?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Al–ZnO thin films are prepared by Silar method and are annealed at 450°C for 1 h. A selected number of samples are irradiated by high-energy electron beam and all are characterized by XRD, SEM and energy-dispersive X-ray spectroscopy. Both irradiated and non-irradiated samples are then placed independently inside a gas chamber kept at rotary vacuum. The gas chamber is maintained at a pressure of 0.20 mb and at a temperature of 350°C. Ethanol vapour is admitted in a controlled manner into the chamber and the resistance of the film is measured continuously before, during and after the admittance of the ethanol vapour. The experiment is repeated for different dosages of irradiation and different doping concentrations of Al and the resistance of the film getting reduced fast and considerably at the admittance of ethanol has been observed. The response and recovery time of the irradiated samples is compared with that of non-irradiated samples of the same doping concentration. It has been noted that both irradiated and non-irradiated samples show a response time of 1 s and recovery time of the irradiated samples is shorter than that of non-irradiated samples.
Public Interest statement
In the present work, ZnO nano-structured thin films irradiated with electron beam have been used for the purpose of gas sensing. The samples show a good and fast response to ethanol vapour. The study made here enables the fabrication of fast response sensors at a reasonable price affordable to common man. All the available sensors are quiet expensive and a farmer in the field or a housewife in her kitchen will not be ready to spend large amounts of money for a sensor in his/her home. The ZnO ethanol sensor as mentioned in the present study makes gas sensors common to all. The same ethanol sensor can be used to sense the presence of LPG, methanol, ethane as well as carbon monoxide.
1. Introduction
Variation in the resistance of material in the presence of any gas can be employed for the sensing of the gas. Such property of ZnO is the reason why it is employed for the sensing of different gases. ZnO thin films with sensing time varying from a few seconds to several minutes have been fabricated (Mithra & Mukopadhyaya, Citation2007). Sensitivity of ZnO to gases, such as methane (Mithra & Mukopadhyaya, Citation2007), carbon dioxide (Hartangel, Dawar, Jain, & Jagadish, Citation1995), carbon monoxide (Gonzales, Olvera, Maldonado, & Melendez, Citation2006) and ethanol (Chou, Teoh, Lai, Su, & Hon, Citation2006), has been reported. The successive ionic layer adsorption and reaction (Silar) method used here is considerably simple and cheap to fabricate ZnO thin films of good surface morphology as well as good electrical properties. Another important advantage of Silar method is that it is easy to perform doping with Al (Chandramohan & Dhanasekaran, Citation2012), Sn (Shelke, Sonawane, Bhole, & Patil , Citation2012), Ga (Joseph & Tabata, Citation1999), N (Joseph & Tabata, Citation1999), Sr (Vijayan & Chandramohan, Citation2008) and more others and we can thereby easily modify and control the properties of ZnO thin films. As the cost of fabrication of ZnO thin films by this method is comparatively less, it will lead to the fabrication of cheap gas sensors as well.
Even if pure ZnO shows good response to ethanol vapour, Al–ZnO thin films are used for the present study as they have more sensitivity and fast recovery than pure ZnO. A comparison has been made between electron-irradiated and non-irradiated Al–ZnO. In the present study, Al–ZnO nano-structured thin films, irradiated and non-irradiated with electron beam have been used as sensors for ethanol gas, and also the response time and recovery time of the irradiated samples are compared with that of non-irradiated samples with the same doping concentration and identical preparation conditions. It is known that the adsorption of reducing gas molecules results in a decrease in the electrical resistance of oxide materials. Among oxide semiconductors, ZnO and SnO2 are mostly studied and they are known to have substantial gas sensitivity (McAleer & Moseley, Citation1987). These materials are non-stoichiometric and independent of the preparation technique used. They are characteristically n-type semiconductors due to non-stoichiometry associated with oxygen vacancy and/or metal excess acting as donor states providing conduction electrons. The surface resistance of metal oxide thin films is greatly influenced by chemisorption of oxygen from the air at the surface and at the grain boundaries. The conduction electrons of the materials are trapped by the chemisorbed oxygen and they form , O− and
depending on the temperature of the surface (Lupan, Citation2006). This leads to an increase in surface resistance of the thin film. When a reducing gas is introduced to the film, the molecules of the reducing gas react with the negatively charged, chemisorbed oxygen species and the conduction electrons of the film become free. This leads to a decrease in the resistance of the film. In this process, the gas molecules get oxidized too. When the reducing gas is removed from the film environment, the film regains its original high resistance.
