Abstract
Cancer tends to be one of the major diseases in the present century affecting global population, and proliferation of cancerous cells needs to be eliminated as the cell growth is uncontrolled. In this present study, a series of facile, high yielding 3-(1H-indol-3-yl)-1, 3-diphenylpropan-1-ones 3(a–j) was designed, synthesized and evaluated for anti-proliferative activity against different human cancer cell lines; MCF-7 (breast cancer), K562 (leukemic cancer), HeLa (cervical cancer), Colo205 (colorectal adreno carcinoma), HepG2 (Hepato cellular carcinoma) cell lines. The compounds 3a, 3c, 3d, 3e, 3f, 3g and 3j exhibited an average inhibition of 35% against Hepatocellular Carcinoma (Hepg2) cell lines whereas, compounds 3a, 3c, 3d and 3e exhibited 33, 31, 35 and 33% inhibition, respectively, against HeLa cells at 10 μm concentrations. Doxorubicin was employed as a positive control. The ADME-TOX data was obtained by subjecting the molecules in silico to quantitatively predict the physicochemical properties. The structure of the title compounds were established by IR, 1H NMR, 13C NMR and Mass analysis.
Public Interest Statement
Cancer in developing countries is still one of the major causes of death. Many drugs with variable structures and mechanisms fail to alleviate the problem. The present study involves stopping the proliferation of cancer cell line by carrying out antiproliferative and ADMET screening of novel 3-(1Hindol-3-yl)-1,3-diphenylpropan-1-one and their derivatives. The in silico ADMET studies for drug likeliness of the molecules has been undertaken to avoid the risk of toxicity of the synthesized molecules. The results indicated that the tested molecules were active against HeLa and Hepg2 cancer cell lines and further help us to identify possible lead moieties as anti-proliferative agent(s). The introduction/modification of active molecules with various functional groups attached exhibited distinctive differences in the biological efficiency and is useful in promoting a potent lead molecule which can be processed into a promising anticancer drug to serve mankind for a better healthy life.
1. Introduction
Indole nucleus has been the central dogma for many natural as well as synthetic pharmaceutical potent molecules (Abdel-Rahman, Citation2010; Kameshwara et al., Citation2011) and are known to exhibit many pharmacological activities such as, anticancer (Chen, Safe, & Bjeldanes, Citation1996; Ekhlass, Citation2010; Vishal et al., Citation2012), antioxidant (Suzen & Buyukbingol, Citation2000), antirhemuatoidal, anti-HIV (Buyukbingol, Suzen, & Klopman, Citation1994; Suzen & Buyukbingol, Citation1998) and has the ability to selectively inhibit farnesyl transferase (Rodney & Fernandes, Citation2008). Indolyl chalcones were found to be promising candidates for in vitro antitumor activity (Magdy & Atef, Citation2009; Magdy, Hanan, Yasmin, & Gamal-Eldeen Amira, Citation2010), as they were previously reported to possess anticancer (Bindu, Mahadevan, & Ravikumar Naik, Citation2012; Bindu, Mahadevan, Satyanarayan, & Ravikumar Naik, Citation2012), immunosuppressant and therapeutic activities for autoimmune diseases (Shum-ichi et al., Citation1999).
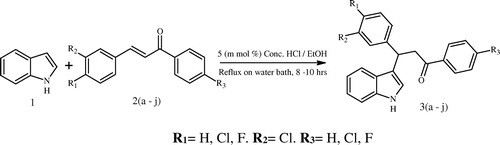
In view of the above studies, the present study prompted us to take indole as moiety and design the structure around with a rationality to be used as potential leads for cancer therapy, because of its ability to interact and inhibit with various enzymes involved in cancer. Hence, it has kept our interest in synthesizing 3-(1H-indol-3-yl)-1, 3-diphenylpropan-1-ones 3(a–j) derivatives (Arrigo, Rosaria, & Maria, Citation2009; Jianwei et al., Citation2010; Xiang, Haibo, & Yu, Citation2011; Zhi-Liang, Shun-Jun, & Teck-Peng, Citation2008) by condensing indole with different chalcones, thereby increasing the characteristic properties of the molecules to obtain a better moiety for the treatment of cancer.
The screening for in vitro anti-proliferative activity against various human cancer cell lines such as normal breast epithelium cells MCF-7 (breast cancer), K562 (leukemic cancer), HeLa (cervical cancer), Colo205 (colorectal adreno carcinoma), HepG2 (Hepatocellular carcinoma) to identify the specificity of the compounds towards highly proliferating cancer cells by MTT assay. The synthesized compound 3(a–j) was subjected for in silico absorption, distribution, metabolism, excretion and toxicity (ADMET) parameters to predict the physicochemical properties such as aqueous solubility (PlogS), Blood-Brain Barrier penetration (QPlogBB), intestinal absorption (logHIA) (Lin & Yamazaki, Citation2003), hepatotoxicity, Caco-2 cell permeability (QPPCaco) which also help to understand drug metabolism studies of the molecules (David, Olivier, Herve, Maria, & Bruno, Citation2008) and optimize using QSAR parameters.
