, Citation1994). Eucalyptus spp. was another predominated honey source plant detected in stingless bee honey samples harvested from the Chalia district of the west Shewa Zone. In addition, in stingless bee honey harvested from Guji Zone (Arda Jila, and Ana Sora district) Eucalyptus spp. was detected as a secondary honey plant source.
Additionally, Coffea arabica and Syzygium guineense were other secondary honey source plants detected in honey stingless bee honey samples harvested in the Guji zone (Arda Jila, and Ana Sora districts) (Table ). The coffee plant (Coffea arabica) is a popular perennial cash crop in southwestern Oromia, providing monofloral honey. According to Adi et al. (Citation2014), Coffea arabica provides abundant and aromatic pollen and nectar that attract honeybees in January. The experiment studied by Bareke et al. (Citation2021) revealed that Coffea arabica has the potential to produce 125 kg/ha of honey. Syzygium guineense flowers have plentiful amounts of nectar and pollen, which bees forage aggressively. This bee forage tree is a major honey source plant in Ethiopia (Fichtl & Adi, Citation1994). Similarly, Syzygium guineense was found to be the predominant honey source plant in the Ana Sora area of Guji from March to April, according to a study done by Bareke and Addi (Citation2018).
Ilex mitis spp. was detected as a secondary honey source plant species in the sample obtained from the Ana Sora district of Guji. Similarly, Bareke and Addi (Citation2018) also reported that Ilex mitis and S. guineense were the main honey source plants in Ana Sora of the Guji zone that yield unifloral honey in the area. Table displays the main, secondary, important minor, and minor pollen species in enriching the stingless bee honey samples.
3.2. Physicochemical properties of Meliponula beccarii honey
The result of the color analysis revealed the color of stingless bee honey samples varied from Water white to Dark amber (Table ). The color of Guji zone (Arda Jila and Ana Sora district) honey was grouped as Extra light amber, whereas the color of honey harvested from Cheliya, Jimma Genete, Sibu Sire, Diga, Didu, and Gera districts were found to be Light amber. The color of the M. beccarii honey harvested from Guduru, Sokoru, and Seka Chekorsa was classified as Amber. Water white color was detected in the sample harvested from the Gechi area of the Illubabor zone. Out total honey samples, 50 % were categorized as Light amber, 21.5% as Extra light amber, and Amber, and 7% as Water white, according to standards of color designations for extracted honey (USDA, Citation1985). This indicates that stingless bee honey varies based on different factors. The color of stingless bee honey may vary depending on methods of production, light exposure, temperature, storage conditions, and enzyme reactions. In general, the color of the stingless bee honey is chosen depending on consumer preferences, which are seen as quality determination and market recognition (da Silva et al., Citation2016).
Table 2. Color of stingless bee (M. beccarii)honey samples categorized according to the Pfund scale
The maximum moisture content was found for the honey sample collected from the Sibu Sire (38.3 ± 0.10%) while the minimum value for the honey sample collected from the Lalo Asabi area (25.17 ± 0.18%) (Table ). A similar finding was reported by Biluca et al. (Citation2016) who suggested moisture value ranged between 23.1 to 43.5% for stingless bee honey samples in Brazil and Chuttong et al. (Citation2016a) reported moisture content ranged from 25 to 47% moisture value for stingless bee honey in Thailand. Souza et al. (Citation2004) also suggested the moisture content ranged from 19.90 to 41.90 % for stingless bee honey obtained from different species. In the present study, the moisture content values of stingless bee honey samples ranged from 24.50 to 39.0% were agree with the finding in the West Shewa zone (25 to 35%) for stingless bee honey produced (Gela et al., Citation2021). According to international quality requirements for Apis mellifera honey (Codex, Citation2001), the overall mean value of the moisture content in current stingless bee honey samples does not comply with the range of the required standards (≤20%).
Table 3. The physicochemical properties of stingless bee (M.Beccarii) honey collected from study areas (mean ± standard error(SE)
pH values varied from 2.38 to 4.08 with a mean value of 3.30 for the M. beccarii honey sample. Significantly, the maximum mean pH value (3.61 ± 0.05) was registered for stingless bee honey from the Ana Sora the Guji area, and the minimum value for the honey sample harvested from Seka Chekorsa of Jimma (2.56 ± 0.06). The overall mean value of pH of the stingless bee honey sample in the current study is consistent with the study reported by Gela et al. (Citation2021) who found the pH values of stingless bee honey produced in West Shoa Oromia region ranged from 3.2 to 4.5 and Alves et al. (Citation2005) who observed the pH values 3.2 ± 0.21 for stingless honey produced in Brazil. Overall, Meliponula beccarii honey exhibits a low pH due to the occurrence of several acids such as gluconic, acetic, lactic, citric, succinic, formic, malic, malic, and oxalic, as well as a lower total solute content (Vit et al., Citation1998). The low pH of stingless bee honey may contribute to its antibacterial properties because it is believed to prevent microbial growth (Malika et al., Citation2005).
Free acidity contents of Meliponula beccarii honey ranged from 15 to 199.87 meq/kg with a mean value of 92.29 ± 4.45 meq/kg. There were significant differences (P < 0.05) in the free acidity of honey between S/Chekorsa and other honey samples. A similar mean of free acidity to the current study result was found in various parts of the world. Accordingly, Kek et al. (Citation2017) found a similar value of free acidity of 36.8 ± 7.6 meq/kg for honey produced by stingless bees in Malaysia stingless bees, Oddo et al. (Citation2008) reported the free acidity of 124.2 ± 22.9 meq/kg for Australian stingless bees and Sousa et al. (Citation2016)observed the free acidity of 103.3 meq/kg and 109 meq/kg for Melipona quadrifasciata and Tetragonisca angustula stingless bee species of respectively, in Brazil
Differences in the acidity of honey may be attributed to the season of harvesting, honey maturation, floral origin, geographical location, storage conditions, and environmental impact (Ahmed et al., Citation2014; Rodrı́guez et al., Citation2004). Apart from moisture content, Meliponula beccarii honey exhibits higher free acidity values than Apis mellifera honey (Almeida‐Muradian et al., Citation2013; Sousa et al., Citation2016). Determination of free acidity is useful for assessing fermentation status in honey and authenticating monofloral honey (Silvano et al., Citation2014). Codex Alimentarius regulation specifies that the free acidity of Apis mellifera honey cannot exceed 50 meq/kg (Codex, Citation2001; EU, Citation2002). The study findings of the current investigation revealed that the free acidity of all stingless bee honey samples failed to satisfy the Codex Alimentarius requirements (2019) for honey produced from Apis mellifera, except for Meliponula beccarii honey harvested from Cheliya of West Shewa zone of Oromia region.