High-energy electron beam irradiation on Al–ZnO thin films has proved that the band gap, and, thereby, the resistivity of the film reduces with irradiation (Sarah, Hassan, Ashour, & Abd El-Rahim, Citation2014). Therefore, films irradiated with high energy electrons have good reduction in resistance than the films that are non-irradiated, but having the same conditions of preparation. The oxide sensing layer is fabricated in different physical forms, such as thin film, thick film, bulk pellet, etc. But as sensing is a surface phenomenon, thin films are found to be most effective for the process (Schiurbaum, Kirner, Geiger, & Gopel, Citation1991).
2. Materials and methods
The preparation of ZnO thin film by Silar method has been reported elsewhere (Schiurbaum et al., Citation1991). The process involves successive dipping of a pre-cleaned microscopic glass substrate in sodium zincate bath and in hot water kept at nearly boiling temperature. A microprocessor-controlled dip coating unit was used to fabricate identical films of good quality. A thin film of sodium zincate deposited on glass substrate during the first dip changed to ZnO during the second dip. The substrate was first immersed in zincate bath for a definite period of time and then in hot water for the same duration. The reaction leading to the formation of ZnO for sodium zincate bath is as follows.
The ZnO thin film thus formed is a white adherent film whose subsequent annealing in air leads to phase pure ZnO (Chandramohan, Dhanasekaran, & Ezhilvizhian, Citation2012). Doping is done by adding desired amount of aluminium sulphate to the sodium zincate bath. The annealing temperature of the film was usually 450°C for the duration of one hour. A microprocessor-controlled annealing chamber was used for the purpose. Here, ZnO films doped with different concentrations of Al varying from 3 atomic % to 7 atomic % in steps of 2 atomic % are used for gas sensing applications, without the help of any catalyst. A number of the samples are irradiated with high-energy electron beam at 6 K Grey and 8 K Grey.
In the present work, the samples are put in a gas chamber for the study of sensitivity to ethanol vapour. The Al–ZnO thin film used for the study has a resistance varying from a few kilo ohms to mega ohms depending on preparation conditions. The electron-irradiated samples show a relatively low resistance varying from a few tens of ohms to kilo ohms. The samples used usually have the dimensions of 1.5 × 1.5 cm. The gas chamber used for admittance of gas for sensing applications has been specially fabricated for the purpose. Resistance of the film was continuously measured using two-probe method before, during and after the admittance of ethanol vapour into the chamber.
The gas chamber was attached to a microprocessor-controlled temperature controller to maintain high temperature inside the chamber. The resistance of the film is measured using Keithley digital multimeter during the course of increasing temperature and also during the admittance and release of ethanol vapour. The inside of the gas chamber is kept at rotary vacuum with a vacuum pump and is attached to a digital Pirani gauge. The experiment is conducted at a pressure of 0.2 mb. The gas chamber is specially designed for admitting gas into it in a controlled manner and for creating vacuum along with the provision for heating.