The addition of indole to α,β-unsaturated ketones was achieved (Marco et al., Citation2002) by using HCl as catalyst in the synthesis of title compounds with excellent yields because of its efficient conversion and can overcome the drawbacks such as low yield, prolong reaction time, solubility of the catalyst in solvents and isolation of the product in good yield without hindrance because of its water solubility.
2. Experimental
2.1. General information
The chemicals and reagents were obtained from Sd-Fine chemicals, India, Hi-Media India, and Sigma-Aldrich, India. Melting points were determined in open capillary and are uncorrected. Purity of the compounds was checked by TLC using precoated silica gel plates procured from Merck, further the synthesized compounds were purified by column chromatography using silicagel (60–120 mesh). FTIR were recorded (400–4,000/cm) using KBr pellet method on Shimadzu-8400S spectrometer;
1H and 13C NMR spectra were recorded on a Bruker supercon FT NMR (400 MHz) spectrometer in CDCl3 using TMS as an internal standard. The chemical shifts are expressed in δ units. Mass spectral data was obtained on a JEOL SX 102/DA-6,000 (10 kV) FAB mass spectrometer.
2.2. Procedure for the synthesis of 3-(1H-indol-3-yl)-1, 3-diphenylpropan-1-one (3a)
To the stirred solution of chalcone (0.25 g, 1 m mol), indole (0.12 g, 1 m mol) in ethanol (5 ml), concentrated HCl 5 m mol% was added and the reaction mixture was kept for reflux on water bath until the completion of reaction indicated by TLC (petroleum ether:ethyl acetate 8:2 v/v). After completion, the reaction mixture was poured into water and further extracted with ethyl acetate (10 × 3 ml). The organic layer collected, was dried over anhydrous Na2SO4, upon evaporation offered product which was further purified by column chromatography over silica gel (60–120 mesh) using petroleum ether:ethyl acetate (8:2 v/v) as mobile phase to yield product 3a (0.319 g, 87%), as crystalline solid, mp 93–94°C. Other derivatives 3(b–j) were prepared by adopting the above procedure mentioned above.
2.3. Spectral data
2.3.1. 3-(1H-indol-3-yl)-1, 3-diphenylpropan-1-one (3a)
1H NMR (400 MHz, CDCl3): δ = 10.10 (s, 1H), 8.01 (s, 1H), 7.78 (d, J = 11.40 Hz, 2H), 7.58 (d, J = 11.44 Hz, 2H), 7.30–7.32 (m, 3H), 7.17 (t, J = 7.56 Hz, 1H), 7.04 (t, J = 10.56 Hz, 2H), 6.92–6.95 (m, 3H), 6.65 (s, 1H), 5.03 (t, J = 9.6 Hz, 1H), 3.79 (d, J = 8.6 Hz, 1H), 3.74(d, J = 8.6 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ = 37.4, 45.0, 76.5, 76.9, 77.3, 111.1, 115.3, 118.9, 119.9, 121.1, 122.2, 126.3, 128.2, 129.0, 129.5, 131.8, 135.6, 136.5, 197.3 ppm; IR (KBr: νmax/cm): 3,459(N–H), 3,014(C=C), 2,932–2,839(C–H), 1,694(C=O), 1,488(C–C); MS: m/z = 326.1.
2.3.2. 3-(4-Chlorophenyl)-3-(1H-indol-3-yl)-1-phenylpropan-1-one (3b)
1H NMR (400 MHz, CDCl3): δ = 10.00 (s, 1H), 8.21 (s, 1H), 7.78 (d, J = 11.16 Hz, 2H), 7.58 (d, J = 11.36 Hz, 2H), 7.26–7.27 (m, 3H), 7.17 (t, J = 9.48 Hz, 1H), 7.04 (t, J = 10.56 Hz, 2H), 6.92–6.95 (m, 3H), 5.03 (t, J = 9.6 Hz, 1H) 3.79 (d, J = 8.6 Hz, 1H), 3.74(d, J = 8.5 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ = 39.4, 42.0, 77.3, 78.9, 79.5, 114.1, 116.9, 117.0, 118.9, 119.5, 120.1, 123.0, 126.3, 127.2, 129.9, 130.0, 133.8, 135.6, 136.5, 199.7 ppm. IR (KBr: νmax/cm): 3,428(N–H), 2.930(C–H), 1,696(C=O), 1,488(C–C), 740(C–Cl); MS: m/z = 361.1(M + 2).