The ash content value analyzed for stingless bee honey samples obtained from Arda Jila showed statistically significant (P < 0.05) from other source samples. The maximum value of ash concentration found for honey collected from Arda Jila of Guji area (0.27 ± 0.04%) and the minimum value documented for sample from Guduru (0.06 ± 0.01%) (Table ). The ash content of the present study (0.01–0.50 g/100 g) was similar to the previous finding for stingless bee honey ash content (0.41 g/100 g) in West Shewa zone of Oromia region (Gela et al., Citation2021). The present study investigation was also compatible with the finding of Suntiparapop et al. (Citation2012) who reported that ash content (0.20%) for Tetragonula laeviceps in Thailand. Gonzalez et al. (Citation2015) reported the variation in ash content of Meliponinae honey ranging from 0.01 to 0.6% with a mean of 0.16 ± 0.12% in honey samples analyzed in Mexico. However, Chuttong et al. (Citation2016a) discovered that stingless bee species honey produced in Thailand has a higher ash level ranging from 0.22 to 3.1%.
Electrical conductivity (EC) values found ranged from 0.16–1.01 mS/cm with a mean value of 0.44 ± 0.02 mS/cm. Statistical differences were observed (p ≤ 0.05) in the EC of M. beccarii of honey samples between the Guduru, Gechi, and Ana Sora districts. The result of this study corroborates the previous study by Gela et al. (Citation2021) who reported that EC of stingless bee honey produced in the West Shoa Oromia region ranged from 0.22–1.52 mS/cm, Alvarez-Suarez et al. (Citation2018) who found the EC of 0.58 ± 0.14 mS/cm for Meliponula beccarii honey produced in Cuban, Berhanu (Citation2014)who observed the EC of 3.27 ± 0.01 mS/cm for honey produced by Trigona species in Ethiopia. The EC value of 0.71 and 0.53 ms/cm was also mentioned by Suntiparapop et al. (Citation2012) for stingless bee species from Southeast Asia. Similar to the present finding, Sousa et al. (Citation2016) and Guerrini et al. (Citation2009) also reported EC of 0.30 and 0.67 mS/cm for stingless bee species honey from South America, respectively. In contrast, the EC of this study was lower than that reported by Biluca et al. (Citation2016), who discovered that the EC of stingless bee honey produced in Brazil ranged from 0.15–1.34 mS/cm. The variations in the electrical conductivity value of stingless bee honey samples were generally associated with changes in the honey samples’ geographical, botanical, and entomological origins (Biluca et al., Citation2016).
In this study, stingless bee honey samples showed HMF values ranging from 0 to 15.78 mg/kg. According to the current findings, the maximum HMF concentration was found for honey samples harvested from the Diga area of East Welega (12.63 ± 0.49 mg/kg). Some studies have reported similar concentrations of HMF in Meliponula beccarii honey. Oddo et al. (Citation2008) found the HMF contents ranging from 0.4 to 2.1 mg/kg in Australian stingless bee honey. Suntiparapop et al. (Citation2012) reported that HMF values ranged from 0.53 to 0.71 mg/kg for honey produced in Thailand from stingless bee species. However, the overall mean value of HMF of this study results (6.58 ± 0.67 mg/kg) was lower than the Gela et al. (Citation2021) finding indicating that the HMF concentration of 18 mg/kg for stingless bee honey collected from the West Shoa zone of Oromia region. All the Meliponula beccarii honey samples in this study exhibited the HMF concentration below established national (ESA, Citation2005) and international (Codex, Citation2001; EU, Citation2002) quality requirements, which establishes a maximum limit of 40 mg/kg. HMF content is an important indicator of honey quality, showing honey freshness, aging excessive heating, and adulteration (Mairaj et al., Citation2008; Subramanian et al., Citation2007).
3.3. Sugar profile of stingless bee (Meliponula beccarii) honey
In these study findings, the maximum fructose content (40.29 ± 0.72%) was detected in a sample of stingless bee honey harvested from Gera and the minimum value (18.27 ± 0.37%) for a sample collected from Sokoru of Jimma area. The overall mean value of fructose concentration in the present study ranged from 16.53–47.50 % with a mean value of 36.48 ± 0.54 %. The content of fructose for the current finding slightly agrees with Almeida‐Muradian et al. (Citation2013) finding who suggested the fructose content 31.61% for M. beccarii honey in the Amazon region of Brazil.
The glucose concentration of the Meliponula beccarii honey sample in this study was 16.88 to 40.24 g/100 g with a mean value of 27.67 g/100. Almeida‐Muradian et al. (Citation2013) discovered 33 g/100 g of glucose concentration in honey produced from Melipona species in Brazil Amazon region, which is comparable to the current study finding. However, Guerrini et al. (Citation2009) discovered a lower glucose concentration of 25.5 g/100 g for stingless bee honey produced in Ecuador, compared to the current finding. The current findings confirm the assumption that fructose was the more dominant sugar than glucose in high-quality Meliponula beccarii honey. The fructose concentration might be responsible for the intensity of the sweet taste and strong hygroscopicity while keeping the honey liquid for an extended period or preventing crystallization.
