?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A sensitive and selective liquid–liquid extraction is carried out using four new ligands L1–L4 as chelating agents for extraction of heavy metals from aqueous solution. Their capacities extraction of Fe2+, Cu2+, Cd2+, Pb2+, Co2+, Zn2+, and Ni2+ were determined by atomic absorption measurement. The high extractive affinity for Fe(II) is 97.28% (L2) and for Pb(II) is about 91.62% (L3). The effects of pH and ligand concentration upon extraction capability were investigated. The results revealed that the extraction of Fe(II) dependents on the pH with maximum in the pH range of 6–7. A back-extraction of Fe(II) and Pb(II) extracted with L3 leads us to recover for Fe(II) 92.12% (using H2SO4) and for Pb(II) 85.66% (using HNO3). The TD-DFT theoretical calculation has been reported.
Public Interest Statement
Industrial wastes, which contain high concentrations of toxic metals, are currently a serious environmental problem. Due to their high toxicity, a great number of researchers worry about it, and look for solution, in particular by developing new robust and reliable detection methods. In our article, we used liquid–liquid extraction method based on organic materials (such as pyrazole and triazole compounds bearing a functional long chain). The percentages of the amount of extracted metal ions (Fe2+, Pb2+, Cu2+, and Cd2+) were determined by atomic absorbance spectroscopy. Also theoretical studies were performed to find out more about the mechanistic of the extraction. The selectivity, the concentration of the extractant, the mechanism of complexation, and the back extraction were also investigated in this study.
1. Introduction
As a result of decades of industrial production involving their use, heavy metal contamination of air, water, and soil is becoming widely found in the environment and will eventually enter into the food chain. Some heavy metals at trace concentrations are essential elements and play important roles in human metabolism. On the other hand, at higher concentrations, most of the heavy metals may be toxic (Khazaeli, Nezamabadi, Rabani, & Panahi, Citation2013). Water contamination by heavy metals is of great importance from a health point of view because of its high toxicity and susceptible carcinogenic effect (Baraka, Hall, & Heslop, Citation2007). One of the most crucial properties of these metals which differentiate them from other toxic pollutants is that they are not biodegradable in the environment (Florence, Citation1982). Heavy metal ions should be accurately evaluated in order to prevent the occurrence of harmful effects (Ghaedi et al., Citation2008). The cycle of trace metal ions from environment to human is also an important part of environmental studies (Afridi et al., Citation2007; Jamali et al., Citation2009; Ramesh, Mohan, Seshaiah, & Jeyakumar, Citation2001; Yurtsever Sarica & Türker, Citation2007). Therefore, accurate determination of trace amounts of heavy metals in the environment is very important. A liquid–liquid extraction (LLE) is a powerful and commonly used sample pretreatment technique for preconcentration and/or separation, which is included in many standard analytical methods especially for determination of metal and metalloids (Mitani & Anthemidis, Citation2013). Chelating agent or organic extractant is an important part when used in coupling with organic solvent to extract metal ions from water. The extraction efficiency and selectivity can be affected by size of the chelate ring and type of its donor atoms, oxidation state and size of the metal ion, and pH of the solvent system (Bond, Dietz, & Chiarizia, Citation2000). Many different kinds of functionalities have been studied for their potential to remove metal ions from wastewater. In recent years, intensive research has focused on pyrazole derivatives, which are used in several fields, for example, pharmacology (Cottineau, Toto, Marot, Pipaud, & Chenault, Citation2002; Park et al., Citation2005; Rapposelli et al., Citation2004; Tewari & Mishra, Citation2001), biology (Calí, Nærum, Mukhija, & Hjelmencrantz, Citation2004; Larsen, Zahran, Pedersen, & Nielsen, Citation1999; Pimerova & Voronina, Citation2001; Radi, Salhi, & Radi, Citation2010; Sechi et al., Citation2005), catalysis (Christenson, Tokar, & Tolman, Citation1995; Slattery, Bare, Jameson, & Goldsby, Citation1999), electronics (Esteruelas, Gómez, López, & Oñate, Citation1998; Marzin, Budde, Steel, & Lerner, Citation1987), and, particularly, the removal of divalent metal ions from aqueous solutions containing either a single metal species or a mixture of metal ions (Bouabdallah, Touzani, Zidane, & Ramdani, Citation2007; Harit, Cherfi, Isaad, Riahi, & Malek, Citation2012; Malachowski & Davidson, Citation1989; Tarrago, Zidane, Marzin, & Tep, Citation1988). The chelating mechanism depends on coordination of the metal ion to active chelating sites present. These active sites characterized by functional groups which are capable of coordination: these would include donor atoms such as O, N, S, and P (Atia, Donia, & Yousif, Citation2003; Samal, Das, Dey, & Acharya, Citation2000). Atomic absorption spectrophotometry (AAS) is extensively employed for quantification of metallic species and presents desirable characteristics, such as ease of operational, high selectivity, and low cost (Khazaeli et al., Citation2013).