At a pressure of 0.20 mb and a temperature of 350°C, ethanol vapour is admitted into the chamber. The pressure inside the chamber rises to 2.00 mb by the admittance of the vapour. As the vacuum pump and the gas admittance valve are connected at opposite ends, the ethanol vapour admitted is being transported throughout the length of the gas chamber. The admitting time of ethanol vapour is varied from a few seconds to two or three minutes. As Al-doped ZnO is having a fast response to ethanol vapour, the resistance of the film gets reduced to a considerable value within one second after the admittance of the gas. The chamber is then closed and the vapour is removed by the vacuum pump. As a result, the resistance of the film starts increasing and it reaches to the initial value, i.e. the value before the admittance of ethanol vapour. The response time and recovery time are noted for different admittance to a single film as well as to films with different doping concentrations, both irradiated and non-irradiated. Apart from the response time, the recovery time and sensitivity are found different for different doping concentrations as well as for the electron-irradiated samples. But all the films regardless of the doping concentration and irradiation show the same response time of 1 s. The sensitivity of the films is determined using the relation (Rair − Rgas/Rair).
3. Results and discussion
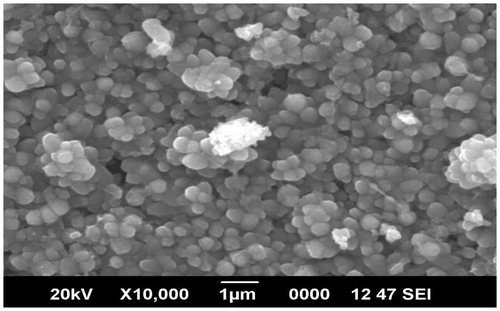
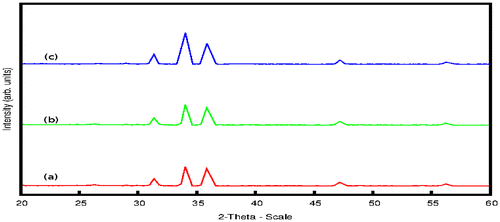
Al–ZnO thin films synthesized by Silar method and irradiated with high-energy electron beams were used as ethanol sensors. The gas sensing property of ZnO thin films with Cd-doping has been reported (Vattappalam & Thomas, Citation2014). Figure represents the XRD patterns of Al–ZnO at different doping concentrations. XRD analysis reveals that the (0 0 2) peak appears with maximum intensity at 2θ = 34.50°. The other peaks at 32° and 36.5° are associated with (1 0 0) and (1 0 1) reflections as for the hexagonal Wurtzite structure (JCPDS No. 36-1-1451). The average grain size of the ZnO crystals in the films was calculated by using Scherrer’s formula as 29.58 nm. The SEM image showing the surface morphology of the film is given in Figure .
Figure 1. XRD pattern of samples with doping concentrations 3 atomic % (a), 5 atomic % (b) and 7 atomic %.
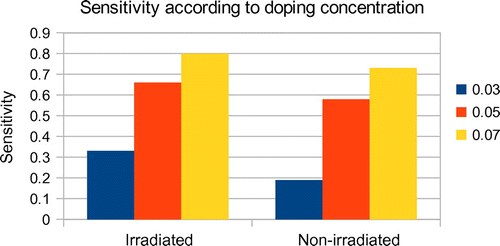
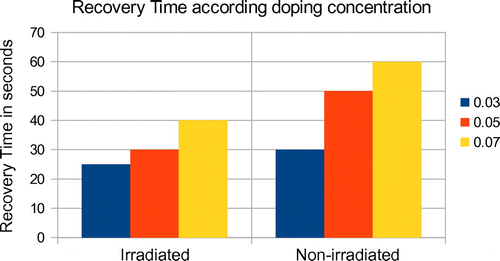
During the admittance of the gas, the response time was found as 1 s for all the samples, regardless of the doping concentration and irradiation, at a temperature and pressure of 350°C and 0.2 mb. The relative resistivity becomes stable after 10–15 s of ethanol vapour exposure. The recovery time, on the other hand, was observed to be varying according to doping concentration as well as irradiation. The 7 atomic %-doped samples showed a recovery time of 1 min, while the 3 atomic %-doped samples showed a response time of 30 s. While in the case of irradiated samples of the same doping concentration, the response time was 40 and 25 s, respectively. A chart showing the comparison of sensitivity and recovery time of the samples is depicted in Figures and , respectively. The variation of resistance in the presence of ethanol vapour, i.e. adsorption–desorption mechanism, is explained on the basis of reversible chemisorption of ethanol vapour on the nano-structured ZnO surface. It produces a reversible variation in resistivity with the exchange of charges between ethanol vapour and ZnO surface leading to changes in the depletion length (Kroger & Schok, Citation1985). It is well known that oxygen is absorbed on ZnO surface as O− and O2− by capturing electrons (Raju & Rao, Citation1991). The sensitivity, response time and recovery time values of 3, 5and 7 at % doping with Al on ZnO nano-structured thin films along with electron-irradiated samples of the same doping concentrations and preparation conditions are tabulated in Table .