Amounts of sucrose ranged from 0.0–9.0 g/100 g with a mean value of 1.24 ± 0.17 g/100 g for Meliponula beccarii honey sample analyzed in this study. There were statistical differences (P < 0.05) in concentrations of sucrose of stingless bee honey harvested from Sokoru from samples collected from the Didu, Guduru, and Diga areas. Sousa et al. (Citation2016) also found similar results of sucrose content ranged from 0.13 to 6.00 g/100 in stingless bee honey produced in Brazil. As part of honey quality parameters, sucrose is linked to honey maturation. High concentrations of sucrose levels that have not been transformed into glucose and fructose might suggest that the product was harvested prematurely (Almeida‐Muradian et al., Citation2013). The findings revealed that the concentration sucrose of in all honey samples did not exceed the maximum recommended limits (5%) specified by international legislation (Codex, Citation2001). With a maximum of 10% for Apis mellifera, the sucrose level of the stingless bee honey complies with Ethiopian Standard Agency quality standards (ESA, Citation2005). Until recently, the standard specifications of honey produced by stingless bees in Ethiopia were not established. Thus a quality parameters comparison was made with the standard suggested by Vit et al. (Citation2004), who reported a sucrose concentration of 6% (maximum recommended limit) for M. beccarii honey. The sucrose content of the current study is consistent with the standard standards specified by Vit et al. (Citation2004). In general, the concentration sucrose of in the current study finding demonstrates that the honey sample was collected at the optimal maturation time since high sucrose concentration might be attributed to the addition of sugar or early honey harvesting.
The highest and lowest maltose concentrations were found in stingless bee honey samples harvested from Seka area Jimma (4.13 ± 0.36 %) and Guduru area of H/G/Welega (0.25 ± 0.01%), respectively. In this study, there was a significant difference (P < 0.05) in maltose concentration between the honey sample collected from the S/Chekorsa and the Didu area of Illubabor. Vit et al. (Citation1998) reported a similar finding, implying that the Meliponula beccarii species honey has a low maltose concentration ranged from 1 to 1.23%, while honey produced by the Trigona species has a high maltose concentration ranged from 24 to 56 %. Kaškonienė et al. (Citation2011) found that authentic (pure) honey was distinguished from artificial (fake) honey by possessing a high level of maltose concentration.
The values of reducing sugar in this study finding ranged from 36.32 to 72.44% with a mean value of 64.15 ± 0.75 % (Table ). Carvalho et al. (Citation2006) also found similar results for reducing the sugar of stingless bee honey ranged from 42.55 % to 55.61%. Similarly, Souza et al. (Citation2004) found that reducing sugar varied from 58 to 75.7% for stingless bee species. The study finding reported by Alves et al. (Citation2005) also discovered the reducing sugar content in Meliponula beccarii varied from 50.6 to 95.6%. However, Chuttong et al. (Citation2016b) found that the average reducing sugar is less than 60% for stingless bee honey. Agus and Umami (Citation2019) revealed that the concentration of sugar stingless bee honey varies based on botanical origin. According to our study results, all the samples of stingless bee honey analyzed in this experiment complied with the minimum reducing sugars (50%) standard established for the quality of Melipona honey (Vit et al., Citation2004). According to the findings of this investigation, except for the honey sample collected from Guji (Ana Sora &Arda Jila), honey sample harvested from Gera, Gechi, L/Asabi, J/Genete, and Sibu Sire area contains reducing sugar lower than 60 g/100 g. As a result, these samples do not meet the minimum quality criteria of the Codex Alimentarius standard, which is 60 g/100 g for A. Mellifera (Codex, Citation2001). The findings suggested that the reducing sugar content varied because samples were taken from various botanical and geographical locations, which can affect the typical carbohydrate composition in honey. Studies demonstrate that Melipona honey possesses fewer sugar components and is less sweet than Apis mellifera honey (Biluca et al., Citation2016; Chuttong et al., Citation2016a; Sousa et al., Citation2016).
Table 4. The sugar concentration(mean ± standard error).Of stingless bee honey(M. beccarii) honey
Table displays the correlation matrix of analyzed physicochemical properties of the Meliponula beccarii honey. The ash concentration has a strong significant positive correlation with Electrical conductivity (r = 0.834, p < 0.001), demonstrating ash content determines electrical conductivity in honey (Table ). Previously, Imtara et al. (Citation2018) found the same correlation between ash content and electrical conductivity. There is a moderate negative correlation between free acidity and pH value of the Meliponula beccarii honey (r = −0.674, p < 0.001) clarifying that stingless bee honey has highly acidic and easily fermentable due to its high free acidity when we compare it to national and international standard of Apis Mellifera. Fructose has a positive strong correlation with reducing sugar (r = 0.820, p < 0.001) showing that the good quality honey sample has dominant fructose and a higher concentration of reducing sugar.
Table 5. Pearson correlation coefficients among physicochemical parameters of the Meliponula beccarii honey
3.4. Microbiological quality of the stingless bee (Meliponula beccarii)honey
Despite honey’s ability to inhibit microbe growth, only a few microorganisms have the ability to proliferate, persist, and cause honey to deteriorate (Pucciarelli et al., Citation2014). Sources of honey contamination with microorganisms include honeybee digestive tracts, pollen, nectar, flowers, air, dirt, and honey manipulation in the processing area and equipment. In the current study, we strive to avoid secondary sources of contamination by harvesting honey with sterile syringes and gloves. The mean count (log CFU/mL) revealed that Aerobic Mesophiles predominated in all stingless bee honey samples except those collected in the Gera of Jimma zone. Among the samples, the highest mean counts (2.545 × 104 CFU/mL) of Aerobic Mesophiles were found in sample harvested from Shabe Sonbo of Jimma Zone while the lowest mean counts(1.9 × 103 CFU/mL) of Aerobic mesophiles were detected in Ana Sora of Guji (Table ). Similar findings have been suggested by Beux et al. (Citation2022), who discovered that the mean total aerobic mesophiles for stingless bee species are 3.78 log CFU/g. In this study, a significant prevalence of Aerobic mesophile count was identified when compared to other microorganisms which could be symptomatic of inappropriate handling during harvesting and processing.
Table 6. The microbial quality of stingless bee (M.Beccarii) honey
The existence of a large variation of aerobic microorganisms in honey indicates a degree of deterioration which helps in determining honey shelf-life (Franco & Landgraf, Citation2008). Moreover, certain qualities of stingless bee honey, including high acidity, minimal water activity, a low protein content, strong antioxidant capacity, and high sugar content, may limit or inhibit bacterial activity, contributing to the product’s extended shelf life (EU, Citation2002; Monte et al., Citation2013). Unfortunately, some of the analyzed samples in this experiment had a high mean count of mesophilic bacteria, which could be attributed to quality and safety issues that could lead to contamination. Source contaminants in honey samples might include honey bees, soil, water, air, pollen, and nectar (Bárbara et al., Citation2015; Oliveira et al., Citation2017). In this study, the number of spore-forming bacteria was undetectable in all Meliponula beccarii honey samples. On the other hand, there is evidence that spore-forming bacteria such as Bacillus cereus (aerobic) and Clostridium spp (anaerobic) have been identified in honey samples, which could cause disease in humans under specific conditions.