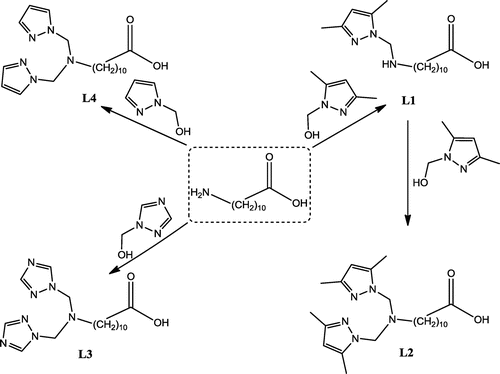
In this contribution, we report the synthesis of a series of pyrazolic compounds 11-(((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)undecanoic acid (L1), 11-(bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)undecanoic acid (L2),11-(bis((1H-1,2,4-triazol-1-yl)methyl)amino)undecanoic acid (L3), and 11-(bis((1H-pyrazol-1-yl)methyl)amino)undecanoic acid (L4) in one step by condensation of one equivalent of (3,5-dimethyl-1H-pyrazol-1-yl)methanol, (1H-pyrazol-1-yl)methanol, or (1H-1,2,4-triazol-1-yl)methanol with one equivalent of 11 aminoundecanoic acid in acetonitrile and the mixture was heated under reflux for 4 h. The present study was aimed to report the results of the capacities extraction of our ligands L1–L4 of heavy metals from aqueous solution, to examine their recovery using different aqueous acidic solutions, to study the effect of varying pH and ligand concentrations upon extraction capability, and to investigate the complexation mechanism between the metal and our ligands.
The theoretical investigation using TD-DFT calculation were performed with Gaussian software version 9 for Windows running under Windows 8.1 (64bit) resident in an Intel Core i5 CPU PC workstation.
2. Experimental section
2.1. General methods
NMR spectra were recorded using a Bruker 300 instrument operating at 300.14 MHz for 1H spectra and 75.47 MHz for 13C spectra (Pz: pyrazole). Infrared (IR) spectra were recorded on a Shimadzu infrared spectrophotometer using the KBr disc technique. Mass spectra were recorded using a Shimadzu GC-MS 2014 Shimadzu Gas Chromatograph-Mass Spectrometer.
2.1.1. 11-(((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)undecanoic acid (L1)
Yield: 75%; IR (KBr), υ (cm−1): 3536 (NH); 1732 (C=O); 1508 (C=N); 1558 (C=C); 1087 (C–N); 1H NMR (300 MHz, CDCl3, d (ppm)): 5.82 (s, 1H, H5Pz); 4.87 (s, 2H, N–CH2–N); 2.53 (t, 2H, H16, J = 3 Hz, J = 9 Hz); 2.32 (t, 2H, H7, J = 3 Hz, J = 9 Hz); 2.24 (s, 3H, CH3Pz); 2.19 (s, 3H, CH3Pz); 1.83 (s, 1H, NH); 1.65–1.09 (m, 16H, H15⋯H8); 13C NMR (75 MHz, CDCl3, d (ppm)): 178.24 (C=O); 144.12–139.67 (C4Pz, C1Pz); 104.43 (C5Pz); 65.36 (N–CH2–N); 48.63(C16-NH); 34.80(C7); 29.46–25.14 (C15⋯C8); 13.43–12.08 (C21Pz C22Pz); ESI-SM m/z: M = 332.02([M + Na]).
2.1.2. 11-(Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)undecanoic acid (L2)
Yield: 87%; IR (KBr), υ (cm−1): 1717 (C=O); 1538 (C=N); 1636 (C=C); 1131 (C–N); 1H NMR (300 MHz, CDCl3, d (ppm)): 5.83 (s, 2H, HPz); 4.89 (s, 4H, N–CH2–N); 2.56 (t, 2H, H21, J = 3 Hz, J = 9 Hz); 2.34 (t, 2H, H12, J = 3 Hz, J = 9 Hz); 2.26 (s, 6H, CH3Pz); 2.20 (s, 6H, CH3Pz); 1.68–1.10 (m, 16H, H20⋯H13); 13C NMR (75 MHz, CDCl3, d (ppm)): 178.19 (C=O); 140.03–139.69 (C1Pz C4Pz C6Pz C9pz); 105.69 (C5Pz C10Pz); 65.35 (N–CH2–N); 48.61 (C21-N); 34.77(C12); 29.48–24.51 (C20⋯C13); 13.41 (C27Pz C30Pz); 12.04 (C28Pz C29Pz); ESI-SM m/z: M = 417([M]).