2.3.3. 3-(4-fluorophenyl)-3-(1H-indol-3-yl)-1-phenylpropan-1-one (3c)
1H NMR (400 MHz, CDCl3): δ = 10.10 (s, 1H), 8.00 (s, 1H), 7.77 (d, J = 7.36 Hz, 2H), 7.57 (d, J = 7.44 Hz, 1H), 7.27–7.27 (m, 3H), 7.17 (t, J = 9.48 Hz, 1H), 7.03 (t, J = 9.44 Hz, 2H), 6.92–6.95 (m, 3H), 6.63 (s, 1H), 5.03 (t, J = 9.60 Hz, 1H), 3.71 (d, J = 8.60 Hz, 1H), 3.70 (d, J = 8.60 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ = 38.9, 44.0, 76.7, 76.9, 77.3, 113.1, 115.3, 118.9, 119.5, 121.9, 122.2, 127.0, 128.2, 129.5, 134.8, 135.6, 136.7, 197.3 ppm; IR (KBr: νmax/cm): 3,653(N–H), 2,992(C–H), 1,692(C=O), 1,088(C–F); MS: m/z = 344.2.
2.3.4. 3-(3-chlorophenyl)-3-(1H-indol-3-yl)-1-phenylpropan-1-one (3d)
1H NMR (400 MHz, CDCl3): δ = 10.01 (s, 1H), 8.12 (s, 1H), 7.70 (d, J = 11.40 Hz, 2H), 7.16 (d, J = 7.16 Hz, 1H), 7.27–7.27 (m, 3H), 7.18 (t, J = 8.36 Hz, 1H), 7.09 (d, J = 3.00 Hz, 2H), 6.72–6.75 (m, 3H), 6.63 (s, 1H), 5.04 (t, J = 4.72 Hz, 1H), 3.80 (d, J = 9.80 Hz, 1H), 3.75(d, J = 8.6 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ = 38.9, 44.0, 76.7, 76.9, 77.3, 113.1, 115.1, 118.9, 119.3, 121.9, 122.2, 127.0, 128.2, 129.5, 134.8, 135.6, 136.7, 197.3 ppm; IR (KBr: νmax/cm): 3,459(N–H), 2,932(C–H), 1,694(C=O), 825(C–Cl); MS: m/z = 361.1(M + 2)
2.3.5. 3-(3-chlorophenyl)-1-(4-chlorophenyl)-3-(1H-indol-3-yl) propan-1-one (3e)
1H NMR (400 MHz, CDCl3): δ = 10.10 (s, 1H), 7.71(s, 1H), 7.65 (t, J = 3.92 Hz, 2H), 7.42 (d, J = 2.88 Hz, 3H), 7.39–7.21 (m, 1H), 7.18 (t, J = 8.56 Hz, 1H), 7.09 (d, J = 11.08 Hz, 1H),7.06–6.90 (m, 1H), 5.04 (t, J = 12.92 Hz, 1H), 3.78 (d, J = 9.12, Hz, 1H), 3.74 (d, J = 5.32, Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ = 37.4, 39.1, 39.7, 40.8, 44.4, 111.8, 115.9, 116.2, 118.8, 119.0, 121.5, 122.4, 126.6, 128.4, 130.7, 131.5, 134.0, 136.8, 138.7, 163.6, 167.1, 197.2 ppm; IR (KBr: νmax/cm): 3,489 (N–H), 3,060 (C=C), 2,923–2,839 (C–H), 1,698 (C=O), 1,488–1,419 (C–C), 748.61 (C–Cl); MS: m/z = 396.2(M + 2).
2.3.6. 1, 3-Bis (4-chlorophenyl)-3-(1H-indol-3-yl) propan-1-one (3f)
1H NMR (400 MHz, CDCl3): δ = 10.01 (s, 1H), 7.71 (s, 1H), 7.67 (d, J = 13.08 Hz, 2H), 7.40 (t, J = 2.84 Hz, 3H), 7.39–7.20 (m, 1H), 7.15 (t, J = 9.88 Hz, 3H), 7.06 (t, J = 6.76 Hz, 1H), 7.01–6.90 (m, 1H), 5.02 (t, J = 9.72 Hz, 1H), 3.80 (d, J = 8.52 Hz, 1H) 3.75 (d, J = 8.52 Hz, 1H) ppm; 13C NMR(100 MHz, CDCl3): δ = 37.4, 39.1, 39.7, 40.8, 44.4, 111.9, 115.9, 116.2, 118.8, 119.0, 121.5, 122.4, 126.6, 128.4, 130.7, 131.5, 134.0, 136.8, 138.7, 163.8, 167.1, 197.2 ppm; IR (KBr: νmax/cm): 3,489(N–H), 3,060(C=C), 2,923–2,839(C–H), 1,698(C=O), 1,488–1,419(C–C), 833–748.61(C–Cl); MS: m/z = 396.2(M + 2).