However, only samples taken from the Horo Guduru Welega Zone Guduru district indicated a Staphylococci count. Both, Beux et al. (Citation2022) and Pucciarelli et al. (Citation2014) confirmed that various Staphylococcus spp. were found in stingless bee species, which is contrary to this finding. According to Pucciarelli et al. (Citation2014), Staphylococcus species is not frequently found in honey produced by A. mellifera species, and it appeared to be unable to survive during processing. Thus it is not expected to grow in honey. The limited prevalence of staphylococci can be linked to aseptic honey collection, equipment, and honey handling, which are considered the primary and secondary sources of contamination (Dümen et al., Citation2013; Pucciarelli et al., Citation2014).
The mean yeast count of the stingless bee (M. beccarii) honey sample collected in the Didu area of the Illubabor was found to be the highest (1.675 × 104 CFU/mL), while honey sample collected in the Arda Jila area of the Guji zone was found to be the lowest (9 × 102 CFU/mL) (Table ). The source of yeast may have originated from the primary cause of contamination since secondary sources of contamination were prevented by using sterilized equipment. Mold and yeast survive in stingless bee honey because fungi, primarily yeasts, can grow in conditions of high sugar concentrations even in the absence of abundant water (Snowdon & Cliver, Citation1996). Pinheiro et al. (Citation2018) found that the average count of fungi and yeast in honey produced by Melipona subnitida in Brazil was 9.12 × 103CFU/g. According to Lani et al. (Citation2017), the yeast count of Meliponini honey’ was low in a fresh sample (104 CFU/g) but dramatically increased to 106 CFU/g after 7 days of storage. Furthermore, Camargo et al. (Citation2017) proposed an acceptable limit of 1.0 × 104 CFU/g for stingless bee honey, highlighting the enhanced humidity of honey as well as the rich fungal microbiota of stingless bee honey, leading to honey with higher counts. As a result, the main problem caused by the presence of microorganisms in honey is fermentation, which may affect the shelf life of the honey (Grabowski & Klein, Citation2017). The concentration of moisture has a direct relation to the fermentation status of the honey as a conducive environment is created for microbial growth. In this regard, Bogdanov (Citation2009) studied the correlation between honey moisture content and fermentation risk and discovered that honey with a moisture content of 17.1 to 18% is safe from fermentation if the yeast count is less than 1000 CFU/g.
Stingless bee honey samples harvested from Ana Sora of the Guji area had the highest mean count (1.8 × 103 CFU/mL) of mold while samples obtained from Guduru and Gera had the lowest(2 × 102 CFU/mL). The prevalence of mold in the current research could be attributed to unsanitary practices during honey harvesting, processing, and storage. Molds, often known as Xerophiles, are capable of growing at or below a water activity (aw) of 0.85 and low water content ranging from 16 to 17.0%. Molds and yeasts may grow easily in Meliponula beccarii honey due to its higher moisture content (Marconi et al., Citation2020). However, the absence of fungal spores and yeast cells in some Meliponula beccarii honey implies that honey possesses intrinsic antimicrobial properties that can delay or prevent the growth of many microorganisms. The yeasts and molds found in honey can resist high concentrations of acids and sugar, which can be a serious problem for honey quality in the honey processing industry (Gois et al., Citation2013). Giraldo et al. (Citation2013) also confirmed this assumption, claiming that the amount of microorganisms in honey is lower than in any other food item because of its high sugar acid concentration. Because stingless bee honey has strong antimicrobial characteristics that hinder or prevent the growth or persistence of many microorganisms honey is expected to have a low number and a limited range of microorganisms (Agbagwa et al., Citation2014; Buba et al., Citation2013). Furthermore, the presence of some phytochemical compounds in honey, especially phenols, terpenes, and pinocembrin, contributes to the control of microbial proliferation (Torres-González et al., Citation2016). Bacteria and fungi identified in Meliponula beccarii honey samples have been linked to cross-contamination during harvesting, straining, transportation, and storage conditions (Tchoumboue et al., Citation2007; Uran et al., Citation2017). The microorganisms in stingless bee honey could have originated in pollen, bee digestive tracts, air, soil, or nectar. On the other hand, the relative availability of water in Meliponula beccarii honey allows microorganisms to survive and multiplicate . Contamination must be controlled during nest, honeycomb, and pollen pot manipulation, as well as honey harvesting, through the implementation of good manufacturing practices. (Snowdon & Cliver, Citation1996).
4. Conclusion
This study examined the quality parameters such as the physicochemical and microbiological quality of honey produced by a stingless bee (Meliponula beccarii) collected from different agroecology of the Oromia region. Apart from moisture content, free acidity, and sugar profile, the other physicochemical parameters of the analyzed honey samples are within the range of recommended quality criteria established by the Ethiopian standard agency and Codex Alimentarius for Apis Mellifera. This variation reveals differences in quality and composition between Apis mellifera and Meliponula beccarii honey samples, as well as the requirement for particular standard standards for stingless bee honey. According to Pearson correlation investigation, a perfect positive relationship between electrical conductivity and ash, as well as fructose and reducing sugar was observed in stingless bee honey. Yet, moderate negative relationship between pH and free acidity of Meliponula beccarii honey.
Although Meliponula beccarii honey is known for its more acidic and powerful antimicrobial properties with a broad range of impacts, some microorganism has been detected in some samples. The high levels of aerobic mesophilic in our evaluated samples indicate a lack of knowledge about good manufacturing practices and honey storage conditioning. The occurrence of yeast, mold, and bacterial microorganisms in some Meliponula beccarii honey samples could be associated with inadequate hygiene practices during harvesting, handling, and storage. Furthermore, the microorganisms in stingless bee honey could have originated from pollen, bee digestive tracts, air, soil, or nectar. The findings of this study demonstrated the microbiological stability of honey produced by Meliponula beccarii. As a result, fresh stingless bee honey directly collected from honey pots has distinctive physicochemical and microbiological qualities that have proven more in demand and are more expensive than Apis Mellifera honey.