2.1.3. 11-(Bis((1H-1,2,4-triazol-1-yl)methyl)amino)undecanoic acid (L3)
Yield: 82%; mp = 52–54°C; IR (KBr), υ (cm−1): 1715 (C=O); 1508 (C=N); 1136–1184 (C–N); 1H NMR (300 MHz, CDCl3, d (ppm)): 8.28 (d, 2H, H1Pz H9Pz, J = 9 Hz); 7.98 (d, 2H, H4Pz H6Pz, J = 9 Hz); 5.15 (s, 4H, N–CH2–N); 2.67 (t, 2H, H21, J = 3 Hz, J = 6 Hz); 2.34 (t, 2H, H12, J = 6 Hz, J = 6 Hz); 1.65–1.18 (m, 16H, H20⋯H13); 13C NMR (75 MHz, CDCl3, d (ppm)): 175.64 (C=O); 149.95 (C4Pz C6Pz); 141.95 (C1Pz C9Pz); 64.05 (N–CH2–N); 48.34 (C21-N); 32.29 (C12); 27.36–22.92 (C20⋯C13); ESI-SM m/z: M = 349.99 ([2M + Na]).
2.1.4. 11-(Bis((1H-pyrazol-1-yl)methyl)amino)undecanoic acid (L4)
Yield: 69%; mp = 46–48°C; IR (KBr), υ (cm−1):1707 (C=O); 1470 (C=N); 1541 (C=C); 1089 (C–N); 1H NMR (300 MHz, CDCl3, d (ppm)): 7.59 (d, 2H, H4Pz H6Pz, J = 3 Hz); 7.53 (d, 2H, H1Pz H9Pz, J = 3 Hz); 6.28 (t, 2H, H5Pz H10Pz, J = 3 Hz, J = 3 Hz); 5.01 (s, 4H, N–CH2–N); 2.61 (t, 2H, H21, J = 6 Hz, J = 6 Hz); 2.34 (t, 2H, H12, J = 3 Hz, J = 6 Hz); 1.67–1.22 (m, 16H, H20⋯H13); 13C NMR (75 MHz, CDCl3, d (ppm)): 176.17 (C=O); 137.67 (C4Pz C6Pz); 127.92 (C1Pz C9Pz); 104.03 (C5Pz C10Pz); 65.82 (N–CH2–N); 48.04 (C21-N); 32.50 (C12); 27.48–23.06 (C20⋯C13).
3. Results and discussion
3.1. Synthesis methodology
The ligands L1–L4 were prepared, in accordance with a literature procedure (Khoutoul et al., Citation2015; Touzani et al., Citation2001; Zerrouki, Touzani, & El Kadiri, Citation2011), by condensation of (3,5-dimethyl-1H-pyrazol-1-yl)methanol, (1H-pyrazol-1-yl)methanol, and (1H-1,2,4-triazol-1-yl)methanol with one equivalent of aminoun decnoic acid in acetonitrile and the mixture was heated under reflux for 4 h, the reaction employed for the synthesis of our ligands is illustrated in Figure .
The observed changes in chemical shifts of RMN1H and RMN13C of δ(N–CH2–N) (Table ) prove that introduction of the donor groups increases the electron density on the nitrogen atoms, and that the presence of acceptor groups lowers the density. This is ascribed to shielding of the methylene proton in the case of donor groups and deshielding for acceptor groups (Khoutoul et al., Citation2015).
