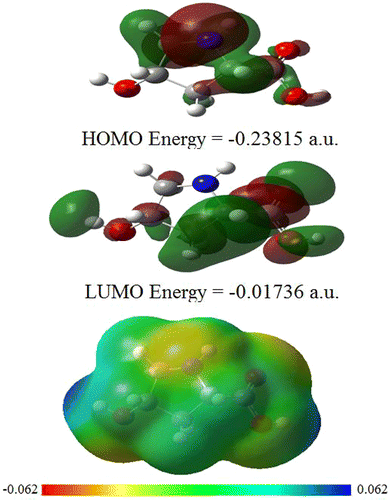?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present study deals with a non-native amino acid, cis-4-hydroxy-d-proline (CHDP) using density functional theory at B3LYP/6-31+G(d,p) level. The potential energy surface scan reveals the global minimum structure of CHDP along with two potential conformers. Highest occupied molecular orbital, lowest unoccupied molecular orbital, and molecular electrostatic potential surfaces are used to explain the chemical reactivity of title molecule. The atomic charge analysis has been carried out using Mulliken and natural population schemes. The equilibrium geometry of CHDP dimer has been obtained and inter-molecular interactions are explored using QTAIM and Natural bonding orbital analyses. Vibrational spectroscopic analysis has been performed on CHDP monomer and dimer at the same level of theory. Assignments to all vibrational modes up to 400 cm−1 have been offered along with their potential energy distribution to the maximum possible accuracy. The calculated frequencies are scaled by an equation, rather than by a constant factor and then compared with experimental FT-IR frequencies obtained by KBr disc and Nujol mull techniques. A number of electronic and thermodynamic parameters have also been evaluated for CHDP monomer and dimer.
Public Interest Statement
Theoretical studies often complement experiments and provide deeper understanding of the systems from both academic and technological perspectives. In this study, we have performed a detailed study on an unnatural amino acid molecule viz. cis-hydroxy-d-proline and its dimer using density functional theory-based calculations. The structural, bonding, and electronic properties of this molecule have been explored which provide deeper insights into its chemical reactivity. The vibrational spectral features have been particularly discussed in relation to the information available in literature.
1. Introduction
Proline is one of the twenty DNA-encoded amino acids but unique in the way that the amine nitrogen is not bound to one but to two alkyl groups, thus making it a secondary amine. The distinctive cyclic structure of proline’s side chain gives proline an exceptional conformational rigidity compared to other amino acids. It also affects the rate of peptide bond formation between proline and other amino acids. When proline is bound as an amide in a peptide bond, its nitrogen is not bound to any hydrogen because it cannot act as a hydrogen bond donor, but can be a hydrogen bond acceptor. Proline and its derivatives are often used as asymmetric catalysts in organic reactions. In brewing, proline-rich proteins combine with polyphenols to produce haze (turbidity) (Lehninger, Nelson, & Cox, Citation2000; Pavlov et al., Citation2010).
Hydroxyproline differs from proline by the presence of a hydroxyl (–OH) group attached to the gamma carbon atom. Although, it is not directly incorporated into proteins, it comprises about 4% of all amino acids found in animal tissue, an amount greater than many other translationally incorporated amino acids (Gorres & Raines, Citation2010). Furthermore, hydroxyproline is a major component of the protein collagen (Szpak, Citation2011) which plays key roles in collagen stability (Nelson & Cox, Citation2005), permitting the sharp twisting of the collagen helix (Brinckmann, Notbohm, & Müller, Citation2005). For this reason, its content has been used as an indicator to determine collagen and/or gelatin amount. Moreover, hydroxyproline-rich glycoproteins are also found in plant cell walls (Cassab, Citation1998). Proline hydroxylation requires ascorbic acid. The most obvious, first effect (gingival and hair problems) of absence of ascorbic acid in humans come from the resulting defect in hydroxylation of proline residues of collagen, with reduced stability of the collagen molecule, causing scurvy. Increased serum and urine levels of hydroxyproline have also been demonstrated in Paget’s disease (http://www.wheelessonline.com/ortho/pagets_disease). cis-4-Hydroxyproline is found in the toxic cyclic peptides from Amanita mushrooms (e.g. alpha-amanitin and phalloidin) (Wieland, Citation1986).