2.3.7. 1-(4-Chlorophenyl)-3-(4-fluorophenyl)-3-(1H-indol-3-yl) propan-1-one (3g)
1H NMR (400 MHz, CDCl3): δ = 10.90 (s, 1H), 8.03 (d, J = 12.00 Hz, 2H), 7.57 (t, J = 11.20 Hz, 2H), 7.42 (d, J = 10.8 Hz, 3H), 7.32 (q, J = 10.8 Hz, 2H), 7.05 (d, J = 7.60 Hz, 2H), 7.00–6.83 (m, 1H), 4.87 (t, J = 9.6 Hz, 1H), 3.91(d, J = 10.4 Hz, 1H), 3.81(d, J = 10.40 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ = 37.3, 39.1, 39.7, 40.2, 40.8, 44.7, 111.8, 115.2, 118.7, 119.1, 121.5, 122.4, 126.7, 129.9, 130.4, 136.8, 138.5, 159.3, 162.5, 197.8 ppm; IR (KBr: νmax/cm): 3,487 (N–H), 3,047–2,970 (C=C), 2,923–2,839 (C–H), 1,658 (C=O), 1,419 (C–C), 1,249 (C–F), 740 (C–Cl); MS: m/z = 376.2.
2.3.8. 3-(3-chlorophenyl)-1-(4-fluorophenyl)-3-(1H-indol-3-yl) propan-1-one (3h)
1H NMR (400 MHz, CDCl3): δ = 10.90 (s, 1H), 8.13 (d, J = 4.00 Hz, 2H), 8.11–7.16 (m, 3H), 7.32 (t, J = 2.80 Hz, 2H), 7.30 (d, J = 6.80 Hz, 2H), 7.04 (t, J = 4.80 Hz, 3H), 7.03–6.88 (m, 1H), 4.87 (t, J = 8.40 Hz, 1H), 3.94 (d, J = 7.20 Hz, 1H), 3.83 (d, J = 8.00 Hz, 1H) ppm; 13CNMR (100 MHz, CDCl3): δ = 37.4, 39.1, 39.7, 40.0, 40.2, 40.5, 40.8, 44.4, 111.8, 115.9, 116.2, 118.8, 119.0, 121.5, 122.4, 126.6, 128.4, 130.1, 131.5, 134.0, 136.8, 144.7, 163.8, 167.1, 197.2 ppm; IR (KBr: νmax/cm): 3,386(N–H), 3,047(C=C), 2,970–2,923(C–H), 1,658(C=O), 1,488(C–C), 1,249(C–F), 740(C–Cl); MS: m/z = 376.2.
2.3.9. 3-(4-Chlorophenyl)-1-(4-fluorophenyl)-3-(1H-indol-3-yl) propan-1-one (3i)
1H NMR (400 MHz, CDCl3): δ = 10.90 (s, 1H), 8.12 (t, J = 4.40 Hz, 2H), 7.25–7.27 (m, 9H), 7.00–7.00 (m, 1H), 6.90 (q, J = 2.00 Hz, 1H), 4.86 (t, J = 7.20 Hz, 1H), 3.96 (d, J = 8.00 Hz, 1H), 3.90 (d, J = 8.00 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ = 37.4, 39.4, 40.2, 40.8, 44.4, 111.8, 115.9, 116.2, 118.81, 119.0, 121.5, 122.4, 126.6, 128.4, 130.1, 131.5, 134.0, 136.8, 144.7, 163.8, 167.1, 197.2 ppm; IR (KBr: νmax/cm): 3,386(N–H), 3,047(C=C), 2,970–2,923(C–H), 1,658(C=O), 1,488(C–C), 1,249(C–F), 740(C–Cl); MS: m/z = 376.2.