Table 1. Comparison of 1H NMR and 13C NMR chemical shifts for the different ligands
Table 2. Efficiency of individual extraction of metal ions E (%) using L1–L4
Table 3. Efficiencies of the competitive extraction E (%) of metal ions using L1–L4
Table 4. Back-extraction of metal ions extracted with using L1–L4
Table 5. Quantum chemical parameters for L1–L4 obtained by using B3LYP/6-31G(d, p)
Table 6. Some experimental and theoretical wavenumbers for L1–L4 obtained by using B3LYP/6-31G (ν: Stretching)
3.2. Solvent extraction methodology
3.2.1. Liquid–liquid individual extraction (metal–ligand)
A 20 mL solution of Dichloromethane (CH2Cl2) containing (7 × 10−5 M) ligand and a 20 mL aqueous solution containing (7 × 10−5 M) metal ions (Fe2+, Cu2+, Cd2+, Pb2+, Co2+, Zn2+ or Ni2+) were placed in a flask. The mixture was shaken for 2 h. LLE experiments were conducted at room temperature and neutral pH (25°C; pH 7). The aqueous phase was separated and analyzed by atomic absorption spectrometry with an air–acetylene flame. The element standard solutions used for calibration were produced by diluting a Fluka 1,000 mg/L stock solutions. All standards were made acidic, using 2% nitric acid (Tokalioǧlu, Kartal, & Elçi, Citation2000), to avoid metal hydrolysis and to match the sample content.
The extractability (Ex %) was determined from the decrease in the metal concentration in the aqueous phase (Equation (1)):(1)
(1)
where [metal]blank and [metal]water denote the metal concentrations in the aqueous phase after extraction with a pure Dichloromethane solution containing extractants (Yilmaz & Deligöz, Citation1996).
For the extraction of Fe2+ and Pb2+ ligands, L2–L4 are very efficient (Fe2+: 97, 90 and 90%, respectively), (Pb2+: 86, 91 and 81%, respectively). For L1, the efficiency of extraction is low (48% for Fe2+ and 23% for Pb2+) (Table ). This may due to the presence of proton attached to the nitrogen atom, this protonation decreases the extractability of L1 of all the metal ions, the presence of two pyrazolic or triazolic rings in each structure of L2–L4 increases the efficiency of extraction of Fe2+ and Pb2+. The methyl group linked to the pyrazol ring of L2 increases the affinity to Fe2+. For extraction of Cu2+, the ligand L3 is relatively efficient, with percentage extraction 65%. L3 gave a good extractability compared to other ligands and this may due to the presence of two sp2 nitrogen atoms in each triazole ring. The complexation mechanism between the metal ions (Fe2+, Pb2+ and Cu2+) and our ligands was investigated in details in the current work. For extraction of Cd2+, Co2+, Ni2+, and Zn2+, the efficiency of extraction is low, between 0.05 and 43%. Based on these results, it seems that our ligands do not have good affinity for these metals.
3.2.2. Liquid–liquid extraction selectivity study (Mixture of metals-ligand)
A solution (7 × 10−5 M) of mixture of metals (Fe2+, Pb2+ Cu2+ and Cd2+) is used. This aqueous solution (20 mL) was stirred for 2 h with 20 mL of an organic solution of the ligand (in CH2Cl2) (7 × 10−5 M). LLE experiments were conducted at room temperature and neutral pH (25°C; pH 7). Diluted standard solutions were prepared from the stock standard solutions. All standards were made acidic, using 2% nitric acid [1]; the aqueous phase was separated and analyzed by atomic absorption spectrometry with an air–acetylene flame measurement. The extractability of the metal cations is expressed by means of Equation (1):
Iron metal is selectively extracted by ligands L2 and L4 (79 and 75%, respectively) and we noticed that these two ligands have high efficiency of extraction in individual extraction test of Pb2+ and Fe2+. However, through a competitive extraction, these two ligands are selective to Fe2+ more than Pb2+ (Table ). The absence of any selectivity to Cu2+ and Cd2+ confirm the previous results obtained with individual liquid–liquid extraction.
3.2.3. Effect of pH on the extraction of metal ion Fe2+
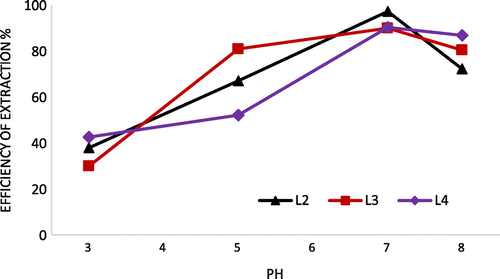
Solution pH is an important factor affecting the efficiency of extraction of metal ions by ligands, especially those containing such functional groups as amino, carboxy, and hydroxy, which can be easily protonated or deprotonated in solutions at different pHs (Khoutoul et al., Citation2015). The effect of pH on the extraction of Fe2+ by the ligands L2–L4 was investigated in the equilibrium pH range (3–8) at a phase ratio of 1:1. It was observed that extraction of metal ions increased with pH in the PH range (3–7), and Fe2+ was highly extracted into the organic phase at equilibrium pH of 7 by our ligands L1–L3 (97, 89 and 90%, respectively) (Figure ). A low extraction values of Fe2+ by the ligands L2–L4 were observed in pH 3 (37, 29 and 42%, respectively) and this can be explained by a rapid and total protonation of coordination sites. At pH values greater than 7.0, the extraction of Fe2+ may decreased because of hydrolysis of Fe2+ (leading to the hydroxide of Fe(II): Fe(OH)+ and Fe(OH)2). There is an increase in Fe2+ during extraction to organic phase as pH increases. When log D-pH graph is examined, there are two pH ranges (3–5 and 5–7), which can be attributed to a different form of protonation or deprotonation. It can be said that there is two structural conformations when forming metal complex at different pHs.