Quantum chemical methods provide a lot of information about the system of chemical and/or biological interests that often complements with the experimental findings. Density functional theory (DFT) calculations provide a better compromise between accuracy and cost. In many instances, the performance of DFT functionals has been found to be in good agreement with experiments (Taşal, Sıdır, Gülseven, Öğretir, & Önkol, Citation2009) and comparable to or even better than high level ab initio method (Srivastava & Misra, Citation2014a). This may explain the reason behind the popularity of DFT functionals and its extensive use in the studies of a variety of biomolecules (Dwivedi, Pandey, & Misra, Citation2011, 2012; Kumar et al., Citation2015; Pandey, Siddiqui, Dwivedi, Raj, & Misra, Citation2011; Siddiqui, Dwivedi, Misra, & Sundaraganesan, Citation2007; Srivastava, Baboo, Narayana, Sarojini, & Misra, Citation2014; Srivastava & Misra, Citation2014b; Srivastava, Narayana, Sarojini, & Misra, Citation2014; Srivastava, Pandey, Gangwar, & Misra, Citation2014). In present study, we perform DFT calculations on cis-4-hydroxy-d-proline (CHDP). The study is organized in following way. Section 2 provides methodology and details of calculations. Section 3, divided into five subsections, deals with the molecular geometry and chemical reactivity surfaces of CHDP, inter-molecular interactions (CHDP dimer), vibrational spectroscopic, electronic and thermodynamic properties. Finally, we conclude our study in the Section 4.
2. Computational details
The initial geometry of CHDP molecule is modeled by Gauss View 5.0 program (Dennington, Keith, & Millam, Citation2005). This geometry is optimized without any symmetry constraint (preference) in the potential energy surface (PES) using a hybrid functional B3LYP (Becke, Citation1993; Lee, Yang, & Parr, Citation1988) in combination with 6-31+G(d,p) basis set (Petersson et al., Citation1988). The vibrational IR frequency calculations are performed using optimized geometry at the same level of theory. All frequencies are found to be positive ensuring the geometry corresponding to true minimum in the PES. The calculated frequencies are scaled by an equation νscaled = 0.9453 νcalculated + 22.1 (Alcolea Palafox, Citation2000; Alcolea Palafox & Rastogi, Citation2002) in order to account for anharmonicity of vibrations and other basis set deficiencies. In a recent investigation, we have verified the superiority of this scaling equation over constant scale factor (Srivastava et al., Citation2016). The properly scaled frequencies are compared with those obtained with experimentally recorded FT-IR spectra via KBr disc and Nujol mull techniques. These FT-IR spectra are already reported in literature and adopted from SDBS website (National Institute of Advanced Industrial Science & Technology, CitationXXXX).
All DFT calculations are performed using Gaussian 09 program package (Frisch et al., Citation2010) using default optimization criteria and relevant graphics are realized with the help of Gauss View 5.0. By default, Gaussian 09 uses fine grid for all DFT-based calculations. Natural bonding orbital (NBO) analysis is performed via NBO 3.1 program (Glendening, Badenhoop, Reed, Carpenter, & Weihold, Citation1996) as implemented in Gaussian 09. The wavefunction of CHDP is employed for quantum theory of atoms in molecule (QTAIM) analysis (Bader, Citation1994) which is performed with AIMAll program (Keith, Citation2012).
3. Results and discussion
3.1. Molecular geometry and PES
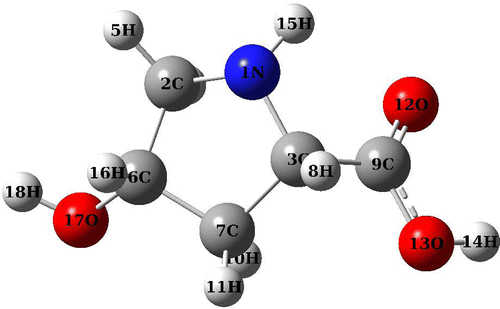
The optimized structure of CHDP is displayed in Figure and related geometrical parameters (bond lengths and angles) are collected in supplementary Table S1. The molecule consists of a five-membered heterocyclic ring system attached with –OH and –COOH groups. The ring deviates from planarity (dihedral, C3–C7–C6–C2 = 31°) due to repulsion created by non-bonding electrons (lone pair) of nitrogen. The ring C6–C7 bond length is 1.53 Å which is found to be slightly changed, i.e. 1.55 Å (C3–C7) in the neighborhood of nitrogen (N1) substituted in the ring.