2.3.10. 1, 3-Bis (4-fluorophenyl)-3-(1H-indol-3-yl) propan-1-one (3j)
1H NMR (400 MHz, CDCl3): δ = 10.87 (s, 1H), 8.11 (t, J = 3.20 Hz, 2H), 7.34–7.36 (m, 3H), 7.31 (d, J = 8.00 Hz, 4H), 7.01–7.02 (m, 3H), 6.90 (t, J = 7.20 Hz, 1H), 4.87 (t, J = 7.20 Hz, 1H), 3.94 (d, J = 8.00 Hz, 1H), 3.90 (d, J = 8.00 Hz, 1H) ppm; 13C NMR(100 MHz, CDCl3): δ = 37.4, 39.1, 39.7, 40.8, 44.4, 111.8, 115.9, 116.2, 118.8, 119.0, 121.5, 122.4, 126.6, 128.4, 130.1, 131.5, 134.0, 136.81, 144.73, 163.61, 167.15, 197.29 ppm; IR (KBr: νmax/cm): 3,402(N–H), 2,923(C–H), 1,666(C=O), 1,411–1,488(C–C), 1,010–1,095(C–F), 740(C–Cl); MS: m/z = 361.1.
3. Results and discussion
3.1. Chemistry
The conjugate addition of indole to α, β-unsaturated ketones was carried out in acetic acid media initially without much success, even after varying the percentage of acetic acid (5, 10 and 100%), complete conversion was achieved with HCl (5 and 10 m mol%) as catalyst using different solvents with stirring under reflux temperature. The method was standardized to achieve complete conversion of the reaction with 5 mmol% of hydrochloric acid in ethanol which was sufficient in the synthesis of 3-(1H-indol-3-yl)-1, 3 diphenylpropan-1-ones with excellent yields (Table ). The selection and variation in the percentage of acid catalyst (HCl) had only marginal difference in the yields and reactions time. Hence, 3-(1H-indol-3-yl)-1, 3-diphenylpropan-1-ones 3(a–j) (Scheme , Table ) were synthesized via two component one pot reaction of indole with differently substituted α, β-unsaturated ketones 2(a–j) in presence of 5 mmol% concentrated hydrochloric acid in ethanol.
Table 1. Standardization of reaction condition for the synthesis of title compounds
Table 2. Synthesis of target compounds 3(a–j) via two component one pot reaction of indole with different α, β-unsaturated ketones
The superiority in using hydrochloric acid as catalyst for conjugate addition of α, β-unsaturated ketones in ethanol as compared with indium(III) sulphate, indium(III) chloride and indium(III) bromide is because the indium salts are soluble in solvent media and cannot be easily purified during reaction workup (Bei-Yao, Miao, Xin-Min, & Yan- Qing, Citation2010; Bimal, Migue, & Claeissa, Citation2005; Brindaban, Suvendu, & Sampak, Citation2005). Thus, concentrated hydrochloric acid catalysed method is found to be economical, feasible and more efficient and can be easily removed by water wash.
3.2. ADME-toxicity prediction
The synthesized molecules were subjected for in silico ADMET parameters to predict the physicochemical properties and optimize using QSAR parameters. The QSAR properties of the compounds significantly help to understand pharmacokinetics and pharmacodynamic behaviour and predict possible biological activity such as ADMET. The ADMET QSAR (Feixiong et al., Citation2012) help to evaluate biologically active molecules and eliminate the biologically poor molecules containing undesirable functional groups based on Lipinski rule, and further statistical calculation of the molecules help to understand biological behaviour of the synthesized molecules.
The molecules have moderate effect on reliability index, and the LD50 dose on different routes of drug administration is found to be safe enough to be investigative further to study the anti-proliferative activity against different types of cancer cell lines. The compounds were found to be good with PlogBB, log HIA, Pcaco, GI, logPGI poor Plog S and solubility and greater membrane permeability aqueous solubility (PlogS), Blood-Brain Barrier Penetration (QPlogBB), intestinal absorption (logHIA) (Lin & Yamazaki, Citation2003) Hepatotoxicity, Caco-2 cell permeability (QPPCaco) also help to understand drug metabolism studies of the molecules (David et al., Citation2008). To predict the toxicity of lead molecules with intraperitoneal, oral, intravenous and subcutaneous toxic effects of blood, cardiovascular system, gastrointestinal, kidney, liver and lungs to calculate sensitivity, specificity and area under the curves (AUC) that predicts the linearity of compounds. The data are represented in Tables and .