3.2.4. Effect of extractant concentration
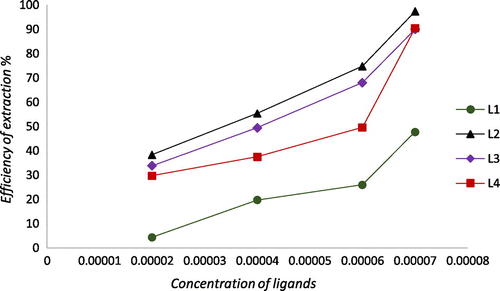
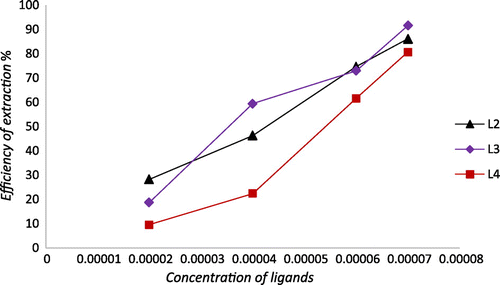
To study the effect of extractant concentration on the extractability of Fe2+ and Pb2+, the concentration of the ligands was varied in the range of (2 × 10−5 M–7 × 10−5 M) and the concentration of the aqueous solution containing metal ions Fe2+ and Pb2+ was fixed to (7 × 10−5 M). The experimental results show that the extraction of Fe2+ increases with an increase in the extractant concentration. As it has been perceived, iron metal extraction increases from 4 to 47% for L1, 38–97% for L2, 43–89% for L3, and 29–90 For L4 (Figure ); whereas, extraction of Pb2+ increases in the range 28–86% for L2, 18–91% for L3, and 10–81% For L3 (Figure ).
The distribution ratio (D) is the ratio of the concentration of the metal ion M in the two ionic liquid phases, at equilibrium, is measured most conveniently by measuring the concentration of the metal ion in the aqueous phase after extraction and by comparing it with the initial concentration (Equations Equation2(2)
(2) and Equation3
(3)
(3) ) (Rout & Binnemans, Citation2014):
(2)
(2)
where Ci and Cf are the concentrations of the metal ions in the feed phase before (initial concentration) and after extraction (final concentration), respectively. VIL1 is the volume of the aqueous phase and VIL2 is the volume of the organic phase [1]. In general, a phase volume ratio of 1:1 was used(3)
(3)
3.2.5. Log–log plot analysis
The complexation mechanism between the metal and our ligands was investigated by slope analysis. The logarithm of the distribution ratio increased with increasing concentration of ligand. The slope of the relationship between the logarithm of the distribution ratio of metals and the logarithm of ligand concentration indicates the number of molecules involved in the extraction of a metal ion.
To characterize the extraction ability, the dependence of the distribution coefficient D of the cation between the two phases upon the ligand concentration was examined. In order to establish the number of moles of ligand participating in the extraction system, a plot of log D vs. log [L] was made as shown in Figures and .
A plot of log D vs. log [L] should be linear (Log [D] = a·Log [L] + b) and its slope should be equal to the number of ligand molecules per metal cation in the extracted species (Gup, Alpoguz, & Beduk, Citation2002). The solvent extracted Fe2+ and Pb2+metal cation from CH2Cl2 at different concentrations of L1–L4 as shown in Figures and .
The obtained log D vs. log [L] plot displayed in Figure is shown as 1:1 ratio of metal–ligand for Fe2+ with L2–L4 and 1:2 ratio of metal: ligand for Fe2+ with L1.
The structures of L2–L4 have an active chelating site, which may indicated that one ligand participated in the extraction process to form iron complex in the organic phase.
As for L1, log D vs. log [L] plot is shown as 1:2 ratio of metal: ligand for Fe2+ which indicated that two ligands participated in the extraction process to form iron complex in the organic phase. L1 is obtained from a monoalkylation of pyrazol cycle that may implied that two molecules of ligand L1 were needed to form an active chelating site.