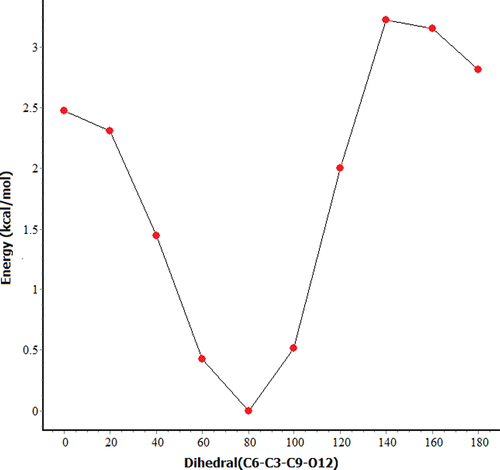
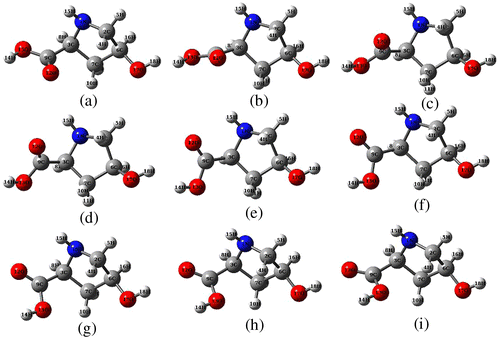
In order to verify whether the optimized structure belong to global minimum of molecular PES, we have considered the rotation of C3–C9 bond with respect to the ring plane, which results in the change in dihedral C6–C3–C9–O12 (which is 80° in Fig. ). Therefore, we have scanned the PES along dihedral C6–C3–C9–O12 and plotted the scan curve plotted in Figure . The PES scan curve clearly reveals that optimized structure (Figure ) indeed corresponds to global minimum. The CHDP conformers obtained are displayed in Figure and corresponding parameters are listed in Table . One can also note that there exist two energetically low lying conformers d and e. The dihedrals of d and e are approximately 60° and 100° and their relative energies are 0.4 and 0.5 kcal/mol, respectively. The ring dihedral angle of these conformers is almost equal to that of global minimum structure (difference being 1° to 2°). Their relative abundances can be calculated using Boltzmann distribution as follows,
Table 1. Conformation analysis of CHDP at B3LYP/6-31+G(d,p) level
where ΔE is the relative energy of a conformer with respect to its global minimum structure and summation runs over the number of possible conformers (n). Using R = 1.987 × 10−3 kcal/mol K and T = 298 K, we obtained, Nf (d) = 0.24 and Nf (e) = 0.21. Therefore, the relative abundances of conformers d and e are 24 and 21%, respectively, whereas that of global minimum structure is 47%.
3.2. Highest occupied molecular orbital, lowest unoccupied molecular orbital, molecular electrostatic potential surfaces, and charge distribution
The most important orbitals in a molecules are the frontier molecular orbitals viz. highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). These orbitals determine the way the molecule interacts with other species. The HOMO primarily acts as an electron donor and the LUMO largely acts as the electron acceptor. The HOMO and LUMO surfaces of CHDP are displayed in Figure with respective energies measured from ionization continuum. One can see that the HOMO is mainly contributed by upper half of the ring including nitrogen, whereas the LUMO is delocalized over the side chains of CHDP. Thus, the transition HOMO→LUMO leads to a charge transfer from the ring to the side chains.
Figure also displays molecular electrostatic potential (MESP) surface of CHDP mapped over uniform electron density. The MESP surface can be used to simultaneously visualize the molecular size, shape as well as positive, negative, and neutral electrostatic potential regions in terms of the color coding. In the present case, the color code ranges between −0.062 a.u. for deepest red and +0.062 a.u. for deepest blue (see Figure ). One can clearly see that the most electropositive regions lie over hydrogen of the side chains. Thus, the nucleophilic attack is more prone to the side chains rather than the ring.
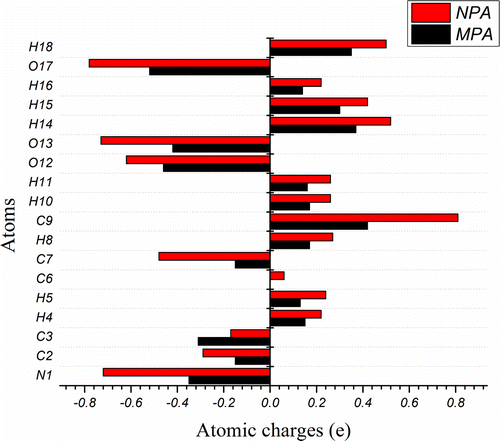
The charge distribution on the atoms in molecule is a feature that can be explored only theoretically. Partial atomic charges of CHDP are computed using Mulliken population analysis (MPA) (Mulliken, Citation1955) as well as natural population analysis (NPA) (Reed, Weinstock, & Weinhold, Citation1985) schemes. MPA is more popular and NPA is more reliable due to its basis set independency, i.e. NPA charges are less sensitive to the change in the basis set. Our recent investigation has also shown the reliability of NPA over various other schemes (Srivastava & Misra, Citation2014c). Figure plots the partial atomic charges of CHDP computed by MPA and NPA. One can note that the NPA charge values are greater in magnitude than MPA charges which is expected due to the fact that NPA assigns the maximum possible occupancy to each atom in a molecule. The carbon atoms are found to carry both positive and negative charges and the maximum value corresponds to positively charged C9. All O and N atoms possess negative charges, thus accept electrons. It is also interesting to note that the charges on hydrogen atoms have only positive values. This may explain the charge transfer from H to N, C and O atoms.