Table 3. ADME and pharmacological parameters prediction for the ligands 3-(1H-indol-3-yl)-1, 3 diphenylpropan-1-ones 3(a–j) using ADMET SAR toolbox
Table 4. LD50 and probability of health effects of 3-(1H-indol-3-yl)-1, 3 diphenylpropan-1-one 3(a–j) using ACD/I-Lab 2.0
3.3. Evaluation of anti-proliferative activity
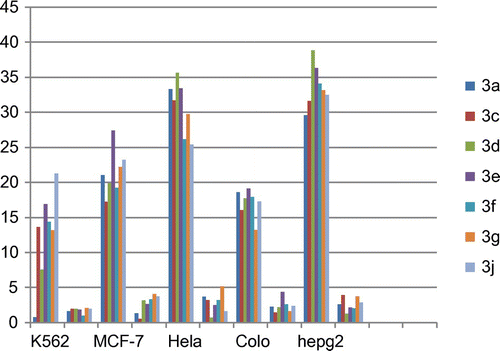
The newly synthesized 3-(1H-indol-3-yl)-1, 3-diphenylpropan-1-ones 3(a–j) were tested for their in vitro cytotoxic effect against five different cancer cell lines such as K562, MCF-7, HeLa, Colo205 and HepG2. Doxorubicin was employed as positive control. The cells lines were obtained from the National Centre for Cell Sciences, Pune, India, and were cultured at a seeding density of 0.2 × 106 in DMEM/RPMI medium supplemented with 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin, respectively, and maintained in a humidified atmosphere with 5% CO2 at 37°C. The compounds were dissolved in dimethyl sulfoxide (DMSO; not exceeding the final concentration of 0.01%) and further diluted in cell culture medium. The anti-proliferative response of the molecules was assessed by MTT assay. Cells (∼10,000) were plated in 200 µL growth medium in the presence or absence of the compounds (10 µmol) in 96-well culture plates for 24 h. Then the culture plates were centrifuged at 2,000 rpm for 10 min at room temperature. 100 µL of supernatant was discarded and 20 µL of MTT (5 mg/mL in PBS) was added to each well and incubated for 4 h at 37°C. The viability of the cells was determined using a spectrophotometer at 570 nm. The IC50, that is, the concentration of the extract required to inhibit cell growth by 50%, was determined. According to the method used for the % inhibition and standard deviation, the concentration of compound required to inhibit 50% of cell growth was determined. The results are summarized in Figure , Table .
Table 5. Anti-proliferative study of different derivatives of 3-(1H-indol-3-yl)-1, 3- diphenylpropan-1-one
3.4. SAR data interpretation: General anti-proliferative activity
Screening for the comparative efficacies of the molecules described in the present work was based on the validated, microtitter plate-based high throughput format MTT assay. The quantitative estimation of MTT assay by spectrophotometer acts as an index for the inhibition study of cancer cells allowing precise estimation of IC50 value for each tested compound.
3.5. Preliminary screening
In view of the well-known anticancer potency of various substituted indole derivatives (Duflos, Kruczynski, & Barrat, Citation2002), we designed and synthesized molecules by coupling chalcones with indole to explore the activity of halogen substituted indole derivative 3-(1H-indol-3-yl)-1, 3-diphenylpropan-1-one (Table ) as representative compounds. However, screening against different cancer cell lines revealed that the molecules showed potency against HepG2 and HeLa cell lines. This promised us to synthesize molecules with different substituents and evaluated for anti-proliferative activity. It is clear (Table ) that introduction on halogen group of the chalcone may lead to significant activity.
Interchanging of halogen functionalities on ring A and ring B is responsible for increased anti-proliferative activity. To our surprise, a reversal of substituent between ring A and B with halogens lead to an increase in average activity (IC50: 33%) of resulting synthesized compound indicating the presence of electronegative functionalities on ring A and B is essential for activity. The compounds with 4-chloro and fluoro (Table ) on ring exhibited progressively better anticancer potential with IC50 values of (35%) at 10 μM, respectively. These observations clearly indicate that increase in the electronegativity on ring A and B significantly enhanced the anticancer activity which is evidently in contrast to earlier reports linking potent anticancer activity with electronegative atoms of chalcones.
3.6. Effect of reduction of double bond in α and β unsaturated ketone unit
In order to evaluate the role of α, β unsaturated ketone moiety, the reduction of double bond in compounds 2(a–j) was carried out by introduction of indole. The resulting compound 3(a–j) (IC50: 35%) showed significant increase in activity.
3.7. Effect of incorporation of heterocyclic moiety
Indole has a history of being a potent anticancer molecule. Indolyl chalcones are proven to be antitumor (Dalip et al., Citation2010). Hence, we were interested in the effect of Michael addition of indole moiety on to chalcone to get structure 3(a–j) (Table ). Thus, addition of indole on to 2(a–j) (Scheme ) yielded a fused saturated target compounds with average (IC50: 33%, Table ).