As it can be seen in Figure , for Pb2+ a slope of about 2 was obtained for L2–L4 which signified that two molecules of ligand participated in the extraction process to form lead complexes in the organic phase.
3.2.6. Back-extraction of Fe2+ and Pb2+
Most metals can be back-extracted from an organic solution into an aqueous solution; this is achieved by acid decomposition of the metal complexes or the complexing agent. The recovery of Fe2+ and Pb2+ from organic solution of ligands L1, L3, and L4, using hydrochloric acid solution, nitric acid solution, and sulfuric acid solution at the same concentration (1 M) was studied.
Based on the obtained results, the recovery was between 73 and 92% for the iron metal, HCl gave a good yield for L1 an L4 (92 and 91, respectively). For L3, the iron metal was recovered with a good yield (92%) in sulfuric acid solution and lead with 86% in nitric acid solution. It was found that the used acids as back-extraction reagents were extremely effective; the ligands could be recycled and reused after stripping the metal ions from the organic phase (Table ).
4. Theoretical calculations
4.1. Computational details
We have utilized DFT theory for the computation of molecular structure, vibrational frequencies, and energies of optimized structures. A considerable effort has been directed to the understanding of organic molecule of theoretical calculations with the DFT (Hohenberg & Kohn, Citation1964), with the Becke’s three-parameter hybrid functional (B3) (Becke, Citation1993) for the exchange part and the Lee–Yang–Parr (LYP) correlation function (Lee, Yang, & Parr, Citation1988). The molecules were first optimized using the B3LYP with 6–31G (d, p) basis set in Gaussian 09. Thereafter, a molecular visualization program, Gaussview 5 was used to study the electronic properties, such as HOMO–LUMO energies and HOMO–LUMO energy visualization contours.
4.2. Quantum chemical parameters
The HOMO and LUMO energy values are related to the ionization potential (IP) and electron affinities (EA): (IP = −EHOMO) (EA = −ELUMO) (Wolinski, Hinton, & Pulay, Citation1990). The difference between HOMO and LUMO energy values gives the HOMO–LUMO energy gap. By using these HOMO–LUMO energy values, the important properties like global hardness (η), global softness (σ), electronegativity (χ), chemical potential (μ), and electrophilicity index (ω) were calculated.
The hardness (η) of a species (atom, ion, or molecule): η = (IP−EA)/2 = −(ELUMO−EHOMO)/2 is a qualitative indication of how polarizable it is, in another way how much its electron cloud is distorted in an electric field.
The softness (σ) is simply the reciprocal of the hardness: σ = 1/η (Gázquez, Martinez, & Méndez, Citation1993; Pearson, Citation1963, Citation1966).
Electronegativity is the tendency of molecules to attract electrons: χ = (IP + EA)/2 = −(ELUMO + EHOMO)/2.
Chemical potential denotes the affinity of an electron to flee and is defined as the first derivative of the total energy with respect to the number of electrons in a molecule (Kavitha, Sundaraganesan, & Sebastian, Citation2010), chemical potential is simply the negative of electronegativity value; μ = −χ.
Electrophilicity index is the capability of a substance to accept electrons, it was proposed as a measure of the electrophilic power of a molecule and it can be measured through the equation: ω = (μ2/2η) (Parr, Szentpály, & Liu, Citation1999).
Molecules with large energy gap are hard which implies higher stability and opposing charge transfer, since they oppose changes in their electron density and distribution. On the contrary, molecules, which require a small energy gap for its excitation, are also termed as soft molecules. Hence, they are highly polarizable in nature. In terms of chemical change, soft molecules are more reactive than hard molecules. (Pilli, Banerjee, & Mohanty, Citation2015).
4.3. HOMO–LUMO analysis
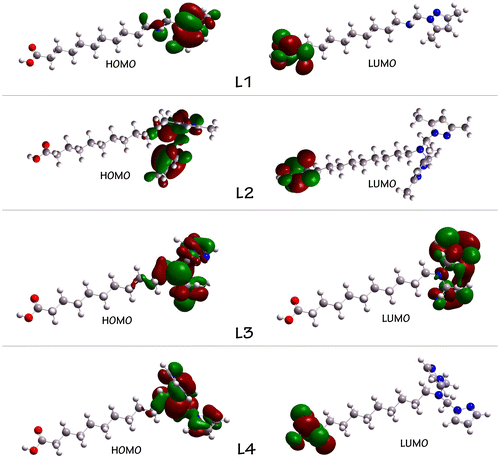
Highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) are very important parameters for quantum chemistry. We can determine the way the molecule interacts with other species; hence, they are called the frontier orbitals. HOMO, which can be thought the outermost orbital containing electrons, tends to act as electron donor. On the other hand, LUMO can be thought the innermost orbital containing free places to accept electrons (Gece, Citation2008). The HOMO and LUMO energies are calculated (Table ). This electronic transition absorption corresponds to the transition from the ground to the first excited state and is mainly described by an electron excitation from the HOMO to the LUMO.