3.3. Inter-molecular interaction, NBO, and QTAIM analyses
Carboxylic acid derivatives generally possess dimeric character. The carboxylic acid dimer is formed by strong hydrogen bonding in the solid and liquid state. In order to explore the inter-molecular interaction, we have studied the formation of dimer of CHDP. The optimized structure of CHDP dimer at B3LYP/6-31+G(d,p) level is displayed in Figure . We have obtained two conformer of CHDP dimer such that both CHDP molecules become parallel to each other (Figure (a)) and interact perpendicularly (Figure (b)). The dimer b is 2.3 kcal/mol higher in energy than conformer a. The binding energies of a and b dimeric structures are 14.3 and 12.0 kcal/mol, respectively. The geometrical parameters of CHDP dimer (a) are listed in supplementary Table S1. In order to gain insight into inter-molecular interaction, we have performed QTAIM and NBO analyses on both dimeric forms.
Figure 6. Optimized structures of CHDP dimer at B3LYP/6-31+G(d,p) level.
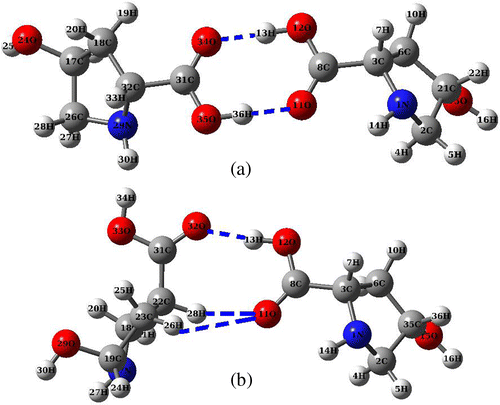
QTAIM analysis is very popular due to its strength in describing various inter- and intra-molecular interactions. In the framework of QTAIM, these interactions are characterized and quantified with the help of some topological parameters at bond critical point (BCP) of interacting atoms. QTAIM analysis performed on CHDP dimers reveals two inter-molecular interaction viz. O34⋯H13 and O11⋯H36 in a, and three inter-molecular interactions viz. O32⋯H13, O11⋯H26, and O11⋯H28 in b (also shown in Figure ). The bond-distances and BCP values of topological parameters associated with these interactions are listed in Table . In dimeric form a, all hydrogen bonded interactions possess moderate strengths and partially covalent nature, as Laplacian of charge density (∇2ρ) > 0 and total energy density (H) < 0 (Rozas, Alkorta, & Elguero, Citation2000). On the contrary, in dimeric form b, all interactions can be characterized as weak and mainly electrostatic due to the fact that ∇2ρ > 0 but H > 0 (Rozas et al., Citation2000). According to Espinosa et al. (Espinosa, Molins, & Lecomte, Citation1988), the interaction energy (ΔE) can be approximated as the half of the potential energy density (V). Thus, calculated ΔE values (Table ) suggest that dimer a is relatively more stabilized due to hydrogen bonding interactions.
Table 2. QTAIM parameters calculated for inter-molecular interactions in CHDP dimer
The NBO analysis is well appreciated for its strength to predict the hybridization of atomic lone‐pairs as well as of the atoms involved in bonding orbitals. The analysis can further be extended for particularly H‐bonded and other van der Waal bonded complexes (Reed, Curtiss, & Weinhold, Citation1988). The donor (filled) NBO σ of the Lewis structure are well adopted to describe covalency effects while non-covalent delocalization effects are associated with σ → σ* interactions where σ* denotes acceptor (empty) NBO of the non‐Lewis structure. The second-order Fock matrix is used to describe the donor–acceptor interactions in NBO basis. The interactions result in a charge transfer (CT) from Lewis into an empty non‐Lewis orbital. We have employed NBO analysis in order to explore the stabilization in CHDP dimer (a and b) caused by lone pair (n) donor and antibond (σ*) interactions. The second-order perturbation theory analysis of n → σ* interactions in the NBO basis is given in Table . NBO quantifies n → σ* delocalization in terms of the perturbative energy lowering (ΔE) and the strength of the Fock interaction matrix element (Fij) from donor to acceptor. One can note that the greatest stabilization (13.56 kcal/mol) in CHDP dimer b is caused by n1(O32), n2(O32) → σ*(O12–H13) interactions whereas analogous interaction in a, n2(O11) → σ*(C8–O12) stabilizes the dimer by 18.75 kcal/mol. This fact is consistent with the relatively higher energy of inter-molecular interactions in dimer a calculated by QTAIM.