3.8. Effect of substituent on ring A and B
After reducing the double bond and retaining the ketone group of chalcone for potent activity, we next ventured to evaluate the effect of various substituents on ring A and B. The structure-activity relationship analysis demonstrated that ring B having no substitution on para position 3(a–d) provided compounds with lower activity as compared to rest 3(e–j) of the target compounds which indicated the importance of electronegative substitutions on ring B. Incorporation of electronegative atoms on chalcones with Cl (3e, 3g, 3f), F (3i, 3j), on ring B have shown an increase in activity by compounds 3e–3g and 3j possessing Cl and F substituent’s, respectively, and exhibited IC50 value of 38.842, 36.344 against Hepg2 and 35.672 against HeLa cell lines, as compared to other compounds (Table ).
4. Conclusion
Here we demonstrated an efficient and simple procedure for getting 3-(1H-indol-3-yl)-1, 3-diphenylpropan-1-ones catalysed by simple acid. The title compounds were tested to identify the cytotoxic effect on cancer cell lines, with the standard drug Doxorubicin. The synthesized compounds 3d and 3e have significant effect particularly against HeLa and Hepg2 with percentage inhibition values of 35 and 33% and 38 and 36%, respectively, when compared to standard (90–93%).
The absorption, distribution, metabolism and excretion, all influence the drug levels and kinetics of drug exposure to the tissues and perform the pharmacological activity of the compounds as a drug. According to in silico ADME analysis, the resultant compounds showed good blood/brain barrier partition coefficient power with PlogBB value nearly 1, good cell permeability with >0.5 PCaco, good intestinal absorption properties with log HIA value of 1, good Glycoprotein substrates and moderate Glycoprotein inhibitors (>0.8), 3e and 3f showed good aqueous soluble property with better LD50 and drug reliability index. All the parameters help us to further explore the molecules on to other parameters of drug discovery to obtain a lead.
Acknowledgements
The author Manjunatha K.S. thanks the OBC Cell and authorities of Kuvempu University for providing student fellowship. The author MK thanks DST WOSA and School of Life Science, University of Hyderabad, Telangana for support. The author also thankful IISc Bangalore for providing spectral data.
Additional information
Funding
Notes on contributors
Nayak D. Satyanarayan
Nayak D. Satyanarayan obtained his PhD in Pharmaceutical Chemistry from Gulbarga University, Gulbarga, India and Post Doctoral research from University of Sunderland, UK. Presently he is working as an assistant professor at Department of Pharmaceutical Chemistry, Kuvempu University, India. His research interest is mainly into drug discovery from natural and synthetic products.
References
- Abdel-Rahman, F. (2010). Synthesis of some new indole derivatives containing pyrazoles with potential antitumor activity. Arkivoc, Xi, 177–187.
- Arrigo, S., Rosaria, V., & Maria, R. A. (2009). Asymmetric Friedel-Crafts alkylation of indole with chalcones catalyzed by chiral phosphoric acids. Molecules, 14, 3030–3036.
- Bei-Yao, L., Miao, Z., Xin-Min, Y., & Yan- Qing, P. (2010). Conjugate addition of indole to α, β -unsaturated ketones catalyzed by Lewis acid immobilized on a bio renewable support. Journal of Chinese Chemical Society, 57, 1243–1247.
- Bimal, K. B., Migue, F., & Claeissa, A. (2005). Iodine-catalyzed highly efficient Michael reaction of indoles under solvent-free condition. Tetrahedron, 46, 2479–2482.
- Bindu, P. J., Mahadevan, K. M., & Ravikumar Naik, T. R. (2012). Sm(III)nitrate-catalyzed one-pot synthesis of furano[3,2c]-1,2,3,4-tetrahydroquinolines and DNA photocleavage studies. Journal of Molecular Structure, 1020, 142–14710.1016/j.molstruc.2012.03.064.
- Bindu, P. J., Mahadevan, K. M., Satyanarayan, N. D., & Ravikumar Naik, T. R. (2012). Synthesis and DNA cleavage studies of novel quinoline oxime esters. Bioorganic and Medicinal Chemistry Letters, 22, 898–90010.1016/j.bmcl.2011.12.037.
- Brindaban, C. R., Suvendu, S. D., & Sampak, S. (2005). Indium (III) chloride catalyzed Michael addition of thioles to chalcones: a remarkable solvent effect. Arkivoc, iii, 44–50
- Buyukbingol, E., Suzen, S., & Klopman, G. (1994). Studies on the synthesis and structure–activity relationships of 5-(30-indolyl)-2-thiohydantoin derivatives as aldose reductase enzyme inhibitors. I l Farmaco, 49, 443–447.
- Chen, I., Safe, S., & Bjeldanes, L. (1996). Indole-3-carbinol and diindolylmethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in T47D human breast cancer cells. Biochemical Pharmacology, 51, 1069–107610.1016/0006-2952(96)00060-3.