To the best of our knowledge, this is the first comprehensive research on the physicochemical and microbiological characterization of honey produced by stingless bee species (Meliponula beccarii) from broader agroecological zones of Oromia in Ethiopia. In general, the current Apis mellifera honey standard requirements are inadequate for all of the stingless bee honey quality parameters analyzed in the present study. This underlines the importance of an appropriate quality standard for honey produced by stingless bees to promote it on a legitimate market and avoid adulteration. Indeed, improved collecting techniques and minimum sanitary requirements for stingless bee honey could be developed to reduce the entry of particular microorganisms. Additionally, standard requirements for the quality of stingless bee honey should be established in Ethiopia
Acknowledgments
The authors would like to thank the Holeta Bee Research Center for providing logistical support. We would also like to express our gratitude to Bee product quality improvement and value addition research staff for their support in this research.
Disclosure statement
The authors declare that they have no known competing financial interests or close relationships that could appear to have influenced the work described in this study. This article does not contain any studies with human or animal subject.
Data availability statement
The authors confirm that the data supporting this study’s findings are available upon request
Additional information
Notes on contributors

Teferi Damto
Teferi Damto has served as Associate Researcher in bee product quality improvement and value addition at the center of Holeta Bee Research at the Oromia Institute of Agricultural Research. He received his MSc in Food Science and Nutrition from Addis Ababa University in 2021, Ethiopia. Over the last six years, Teferi has worked broadly on bee product quality improvement and value addition, food and nutrition research, and training. Mr. Teferi published several papers in favorite Journals and chapters in books and participated in a range of forums on honey bee product quality and value addition. He currently works in the bee product quality improvement and values addition by conducting different innovative and technological research on areas of quality improvement and value addition of bee products. His current research interests include beekeeping, bee product (honey, beeswax, pollen grain, propolis, and royal jelly) quality improvement, value addition and processing, and Apitherapy. E-mail address: [email protected]
References
- Adgaba, N. (2002). Geographical races of the Honeybees (Apis mellifera L.) of the Northern Regions of Ethiopia Doctoral dissertation, Rhodes University.
- Adi, A., Wakjira, K., Kelbessa, E., & Bezabeh, A. (2014). Honey bee forages of Ethiopia. Holeta Bee Research Center, Addis Ababa.
- Agbagwa, O. E., Otokunefor, T. V., & Frank-Peterside, N. (2014). Preliminary detection of bacillus species in commercial honey. Microbiology Research Journal International, 4(12), 1370–21. https://doi.org/10.9734/BMRJ/2014/7217
- Agus, A., & Umami, N. (2019). The sugar content profile of honey produced by the Indonesian stingless bee, tetragonula laeviceps, from different regions. Livestock Research for Rural Development, 31(6).
- Ahmed, M., Khiati, B., Meslem, A., Aissat, S., & Djebli, N. (2014). Evaluation of physicochemical and antioxidant properties of raw honey from Algeria. Journal of Microbial & Biochemical Technology, s1(01), 006. https://doi.org/10.4172/1948-5948.S4-006
- Alemu, T., Seifu, E., & Bezabih, A. (2010). Physicochemical properties of honey produced in Sekota district, Northern Ethiopia. International Food Research Journal, 20(6), 3061–3067.
- Almeida‐Muradian, L. B., Stramm, K. M., Horita, A., Barth, O. M., da Silva de Freitas, A., & Estevinho, L. M. (2013). Comparative study of the physicochemical and palynological characteristics of honey from M elipona subnitida and a pis mellifera. International Journal of Food Science & Technology, 48(8), 1698–1706. https://doi.org/10.1111/ijfs.12140
- Alqarni, A. S., Balhareth, H. M., & Owayss, A. A. (2014). Performance evaluation of indigenous and exotic honey bee (Apis mellifera L.) races in Assir region, southwestern Saudi Arabia. Saudi Journal of Biological Sciences, 21(3), 256–264. https://doi.org/10.1016/j.sjbs.2013.10.007
- Alvarez-Suarez, J. M., Giampieri, F., Brenciani, A., Mazzoni, L., Gasparrini, M., González-Paramás, A. M., Santos-Buelga, C., Morroni, G., Simoni, S., Forbes-Hernández, T. Y., Afrin, S., Giovanetti, E., & Battino, M. (2018). Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT, 87, 272–279. https://doi.org/10.1016/j.lwt.2017.08.079
- Alves, R. M. D. O., Carvalho, C. A. L. D., Souza, B. D. A., Sodré, G. D. S., & Marchini, L. C. (2005). Características físico-químicas de amostras de mel de Melipona mandacaia Smith (Hymenoptera: Apidae). Ciência e Tecnologia de Alimentos, 25(4), 644–650. https://doi.org/10.1590/S0101-20612005000400004
- Arega, A., Tusa, G., & Megersa, D. (2020). Assessment on honeybee flora species with their time of flowering in East and Horo Guduru Wollega, Oromia regional state, Ethiopia. International Journal of Fauna and Biological Studies, 7(4), 156–163.
- Ashenafi, M. (1994). The in vitro antibacterial activity of” tazma Mar” honey produced by the stingless bee (apis mellipodae). Ethiopian Journal of Health Development, 8(2), 109–117.
- Bárbara, M. S., Machado, C. S., Sodré, G. D. S., Dias, L. G., Estevinho, L. M., & De Carvalho, C. A. L. (2015). Microbiological assessment, nutritional characterization and phenolic compounds of bee pollen from Mellipona mandacaia Smith, 1983. Molecules, 20(7), 12525–12544. https://doi.org/10.3390/molecules200712525
- Bareke, T., & Addi, A. (2018). Honeybee flora resources of Guji zone, Ethiopia. Journal of Biology, Agriculture and Healthcare, 8(21), 1–9.
- Bareke, T., & Addi, A. (2019). Bee flora resources and honey production calendar of Gera Forest in Ethiopia. Asian Journal of Forestry, 3(2). https://doi.org/10.13057/asianjfor/r00300204
- Bareke, T., Addi, A., Wakjira, K., & Kumsa, T. (2021). Dynamics of nectar secretion, honey production potential and colony carrying capacity of Coffea arabica L., rubiaceae. Journal of Agriculture and Environment for International Development (JAEID), 115(1), 125–138.