Table 3. Second-order perturbation theory analysis of Fock matrix for various lone pairs’ interactions in the NBO basisTable Footnote* for CHDP dimer
3.4. Vibrational spectroscopic analysis
One of strengths of DFT calculations is the interpretation of the vibrational spectra of molecules to a reasonable accuracy. Some minor discrepancies between calculated (scaled) and observed frequencies may exist due to the fact that experimentally FT-IR spectra of the solid sample are recorded after dissolving in some solvents. There are two common strategies to record FT-IR spectra, KBr disc, and Nujol mull. In KBr disc technique, bands at ~3,450 and ~1,640 cm−1 may appear frequently because of the moisture in the sample. The spectra obtained by Nujol mull technique are often free of interfering bands in 4,000–250 cm−1 region, however one has to pay attention to the CH bands due to Nujol (~2,950 and 1,400 cm−1) during assignments. On the contrary, the calculated spectra are obtained for an isolated molecule (in the gas phase), therefore inter-molecular interactions cannot be taken into account.
In the present work, we have calculated the vibrational spectra of the lowest energy structure of CHDP (Figure ) as well as its dimer (a, see Figure ) in order to account for inter-molecular interactions. The choice of lowest energy structures is due to the fact that these are the most likely to be found experimentally. The calculated spectra of CHDP monomer and dimer are plotted in Figure along with FT-IR spectra recorded by KBr disc and Nujol mull techniques for a comparison. This provides us an opportunity to offer a more reliable and accurate assignments to the vibrational modes of CHDP. In Table , we have collected the calculated and scaled frequencies with respective intensities of CHDP monomer and dimer. All vibrational modes up to 400 cm−1 are assigned on the basis of potential energy distribution (PED) and compared with observed FT-IR wavenumbers. The PED analysis is carried out with gar2ped program (Martin, Alsenoy, & Alsenoy, Citation1995). For simplicity of discussion, we classify the vibrational modes into two broad categories.
Figure 7. Experimental FT-IR spectra (a) KBr disc, (b) Nujol mull, and calculated IR spectra, (c) monomer, (d) dimer of CHDP at B3LYP/6-31+G(d,p) level.
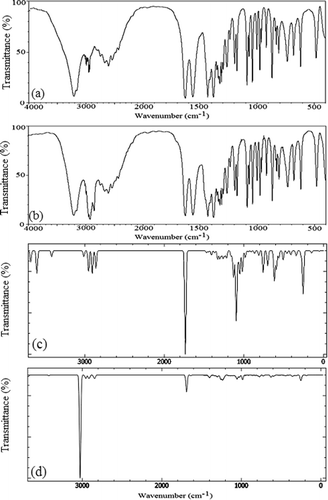
Table 4. Vibrational analysis of CHDP at B3LYP/6-31+G(d,p) level
3.4.1. Ring vibrations
The ring vibrations mainly include CH stretching, CC stretching, and bending modes. In addition, some modes associated with substituent (N) also obtained with significant intensity. CH vibrations are generally observed in the range 3,100–3,000 cm−1 (Silverstein, Basseler, & Morill, Citation1981), which are not much affected by substituent. The IR active bands at 2,993–2,888 cm−1 in monomer and 2,997–2,825 cm−1 in dimer have been assigned to pure CH stretching modes. This is consistent with correspond observed values (see Table ). Most of CH stretching modes is found to be moderate or weak due to charge transfer from hydrogen to carbon atoms, as discussed in earlier section. Pure NH stretching is found to be at 3,393 cm−1 in CHDP monomer and 3,395–3,386 cm−1 in CHDP dimer.
The vibrational modes associated with CHH, CCH, CNC, etc. in-plane and out-of-plane bendings are found to be below 1,500 cm−1. For instance, some out-of-plane bending vibrations present at 1,398 and 1,358 cm−1 in CHDP monomer are found to be at 1,398–1,364 cm−1 in its dimer. Furthermore, in monomer, in-plane bending vibrations occur at 1,320 and 1,295 cm−1 while in dimer, these are at 1,338 and 1,303 cm−1. In the lower frequency region, torsion modes, some ring deformation modes and various coupled vibrations have been observed. For monomer, two intense torsion modes having significant PED contributions are assigned at 695 and 586 cm−1 whereas that of the dimer at 736 and 591 cm−1. These modes correspond to 681 cm−1 in FT-IR spectra.