Table 1. Sensitivity, response time and recovery time with doping concentrations
4. Elemental analysis by energy-dispersive X-ray spectroscopy
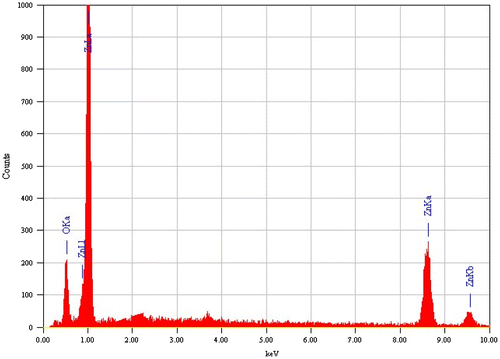
Energy-dispersive X-ray spectroscopy (EDX) is one of the analytical techniques used for the elemental analysis or chemical characterization of a sample. Theoretically, expected stoichiometric mass percentage of zinc and oxygen are 80.3 and 19.7. The atomic percentage of oxygen in the pure and doped samples is 27.39 and 23.92, respectively. From the above values, it is clear that as the doping concentration increases, the oxygen content of the samples decreases. This means that oxygen vacancy is more in samples that have more doping concentrations. Native or intrinsic defects are the reason behind this vacancy creation (Thomas, Vattappalam, Mathew, & Augustine, Citation2014). It is reported that Al doping will create oxygen vacancies due to the substitution of Zn by Al. The EDX of the sample is depicted in Figure .
5. Conclusion
Al–ZnO nano-structured thin films synthesized by Silar method are found to have fast and considerable reduction in resistance in the presence of ethanol vapour, and hence can be used as fast response sensors to ethanol vapour. The samples irradiated with high-energy electron irradiation show more sensitivity than the non-irradiated samples having the same preparation conditions and doping concentrations. The response time of the films is found to be 1 s regardless of doping concentrations and electron irradiation. Average sensitivity of the irradiated samples is found to be 0.600 and that of non-irradiated samples 0.50. An increase in doping concentration with Al increases the sensitivity of the films as well as the recovery time. Irradiation with high-energy electron beam can also increase the sensitivity of the films.
Cover image
Source: Author.
Acknowledgement
The authors are also thankful to Dr Sanjeev Ganesh of Microtron centre, Department of studies in Physics, Manglore University, for the facility provided for electron irradiation.
Additional information
Funding
Notes on contributors
Sunil C. Vattappalam
The author and co-workers have been working on ZnO for many years. We already have studied and published the gas sensing property of Cd–ZnO, the change in orientation index of ZnO due to doping and annealing, the anti-bacterial and anti-microbial properties of iodine-doped and calcium-doped ZnO, band gap and conductivity variations of ZnO due to Al-doping and band gap and conductivity variations of Al–ZnO due to vacuum annealing. Presently, we are working on the sensitivity studies of electron-irradiated Cd–ZnO and Sn–ZnO as well as on the sensitivity properties of Al, Sn and Cd–ZnO to LPG. In future we are planning to fabricate a gas sensing device.