The HOMO is located over the pyrazole ring. For L1, L2, and L4, the HOMO→LUMO transition implies an electron density transfer to the carboxylic group from pyrazole ring. For L3, the LUMO is located over the triazole ring and it can be explained by the presence of two sp2-hybridized nitrogen atoms, which increases the acceptor character of the ring cycle, and the HOMO→LUMO transition in this situation implies an electron density transfer in the same triazole ring. The atomic compositions of the frontier molecular orbital are shown in Figure .
4.4. Vibrational spectral analysis
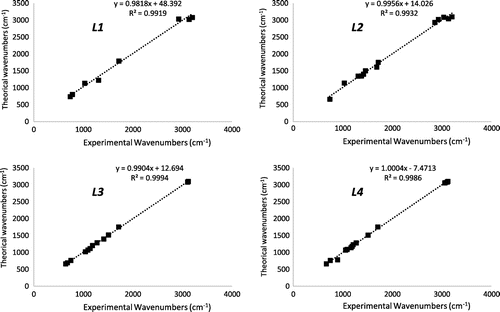
IR spectra were performed by a comparison of experimental data with those calculated by Gaussian using B3LYP/6-31G. The experimental and calculated IR spectra are shown in Table . The vibrational bands assignments have been made using Gauss- View molecular visualization program. Table presents the calculated vibrational frequencies and their experimentally measured values. We have compared our calculation with the experimental results. To make comparison with experiment, we present correlation graphics in Figure based on the calculations. As it seen from calculation graphic Figure , good agreement is seen between calculated and experimental IR spectra.
NH theoretical stretching mode was observed at 3,589 cm− 1, C=N at 1,501 cm−1. For L1; OH stretching modes are observed in the region 3,412–3,649 cm−1, CH ring stretching vibrations are observed in the region 3,284–3,333 cm−1; experimental C=O stretching modes are observed at 1,732, 1,717, 1,715, and 1,707 cm−1 for L1–L4, respectively, and are in better agreement with theoretical wavenumbers 1,792, 1,751, 1,751, and 1,751 cm−1 respectively.
Other essential characteristic vibrations of our compounds are CH2 (in N–C–N) symmetric and asymmetric stretching. These modes have been calculated at 3,017–3,074 cm−1 for symmetric stretching and at 3,100–3,143 cm−1 for asymmetric stretching; these results are in correlation with experimental wavenumbers. The other modes are shown in Table .
To see correlation between the experimental and calculated wavenumbers were plotted correlation graphics and given in Figure . The relation between experimental and calculated wavenumbers is usually linear and described for L1–L4 by the following equations:
The IR calculations gave a slightly better coefficient and lower standard error R2 (Figure ). However, the small deviation between the experimental and computed wavenumbers may be due to the difference between the molecular geometry in the gas phase and in its solid phase.
5. Conclusion
Some conclusive remarks can be tentatively derived from the results mentioned above:
| (1) | Extraction of heavy metals with pyrazole and triazole N-ligand L1–L4 shows high extractability of Fe(II) and Pb(II) for L2–L4 and a significant extractability of Copper (II), while lowest extraction efficiency was found for Ni(II), Cd(II), Co(II), and Zn(II). | ||||
| (2) | pH is one of the determining factors for metals extraction by ligands. The extractability of Fe2+ and Pb2+ increased with pH values, it becomes more and more important while approaching the neutral pH. | ||||
| (3) | The effect of varying ligand concentrations upon extraction capability of Fe(II) and Pb(II) was investigated for establishing the number of moles of ligand participating in each extraction system. | ||||
| (4) | Very good agreement has been noticed between experimental and calculated IR spectra using TD-DFT (B3LYP/6-31G) and the structural geometries of ligands were investigated. | ||||
Acknowledgments
The authors gratefully acknowledge Université de Rennes 1 France for recording NMR and Mass spectra. We would also like to thank Ahmed Mrabti, technician at COSTE, Université Mohamed Premier, Oujda, Morocco for atomic absorption measurements.