3.4.2. Groups vibrations
CHDP contains two groups –OH (G1) and –COOH (G2) attached to the ring. The OH stretching of G1 is obtained at 3,656 cm−1 in monomer, whereas 3,644 cm−1 in dimer. In fact, OH stretching absorption of the hydroxyl group is sensitive to hydrogen bonding. In the gas phase alcohols exhibit a sharp absorption in the 3,670–3,620 cm−1 region, whereas hydrogen bonded OH stretching occurs in the range 3,500–3,200 cm−1. Thus, the calculated frequencies of CHDP suggest that OH of G1 does not participate in hydrogen bonding, which is reflected in its dimeric structure (see Figure (a)).
The vibrations of G2 include CO and OH stretching as well as COH and COO bending. These OH bands are found to be at lower frequencies as compared to alcohols due to their involvement in hydrogen bonding. For instance, OH stretching of G2 is found to be at 3,578 cm−1 in monomer, which is reduced to 3,002 cm−1 in dimer. The IR intensity of hydrogen bonded OH is particularly high due to appreciable hydrogen bonding strengths as mentioned earlier. A similar case has already been observed by Ghalla et al. (Citation2015) in case of 2-furanacetic acid dimer. The CO absorption bands occur in the region 1,450–1,800 cm−1. Two intense stretching vibrations of CO are obtained at 1,723 and 1,459 cm−1 in monomer and at 1,684 and 1,432 cm−1 in CHDP dimer. Other modes associated with in-plane and out-of-plane bending of G2 are obtained in middle and lower frequency region.
3.5. Electronic and thermodynamic parameters (monomer and dimer)
The energies of HOMO and LUMO are very useful in determining many electronic parameters describing chemical reactivity of the molecules. Within the framework of Koopmans’ theorem, the ionization potential (I) and electron affinity (A) are defined as the negative of energy eigenvalues of HOMO and LUMO, respectively. Furthermore, the HOMO–LUMO energy gap (Eg) helps to characterize the chemical reactivity and kinetic stability of the molecule. A large HOMO–LUMO gap can be associated with high-kinetic stability because it is energetically unfavorable to add electrons to a high-lying LUMO or to extract electrons from a low-lying HOMO and so to form the activated complexes of any potential reaction (Manolopoulos, May, & Down, Citation1991). The molecular dipole moment (μ) gives a signature of geometry and charge distribution within the molecule. The molecules with higher μ are more polar and vice versa.
The calculated electronic parameters of CHDP monomer (Figure ) and dimer (Figure (a)) are listed in Table . The ionization potential and electron affinity of CHDP dimer are slightly smaller and higher than its monomer, respectively. This may imply that CHDP dimer is relatively more reactive. This fact is further supported by its lower HOMO–LUMO gap and higher dipole moment. Table also lists a number of thermodynamic parameters viz. the zero point energy (ZPE), thermal energy (E), constant volume heat capacity (Cv), and entropy (S) for monomer as well as dimer of CHDP. These parameters are related to one another by standard thermodynamic relations and can be very useful in determining chemical reaction paths.
Table 5. Electronic and thermodynamic parameters of CHDP monomer and dimer calculated at B3LYP/6-31+G(d,p) level
4. Conclusions
Using B3LYP/6-31+G(d,p) level, we have performed a detailed study on cis-4-hydroxy-d-proline (CHDP). The global minimum structure of CHDP has been obtained by scanning the PES. HOMO, LUMO, and MESP surfaces are used to explain the chemical reactivity of title molecule. Partial atomic charges are evaluated using Mulliken and natural population schemes. The equilibrium geometry of CHDP dimer has been determined and inter-molecular interactions are studied using QTAIM and NBO analyses. Vibrational spectroscopic analysis has been performed on CHDP monomer and dimer at the same level of theory to the maximum possible accuracy. The calculated frequencies are scaled by an equation, rather than by a constant factor and compared with experimental FT-IR frequencies obtained by KBr disc and Nujol mull techniques. Various electronic and thermodynamic parameters are also calculated for CHDP monomer and dimer.
Cover image
Source: Authors.
Supplementary_material_doc.docx
Download MS Word (22.9 KB)Acknowledgment
A.K. Srivastava acknowledges Council of Scientific and Industrial Research (CSIR), New Delhi, India for upgrading his fellowship to SRF. The Central Facility for Computational Research (CFCR), University of Lucknow is also acknowledged.
Additional information
Funding
Notes on contributors
Ambrish Kumar Srivastava
Ambrish Kumar Srivastava obtained his BSc in 2008 and MSc (Physics) in 2010 from University of Lucknow, India. He is currently a senior research fellow of CSIR, India pursuing PhD in Department of Physics, University of Lucknow, Lucknow, India under the supervision of Neeraj Misra. His research topic is “Computational Studies of Biologically Active Molecules and Small Clusters: DFT and TD-DFT Approaches”. The research interests of Misra’s group include simulation of biologically relevant molecules as well as novel atomic and molecular clusters including superatoms and nanoclusters. His group has contributed more than 100 research publications in various scientific journals of international repute. In continuation to ongoing research on biologically active molecules, this paper reports computational study on various aspects of cis-hydroxy-d-proline (CHDP), an unnatural amino acid molecule. Using density functional theory, conformational analysis, inter-molecular interactions, electronic and vibrational spectroscopic properties of CHDP have been explored.