- Belay, A., Haki, G. D., Birringer, M., Borck, H., Lee, Y. C., Cho, C. W., Kim, K.-T., Bayissa, B., Baye, K., & Melaku, S. (2017). Sugar profile and physicochemical properties of Ethiopian monofloral honey. International Journal of Food Properties, 20(11), 2855–2866. https://doi.org/10.1080/10942912.2016.1255898
- Berhanu, A. (2014). Physico-chemical, microbiological and antibacterial properties of Apis mellipodae and Trigona spp. Honey against bacterial pathogens. The Journal of Agricultural Science, 10(3), 112–120.
- Beux, M. R., Ávila, S., Surek, M., Bordin, K., Leobet, J., Barbieri, F., & Rosa, E. A. R. (2022). Microbial biodiversity in honey and pollen pots produced by Tetragonisca angustula (Jataí). Brazilian Archives of Biology and Technology, 65, 65. https://doi.org/10.1590/1678-4324-2022210440
- Biluca, F. C., Braghini, F., Gonzaga, L. V., Costa, A. C. O., & Fett, R. (2016). Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (meliponinae). Journal of Food Composition and Analysis, 50, 61–69. https://doi.org/10.1016/j.jfca.2016.05.007
- Bogdanov, S. (2009). Honey composition. Bee product Science. http://www.beehexagon.net/.
- Buba, F., Gidado, A., & Shugaba, A. (2013). Physicochemical and microbiological properties of honey from North East Nigeria. Biochemistry & Analytical Biochemistry, 2(142), 61–67. https://doi.org/10.4172/2161-1009.1000142
- Camargo, R. C. R., De Oliveira, K. L., & Berto, M. I. (2017). Stingless bee honey: Technical regulation proposal. Brazilian Journal of Food Technology, 20. https://doi.org/10.1590/1981-6723.15716
- Carvalho, C. A., Sodré, G. S., Fonseca, A. A., Alves, R. M., Souza, B. A., & Clarton, L. (2006). Physicochemical characteristics and sensory profile of honey samples from stingless bees (Apidae: Meliponinae) submitted to a dehumidification process. Anais da Academia Brasileira de Ciências, 81(1), 143–149. https://doi.org/10.1590/S0001-37652009000100015
- Chuttong, B., Chanbang, Y., Sringarm, K., & Burgett, M. (2016a). Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South east Asia (Thailand). Food Chemistry, 192, 149–155. https://doi.org/10.1016/j.foodchem.2015.06.089
- Chuttong, B., Chanbang, Y., Sringarm, K., & Burgett, M. (2016b). Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand). Journal ofFood Chemistry, 192, 149–155. https://doi.org/10.1016/j.foodchem.2015.06.089
- Codex. (2001). Codex Alimentarius standard for honey 12-1981. Revised Codex standard for honey. Standards and standard methods (Vol. 11). Retrieved December , 2014, from http://www.codexalimentarius.net
- da Silva, P. M., Gauche, C., Gonzaga, L. V., Costa, A. C. O., & Fett, R. (2016). Honey: Chemical composition, stability and authenticity. Food Chemistry, 196, 309–323. https://doi.org/10.1016/j.foodchem.2015.09.051
- Degaga, A. H. (2017). Identification of honey source bee floras during major and minor honey harvesting seasons in Jimma zone, Southwest Ethiopia. Journal of Environment and Earth Science, 7(3), 25–32.
- Dümen, E., Akkaya, H., Öz, G. M., & Sezgin, F. H. (2013). Microbiological and parasitological quality of honey produced in İstanbul. Turkish Journal of Veterinary and Animal Sciences, 37(5), 602–607. https://doi.org/10.3906/vet-1301-46
- Ethiopia Standard. (2005). Honey specification: Ethiopian standard, ES 1202: 2005. Addis Ababa.
- European Commission. (2002). European commission council directive 2001/110/EC of 20 December 2001 relating to honey. Official Journal of the European Communities. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:010:0047:0052(EN:PDF).
- Fichtl, R., & Adi, A. (1994). Honeybee flora of Ethiopia. Margraf Verlag.
- Franco, B. D. G. M., & Landgraf, M. (2008). In Microbiologia dos alimentos (pp. 182–182). Ateneu.
- Gela, A., Hora, Z. A., Kebebe, D., & Gebresilassie, A. (2021). Physico-chemical characteristics of honey produced by stingless bees (Meliponula beccarii) from West Showa zone of Oromia region, Ethiopia. Heliyon, 7(1), e05875. https://doi.org/10.1016/j.heliyon.2020.e05875
- Gheldof, N., Wang, X. H., & Engeseth, N. J. (2002). Identification and quantification of antioxidant components of honeys from various floral sources. Journal of Agricultural and Food Chemistry, 50(21), 5870–5877. https://doi.org/10.1021/jf0256135
- Giraldo, A. M. V. A., Costa, V. L. M., & Gallego, R. Z. (2013). Physicochemical and microbiological characterization of Apis Mellifera sp. honey from showest of Antioquia in Colombia. Ingenieria y Ciencia, 9(18), 61–74. https://doi.org/10.17230/ingciecia.9.18.3
- Gobessa, S., Seifu, E., & Bezabih, A. (2012). Physicochemical properties of honey produced in the Homesha district of Western Ethiopia. Journal of Apicultural Science, 56(1), 33–40. https://doi.org/10.2478/v10289-012-0004-z
- Gois, G. C., Rodrigues, A. E., de LIma, C. A. B., & Silva, L. T. (2013). Composição do mel de Apis mellifera: Requisitos de qualidade. Acta Veterinaria Brasilica, 7(2), 137–147.