References
- Chandramohan, R., & Dhanasekaran, V. (2012). Spectral properties of aluminium doped zinc oxide thin films prepared by SILAR method. Journal of Materials Science: Materials in Electronics, 23, 390–397.10.1007/s10854-011-0439-1
- Chandramohan, R., Dhanasekaran, V., Ezhilvizhian, S., & Vijayan, T. A. (2012). Spectral properties of aluminium doped zinc oxide thin films prepared by SILAR method. Journal of Materials Science: Materials in Electronics, 23, 390–397. 10.1007/s10854-011-0439-1
- Chou, S. M., Teoh, L. G., Lai, W. H., Su, Y. H., & Hon, M. H. (2006). ZnO:Al thin film gas sensor for detection of ethanol vapor. Sensors, 6, 1420. 10.3390/s6101420
- Gonzales, J. L., Olvera, L., Maldonado, A., & Melendez, M. (2006). CO sensitivity of undoped-ZnO, Cr-ZnO and Cu-ZnO thin films obtained by spray pyrolysis. Revista Mexicana De Fisica, S52, 2.
- Hartangel, H. L., Dawar, A. L., Jain, A. K., & Jagadish, C. (1995). Semiconducting transparent thin films. Bristol: IOP.
- Joseph, M., & Tabata, H. (1999). p-type electrical conduction in ZnO thin films by Ga and N codoping. Japanese Journal of Applied Physics, 38, L1205–L1207.
- Kroger, F. A., & Schok, R. N. (1985). Point defects in minerals (p. 1). Washington, DC: American Geophysical Union.
- Lupan, O. (2006). Nanostructured zinc oxide films synthesized by successive chemical solution deposition for gas sensor applications. Materials Research Bulletin, 10, 1016.
- McAleer, J. F., & Moseley, P. T. (1987). Tin dioxide gas sensors. Part 1.—Aspects of the surface chemistry revealed by electrical conductance variations. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 83, 1323–1346.10.1039/f19878301323
- Mithra, P., & Mukopadhyaya, A. K. (2007). ZnO thin film as methane sensor. Bullettin of Polish Academy of Sciences, 55, 281–285.
- Raju, A. R., & Rao, C. N. R. (1991). Gas-sensing characteristics of ZnO and copper-impregnated ZnO. Sensors and Actuators, 3, 305–310.
- Sarah, M. A.-S., Hassan, H. E., Ashour, A. H., & Abd El-Rahim, M. M. (2014). Study of γ-rays enhanced changes of the ZnO:Al thin film structure and optical properties. International Journal of Electrochemical Science, 9, 3209–3221.
- Schiurbaum, K. D., Kirner, U. K., Geiger, J. F., & Gopel, W. (1991). Shottky barrier and conductivity gas sensors based upon Pd/SnO2and Pt/TiO2. Sensors and Actuators, B5, 87–94.
- Shelke, V., Sonawane, B. K., Bhole, M. P., & Patil, D. (2012). Electrical and optical properties of transparent conducting tin doped ZnO thin films. Journal of Materials Science: Materials in Electronics, 23, 451–456.10.1007/s10854-011-0462-2
- Thomas, D., Vattappalam, S. C, , Mathew, S., & Augustine, S. (2014). Appraisal on textured grain growth and photoconductivity of ZnO thin film SILAR. Advances in Chemistry. 549019.
- Vattappalam, S. C., & Thomas, D. (2014). Cd doped ZnO thin films by Silar for gas sensing applications. IOSR Journal of Applied Physics, 6, 32–35.
- Vijayan, T. A., & Chandramohan, R. (2008). Comparative investigation on nanocrystal structure, optical, and electrical properties of ZnO and Sr-doped ZnO thin films using chemical bath deposition method. Journal of Materials Science, 43, 1776–1782.10.1007/s10853-007-2404-1