References
- Alcolea Palafox, M. A. (2000). Scaling factors for the prediction of vibrational spectra. I. Benzene molecule. International Journal of Quantum Chemistry, 77, 661–684.10.1002/(ISSN)1097-461X
- Alcolea Palafox, M. A., & Rastogi, V. K. (2002). Quantum chemical predictions of the vibrational spectra of polyatomic molecules. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 58, 411–440.10.1016/S1386-1425(01)00509-1
- Bader, R. F. W. (1994). Atoms in molecules: A quantum theory (2nd ed.). Oxford: Oxford University Press.
- Becke, A. D. (1993). Density‐functional thermochemistry. III. The role of exact exchange. The Journal of Chemical Physics, 98, 5648–5652.10.1063/1.464913
- Brinckmann, J., Notbohm, H., & Müller, P. K. (2005). Collagen: Topics in current chemistry 247. Berlin: Springer.
- Cassab, G. I. (1998). Plant cell wall proteins. Annual Review of Plant Physiology and Plant Molecular Biology, 49, 281–309.10.1146/annurev.arplant.49.1.281
- Dennington, R., Keith, T., & Millam, J. (2005). GaussView, Version 5.0. Shawnee, KS: Semichem.
- Dwivedi, A., Pandey, A. K., & Misra, N. (2011). Electronic structure, optical properties and vibrational analysis of 2-decenoic acid and its derivative by density functional theory. Journal of Spectroscopy, 26, 367–385.
- Dwivedi, A., Pandey, A. K., & Misra, N. (2012). Comparative study of vibrational spectra of two bioactive natural products lupeol and lupenone using MM/QM method. Journal of Spectroscopy, 27, 155–166.
- Espinosa, E., Molins, E., & Lecomte, C. (1988). Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chemical Physics Letters, 285, 170–173.
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., … Fox, D. J. (2010). Gaussian 09, Revision B.01. Wallingford, CT: Gaussian.
- Ghalla, H., Govindarajan, M., Flakus, H. T., Issaoui, N., Yaghmour, S. J., & Oujia, B. (2015). Molecular structure and vibrational spectroscopic studies on 2-furanacetic acid monomer and dimer. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,136, 579–593.10.1016/j.saa.2014.09.071
- Glendening, E. D., Badenhoop, J. K., Reed, A. E., Carpenter, J. E., & Weihold, F. (1996). NBO. 3.1 Program. Madison, WI: Theoretical Chemistry Institute, University of Wisconsin.
- Gorres, K. L., & Raines, R. T. (2010). Prolyl 4-hydroxylase. Critical Reviews in Biochemistry and Molecular Biology, 45, 106–124.10.3109/10409231003627991
- Keith, T. A. (2012). T K Gristmill Software. AIMAll (Version 12.09.23). Overland Park KS.
- Kumar, A., Srivastava, A. K., Gangwar, S., Misra, N., Mondal, A., & Brahmachari, G. (2015). Combined experimental (FT-IR, UV–visible spectra, NMR) and theoretical studies on the molecular structure, vibrational spectra, HOMO, LUMO, MESP surfaces, reactivity descriptor and molecular docking of phomarin. Journal of Molecular Structure, 1096, 94–101.10.1016/j.molstruc.2015.04.031
- Lee, C., Yang, W., & Parr, R. G. (1988). Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Physical Review B, 37, 785–789.10.1103/PhysRevB.37.785
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Principles of biochemistry (3rd ed.). New York, NY: W. H. Freeman.
- Manolopoulos, D. E., May, J. C., & Down, S. E. (1991). Theoretical studies of the fullerenes: C34 to C70. Chemical Physics Letters, 181, 105–111.10.1016/0009-2614(91)90340-F
- Martin, J. M. L., Alsenoy, V., & Alsenoy, C. V. (1995). Gar2ped. Antwerp: University of Antwerp.
- Mulliken, R. S. (1955). Electronic population analysis on LCAO–MO molecular wave functions. I. The Journal of Chemical Physics, 23, 1833.10.1063/1.1740588
- National Institute of Advanced Industrial Science and Technology. (XXXX). Retrieved January, 2014, from http://sdbs.db.aist.go.jp
- Nelson, D. L., & Cox, M. M. (2005). Lehninger’s principles of biochemistry (4th ed.). New York, NY: W. H. Freeman.