- Gonzalez, V. H., Amith, J. D., & Stein, T. J. (2015). Nesting ecology and the cultural importance of stingless bees to speakers of Yoloxóchitl mixtec, an endangered language in Guerrero, Mexico. Apidologie, 49(5), 625–636. https://doi.org/10.1007/s13592-018-0590-2
- Grabowski, N. T., & Klein, G. (2017). Microbiology and foodborne pathogens in honey. Critical Reviews in Food Science and Nutrition, 57(9), 1852–1862. https://doi.org/10.1080/10408398.2015.1029041
- Guerrini, A., Bruni, R., Maietti, S., Poli, F., Rossi, D., Paganetto, G., Muzzoli, M., Scalvenzi, L., & Sacchetti, G. (2009). Ecuadorian stingless bee (meliponinae) honey: A chemical and functional profile of an ancient health product. Food Chemistry, 114(4), 1413–1420. https://doi.org/10.1016/j.foodchem.2008.11.023
- Imtara, H., Elamine, Y., & Lyoussi, B. (2018). Physicochemical characterization and antioxidant activity of Palestinian honey samples. Food Science and Nutrition, 6(8), 2056–2065. https://doi.org/10.1002/fsn3.754
- International Honey Commission. (2009). Harmonised methods of the international honey commission. https://www.ihc-platform.net/ihcmethods2009.pdf
- Kaškonienė, V., Venskutonis, P., & Čeksterytė, V. (2011). Sugar analysis for authenticity evaluation of honey in Lithuanian market. Acta Alimentaria, 40(2), 205–216. https://doi.org/10.1556/AAlim.40.2011.2.4
- Kebede, N., Subramanian, & Gebrekidan, M. (2012). Physicochemical analysis of Tigray honey: An attempt to determine major quality markers of honey. Bulletin of the Chemical Society of Ethiopia, 26(1), 127–133. https://doi.org/10.4314/bcse.v26i1.14
- Kek, S. P., Chin, N. L., Yusof, Y. A., Tan, S. W., & Chua, L. S. (2017). Classification of entomological origin of honey based on its physicochemical and antioxidant properties. International Journal of Food Properties, 20(sup3), S2723–S2738. https://doi.org/10.1080/10942912.2017.1359185
- Kinati, C., Tolemariam, T., & Kebede, D. (2011). Quality evaluation of honey produced in Gomma Woreda of South-Western Ethiopia. Livestock Research for Rural Development, 23(9).
- Lani, M. N., Zainudin, A. H., Razak, S. B. A., Mansor, A. Z. L. I. N. A., & Hassan, Z. A. I. T. O. N. (2017). Microbiological quality and pH changes of honey produced by stingless bees, Heterotrigona itama and geniotrigona thoracica stored at ambient temperature. Malaysian Applied Biology, 46(3), 89–96.
- Louveaux, J., Maurizio, A., & Vorwohl, G. (1978). Methods of melissopalynology. Bee World, 59(4), 139–157. https://doi.org/10.1080/0005772X.1978.11097714
- Mairaj, G., Akhtar, S., Khan, A. R., Ullah, Z., Bibi, S., & Ali, S. (2008). Quality evaluation of different honey samples produced in Peshawar valley. Pakistan Journal of Biological Sciences, 11(5), 797. https://doi.org/10.3923/pjbs.2008.797.800
- Malika, N. A. M. A. N., Mohamed, F. C. E. A., & Chakib, E. A. (2005). Microbiological and physico-chemical properties of Moroccan honey. International Journal of Agriculture and Biology, 7(5), 773–776.
- Marconi, M., Luna, J. O., & Giove, C. D. V. (2020). Physicochemical and microbiological quality of honeys produced by stingless bees scaptotrigona polysticta, Melipona illota and Tetragonisca angustula (Apidae: Meliponini) in San Martín, Peru. Peruvian Journal of Agronomy, 4(2), 55–60. https://doi.org/10.21704/pja.v4i2.1541
- Monte, A. M., Azevedo, M. L. X., Das Chagas Cardoso Filho, F., Rodrigues, A. M. D., de Moura, S. G., & Muratori, M. C. S. (2013). Qualidade de méis de abelhas nativas sem ferrão do estado do Piauí, Brasil. Brazilian Journal of Veterinary Medicine, 35(1), 48–54.
- Moo-Huchin, V. M., González-Aguilar, G. A., Lira-Maas, J. D., Pérez-Pacheco, E., Estrada-León, R., Moo-Huchin, M. I., & Sauri-Duch, E. (2015). Physicochemical properties of Melipona beecheii honey of the Yucatan Peninsula. Journal of Food Research, 4(5), 25. https://doi.org/10.5539/jfr.v4n5p25
- Muleta, D., & Ashenafi, M. (2001). Salmonella, shigella and growth potential of other food-borne pathogens in Ethiopian street vended foods. East African Medical Journal, 78(11), 576–580. https://doi.org/10.4314/eamj.v78i11.8946
- Mulugeta, E., & Tadese, M. (2017). Physicochemical characterization and Pesticide Residue analysis of honey produced in West Shewa zone, Oromia. American Journal of Applied Chemistry, 5(6), 101–109. https://doi.org/10.11648/j.ajac.20170506.13
- Oddo, L. P., Heard, T. A., Rodríguez-Malaver, A., Pérez, R. A., Fernández-Muiño, M., Sancho, M. T., Sesta, G., Lusco, L., & Vit, P. (2008). Composition and antioxidant activity of Trigona carbonaria honey from Australia. Journal of Medicinal Food, 11(4), 789–794. https://doi.org/10.1089/jmf.2007.0724
- Oliveira, A., Ribeiro, H. G., Silva, A. C., Silva, M. D., Sousa, J. C., Rodrigues, C. F., Melo, L. D. R., Henriques, A. F., & Sillankorva, S. (2017). Synergistic antimicrobial interaction between honey and phage against Escherichia coli biofilms. Frontiers in Microbiology, 8, 2407. https://doi.org/10.3389/fmicb.2017.02407
- Pauly, A., & Hora, Z. A. (2013). Apini and Meliponini from Ethiopia (Hymenoptera: Apoidea: Apidae: Apinae). Belgian Journal of Entomology, 16, 1–35.