- Dalip, K., Maruthi, K. N., Kanako, A., Eriko, K., Hiroshi, H., & Takeo, I. (2010). Synthesis and biological evaluation of indolyl chalcones as antitumor agents. Bioorganic and Medicinal Chemistry Letters, 20, 3916–3919.
- David, L., Olivier, S., Herve, G., Maria, A. M., & Bruno, O. V. (2008). FAF-drugs: Free ADME/tox filtering tool to assist drug discovery and chemical biology projects. BMC Bioinformatics, 9, 396. doi:10.1186/1471-2105-9-396
- Duflos, A., Kruczynski, A., & Barrat, J. M. (2002). Novel aspects of natural and modified vinca alkaloids. Current Medicinal Chemistry Anticancer Agents, 2, 55–70.
- Ekhlass, N. (2010). Synthesis, (in vitro) antitumor and antimicrobial activity of some pyrazoline, pyridine, and pyrimidine derivatives linked to indole moiety. Journal of American Science, 6, 338–347.
- Feixiong, C., Weihua, L., Yadi, Z., Jie, S., Zengrui, W., & Guixia, L. (2012). Admetsar: A comprehensive source and free tool for assessment of chemical ADMET properties. Journal of Chemical Information and Modeling, 52, 3099–3105.
- Jianwei, X., Xinhai, Z., Manna, H., Fei, M., Man, W., & Yiqian, W. (2010). Sc(OTf)3: A highly efficient and renewable catalyst for Michael addition of indoles to nitroolefins in water. Synthetic Communication, 40, 3259–3267.
- Kameshwara, R. V., Bhupender, S. C., Amir, N. S., Rakesh, T., Keykavous, P., & Anil, K. (2011). 3-Substituted indoles: One-pot synthesis and evaluation of anticancer and Src kinase inhibitory activities. Bioorganic Medicinal Chemistry Letters, 21, 3511–3514.
- Lin, J. H., & Yamazaki, M. (2003). Role of P-Glycoprotein in pharmacokinetics. Clinical Pharmacokinetics, 42, 59–9810.2165/00003088-200342010-00003.
- Magdy, A. H. Z., & Atef, M. I. (2009). Synthesis and cellular cytotoxicities of new N-substituted indole-3-carbaldehyde and their indolylchalcones. Journal of Chemical Science, 121, 455–462.
- Magdy, A. H. Z., Hanan, F. S., Yasmin, G. A., & Gamal-Eldeen Amira, M. (2010). Efficient microwave irradiation enhanced stereoselective synthesis and antitumor activity of indolylchalcones and their pyrazoline analogs. Journal of Chemical Science, 122, 587–595.
- Marco, B., Pier, G. C., Massimo, G., Paolo, M., Simona, S., & Achille, U. R. (2002). Sequential one-pot InBr 3-catalyzed 1, 4-then 1, 3-nucleophilic addition to enones. Journal of Organic Chemistry, 67, 3700–3704.
- Rodney, A., & Fernandes, B. (2008). A short synthesis of (?)-(S)-kurasoin. Tetrahedron Asymmetry, 19, 15–18.
- Shum-ichi, I., Uichiro, K., Tadashi, A., Katsushige, G., Hiromitsu, S., Masaji, K., … Soichiro, S. (1999). Propenone derivatives. US Patent, Cont-in-part of US Ser., 08/641, 699.
- Suzen, S., & Buyukbingol, E. (1998). Evaluation of anti-HIV activity of 5-(2-phenyl-3′-indolal)-2-thiohydantoin. Il Farmaco, 53, 525–52710.1016/S0014-827X(98)00053-6.
- Suzen, S., & Buyukbingol, E. (2000). Anti-cancer activity studies of indolalthiohydantoin (PIT) on certain cancer cell lines. Il Farmaco, 55, 246–24810.1016/S0014-827X(00)00028-8.
- Vishal, S., Raman, K., Tilak, R., Vivek, K. G., Nitasha, S., Ajit, K. S., … Mohan, P. S. I. (2012). Synthesis and cytotoxic evaluation of substituted 3-(30-indolyl-/30-pyridyl)-isoxazolidines and bis-indoles. Acta pharmaceutica Sinica B, 2, 32–41.
- Xiang, J., Haibo, T., & Yu, Y. (2011). Facile and efficient Michael addition of indole to nitroolefins catalyzed by Zn(OAc)2 2H2O. Synthetic Communication, 41, 372–379.
- Zhi-Liang, S., Shun-Jun, J., & Teck-Peng, L. (2008). Indium (III) iodide mediated Strecker reaction in water: an efficient and environmentally friendly approach for the synthesis of a-aminonitrile via a three-component condensation. Tetrahedron, 64, 8159–8163.