- Pandey, A. K., Siddiqui, S. A., Dwivedi, A., Raj, K., & Misra, N. (2011). Density functional theory study on the molecular structure of loganin. Journal of Spectroscopy, 25, 287–302.
- Pavlov, M. Y., Watts, R. E., Tan, Z., Cornish, V. W., Ehrenberg, M., & Forster, A. C. (2010). Slow peptide bond formation by proline and other N-alkyl amino acids in translation. Proceedings of the National Academy of Sciences, 106, 50–54.
- Petersson, G. A., Bennett, A., Tensfeldt, T. G., Al-Laham, M. A., Shirley, W. A., & Mantzaris, J. (1988). A complete basis set model chemistry. I. The total energies of closed‐shell atoms and hydrides of the first‐row elements. The Journal of Chemical Physics, 89, 2193–2218.10.1063/1.455064
- Reed, A. E., Curtiss, L. A., & Weinhold, F. (1988). Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chemical Reviews, 88, 899–926.10.1021/cr00088a005
- Reed, A. E., Weinstock, R. B., & Weinhold, F. (1985). Natural population analysis. The Journal of Chemical Physics, 83, 735–746.10.1063/1.449486
- Rozas, I., Alkorta, I., & Elguero, J. (2000). Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. Journal of the American Chemical Society, 122, 11154.10.1021/ja0017864
- Siddiqui, S. A., Dwivedi, A., Misra, N., & Sundaraganesan, N. (2007). Computational note on vibrational spectra of tyramine hydrochloride: DFT study. Journal of Molecular Structure: THEOCHEM, 847, 101–102.10.1016/j.theochem.2007.09.003
- Silverstein, M., Basseler, G. C., & Morill, C. (1981). Spectrometric identification of organic compounds. New York, NY: Wiley.
- Srivastava, A. K., Baboo, V., Narayana, B., Sarojini, B. K., & Misra, N. (2014). Comparative DFT study on reactivity, acidity and vibrational spectra of halogen substituted phenylacetic acids. Indian Journal of Pure & Applied Physics, 52, 507–519.
- Srivastava, A. K., Kumar, A., Misra, N., Manjula, P. S., Sarojini, B. K., & Narayana, B. (2016). Synthesis, spectral (FT-IR, UV-visible, NMR) features, biological activity prediction and theoretical studies of 4-Amino-3-(4-hydroxybenzyl)-1H-1,2,4-triazole-5(4H)-thione and its tautomer. Journal of Molecular Structure, 1107, 137–144.10.1016/j.molstruc.2015.11.042
- Srivastava, A. K., & Misra, N. (2014a). Novel planar chain like Li7F7 and Li9F9 nanostructures. Chemical Physics Letters, 612, 302–305.10.1016/j.cplett.2014.08.045
- Srivastava, A. K., & Misra, N. (2014b). A comparative theoretical study on the biological activity, chemical reactivity, and coordination ability of dichloro-substituted (1,3-thiazol-2-yl)acetamides. Canadian Journal of Chemistry, 92, 234–239.10.1139/cjc-2013-0335
- Srivastava, A. K., & Misra, N. (2014c). Structures, stabilities, electronic and magnetic properties of small RhxMny (x+y=2–4) clusters. Computational and Theoretical Chemistry, 1047, 1–5.10.1016/j.comptc.2014.08.008
- Srivastava, A. K., Narayana, B., Sarojini, B. K., & Misra, N. (2014). Vibrational, structural and hydrogen bonding analysis of N′-[(E)-4-hydroxybenzylidene]-2- (naphthalen-2-yloxy) acetohydrazide: Combined density functional and atoms-in-molecule based theoretical studies. Indian Journal of Physics, 88, 547–556.10.1007/s12648-014-0449-y
- Srivastava, A. K., Pandey, A. K., Gangwar, S. K., & Misra, N. (2014). Structural, vibrational and electronic properties of cis and trans conformers of 4-hydroxy-l-proline: A density functional approach. Journal of Atomic and Molecular Sciences, 5, 279–288.
- Szpak, P. (2011). Fish bone chemistry and ultrastructure: Implications for taphonomy and stable isotope analysis. Journal of Archaeological Science, 38, 3358–3372.10.1016/j.jas.2011.07.022
- Taşal, E., Sıdır, İ, Gülseven, Y., Öğretir, C., & Önkol, T. (2009). Vibrational spectra and molecular structure of 3-(piperidine-1-yl-methyl)-1,3-benzoxazol-2(3H)-one molecule by density functional theory and Hartree–Fock calculations. Journal of Molecular Structure, 923, 141–152.10.1016/j.molstruc.2009.02.017
- Wieland, T. (1986). Peptides of poisonous amanita mushrooms. New York, NY: Springer.