- Pinheiro, C. D. G. M. D. E., Abrantes, M. R., Silva, R. O. S., Oliveira, C. A., Lobato, F. C. F., & Silva, J. B. A. D. (2018). Microbiological quality of honey from stingless bee, jandaíra (Melipona subnitida), from the semiarid region of Brazil. Ciência Rural, 48(9). https://doi.org/10.1590/0103-8478cr20180151
- Pucciarelli, A. B., Schapovaloff, M. E., Kummritz, S., Señuk, I. A., Brumovsky, L. A., & Dallagnol, A. M. (2014). Microbiological and physicochemical analysis of yateí (Tetragonisca angustula) honey for assessing quality standards and commercialization. Revista Argentina de Microbiología, 46(4), 325–332. https://doi.org/10.1016/S0325-7541(14)70091-4
- Rao, P. V., Krishnan, K. T., Salleh, N., & Gan, S. H. (2016). Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Revista Brasileira de Farmacognosia, 26(5), 657–664. https://doi.org/10.1016/j.bjp.2016.01.012
- Rodrı́guez, G. O., de Ferrer, B. S., Ferrer, A., & Rodrı́guez, B. (2004). Characterization of honey produced in Venezuela. Food Chemistry, 84(4), 499–502. https://doi.org/10.1016/S0308-8146(02)00517-4
- Shenkute, A. G., Getachew, Y., Assefa, D., Adgaba, N., Ganga, G., & Abebe, W. (2012). Honey production systems (apis mellifera L.) in Kaffa, Sheka and bench-maji zones of Ethiopia. Journal of Agricultural Extension & Rural Development, 4(19), 528–541. https://doi.org/10.5897/JAERD12.088
- Silvano, M. F., Varela, M. S., Palacio, M. A., Ruffinengo, S., & Yamul, D. K. (2014). Physicochemical parameters and sensory properties of honeys from Buenos Aires region. Food Chemistry, 152, 500–507. https://doi.org/10.1016/j.foodchem.2013.12.011
- Snowdon, J. A., & Cliver, D. O. (1996). Microorganisms in honey. International Journal of Food Microbiology, 31(1–3), 1–26. https://doi.org/10.1016/0168-1605(96)00970-1
- Sousa, J. M. B., de Souza, E. L., Marques, G., de Toledo Benassi, M., Gullón, B., Pintado, M. M., & Magnani, M. (2016). Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT-Food Science and Technology, 65, 645–651. https://doi.org/10.1016/j.lwt.2015.08.058
- Souza, B. A., Carvalho, C., Sodre, G., & Marchini, L. (2004). Características físico-químicas de amostras de mel de Melipona asilvai (Hymenoptera: Apidae). Ciência Rural, 34(5), 1623–1624. https://doi.org/10.1590/S0103-84782004000500048
- Subramanian, R., Umesh Hebbar, H., & Rastogi, N. K. (2007). Processing of honey: A review. International Journal of Food Properties, 10(1), 127–143. https://doi.org/10.1080/10942910600981708
- Suntiparapop, K., Prapaipong, P., & Chantawannakul, P. (2012). Chemical and biological properties of honey from Thai stingless bee (tetragonula leaviceps). Journal of Apicultural Research, 51(1), 45–52. https://doi.org/10.3896/IBRA.1.51.1.06
- Tchoumboue, J., Awah-Ndukum, J., Fonteh, F. A., Dongock, N. D., Pinta, J. M. Z. A., & Mvondo, Z. A. (2007). Physico-chemical and microbiological characteristics of honey from the Sudano-Guinean zone of West Cameroon. African Journal of Biotechnology, 6(7).
- Temaru, E., Shimura, S., Amano, K., & Karasawa, T. (2007). Antibacterial activity of honey from stingless honeybees (Hymenoptera; Apidae; meliponinae). Polish Journal of Microbiology, 56(4), 281.
- Tesfaye, B., Begna, D., & Eshetu, M. (2016). Evaluation of physico-chemical properties of honey produced in Bale Natural Forest, Southeastern Ethiopia. International Journal of Agricultural Science and Food Technology, 2(1), 21–27. https://doi.org/10.17352/2455-815X.000010
- Torres-González, A., López-Rivera, P., Duarte-Lisci, G., López-Ramírez, Á., Correa-Benítez, A., & Rivero-Cruz, J. F. (2016). Analysis of volatile components from Melipona beecheii geopropolis from Southeast Mexico by headspace solid-phase microextraction. Natural Product Research, 30(2), 237–240. https://doi.org/10.1080/14786419.2015.1043631
- Uran, H., Aksu, F., & Dülger, A. D. (2017). A research on the chemical and microbiological qualities of honeys sold in Istanbul. Food Science & Technology, 37(suppl 1), 30–33. https://doi.org/10.1590/1678-457x.32016
- USDA. (1985). United States standards for grades of extracted honey. Retrieved December 22, 2017 Retrieved fromhttp://www.honey.com/images/downloads/exhoney.pdf.
- Vit, P., Bogdanov, S., & Kilchenmann, V. (1994). Composition of Venezuelan honeys from stingless bees (Apidae: Meliponinae) and Apis mellifera L. Apidologie, 25(3), 278–288. https://doi.org/10.1051/apido:19940302
- Vit, P., Medina, M., & Eunice Enríquez, M. (2004). Quality standards for medicinal uses of meliponinae honey in Guatemala, Mexico and Venezuela. Bee World, 85(1), 2–5. https://doi.org/10.1080/0005772X.2004.11099603
- Vit, P., Oddo, L. P., Marano, M. L., & de Mejias, E. S. (1998). Venezuelan stingless bee honeys characterized by multivariate analysis of physicochemical properties. Apidologie, 29(5), 377–389. https://doi.org/10.1051/apido:19980501
- Von Der Ohe, W., Oddo, L. P., Piana, M. L., Morlot, M., & Martin, P. (2004). Harmonized methods of melissopalynology. Apidologie, 35(Suppl. 1), S18–S25. https://doi.org/10.1051/apido:2004050
- Yadata, D. (2014). Detection of the electrical conductivity and acidity of honey from different areas of Tepi. Food Science & Technology, 2(5), 59–63. https://doi.org/10.13189/fst.2014.020501
- Yalemwork, E., Lemma, W., & Birhane, N. (2013). Antibacterial effects of apis mellifera and stingless bees honeys on susceptible and resistant strains of Escherichia coli, staphylococcus aureus and Klebsiella pneumoniae in Gondar, Northwest Ethiopia. BMC Complementary and Alternative Medicine, 13, 269. https://doi.org/10.1186/1472-6882-13-269