Additional information
Funding
Notes on contributors
Morad Lamsayah
My group work is focussed on green chemistry and sustainable topics. We want to achieve many goals with our fructuous collaborations: the invention of easier and less expensive methods for the preparation of materials with high added value; the development of libraries of heterocyclic compounds such as pyrazole, triazole, quinoxaline, pyridine, and their coordinated complexes. the exploitation of these materials in many applications such as medicinal, pharmaceutical, and bioactive molecules in the area of anticancer, antifungal, antibacterial, antimicrobial, and antioxidant; biomimetic enzyme and homogeneous catalysis, for oxidation reactions and activation of the C-H and C-C bonds (organic and organometallic chemistry); -the inhibition of corrosion in acidic media, and the liquid–liquid extraction of metal ions and for the environmental protection (pesticides); the enhancement of natural products from our region (aromatic medicinal plants). We enjoy being challenged for new collaborations and combinatorial projects.
References
- Afridi, H. I., Kazi, T. G., Arain, M. B., Jamali, M. K., Kazi, G. H., & Jalbani, N. (2007). Determination of cadmium and lead in biological samples by three ultrasonic-based samples treatment procedures followed by electrothermal atomic absorption spectrometry. Journal of AOAC International, 90, 470–478.
- Atia, A. A., Donia, A. M., & Yousif, A. M. (2003). Synthesis of amine and thio chelating resins and study of their interaction with zinc(II), cadmium(II) and mercury(II) ions in their aqueous solutions. Reactive and Functional Polymers, 56, 75–82.10.1016/S1381-5148(03)00046-4
- Baraka, A., Hall, P., & Heslop, M. (2007). Melamine–formaldehyde–NTA chelating gel resin: Synthesis, characterization and application for copper(II) ion removal from synthetic wastewater. Journal of Hazardous Materials, 140, 86–94.10.1016/j.jhazmat.2006.06.051
- Becke, A. D. (1993). Density-functional thermochemistry. III. The role of exact exchange. The Journal of Chemical Physics, 98, 5648–5652.10.1063/1.464913
- Bond, A. H., Dietz, M. L., & Chiarizia, R. (2000). Incorporating size selectivity into synergistic solvent extraction: A review of crown ether-containing systems. Industrial & Engineering Chemistry Research, 39, 3442–3464.10.1021/ie000356j
- Bouabdallah, I., Touzani, R., Zidane, I., & Ramdani, A. (2007). Synthesis of new tripodal ligand: N,N-bis[(1,5-dimethylpyrazol-3-yl)methyl]benzylamine. Catalysis Communications, 8, 707–712.10.1016/j.catcom.2006.08.034
- Calí, P., Nærum, L., Mukhija, S., & Hjelmencrantz, A. (2004). Isoxazole-3-hydroxamic acid derivatives as peptide deformylase inhibitors and potential antibacterial agents. Bioorganic & Medicinal Chemistry Letters, 14, 5997–6000.10.1016/j.bmcl.2004.09.087
- Christenson, D. L., Tokar, C. J., & Tolman, W. B. (1995). New Copper and Rhodium Cyclopropanation catalysts supported by Chiral Bis (pyrazolyl) pyridines. A Metal-Dependent Enantioselectivity Switch. Organometallics, 14, 2148–2150.
- Cottineau, B., Toto, P., Marot, C., Pipaud, A., & Chenault, J. (2002). Synthesis and hypoglycemic evaluation of substituted pyrazole-4-carboxylic acids. Bioorganic & Medicinal Chemistry Letters, 12, 2105–2108.10.1016/S0960-894X(02)00380-3
- Esteruelas, M. A., Gómez, A. V., López, A. M., & Oñate, E. (1998). 1, 2, 3-Diheterocyclization reactions on the Allenylidene ligand of a Ruthenium complex. Organometallics, 17, 3567–3573.10.1021/om980230a
- Florence, T. (1982). The speciation of trace elements in waters. Talanta, 29, 345–364.10.1016/0039-9140(82)80169-0
- Gázquez, J. L., Martinez, A., & Méndez, F. (1993). Relationship between energy and hardness differences. Journal of Physical Chemistry, 97, 4059–4063.
- Gece, G. (2008). The use of quantum chemical methods in corrosion inhibitor studies. Corrosion Science, 50, 2981–2992.10.1016/j.corsci.2008.08.043
- Ghaedi, M., Niknam, K., Shokrollahi, A., Niknam, E., Rajabi, H. R., & Soylak, M. (2008). Flame atomic absorption spectrometric determination of trace amounts of heavy metal ions after solid phase extraction using modified sodium dodecyl sulfate coated on alumina. Journal of Hazardous Materials, 155, 121–127.10.1016/j.jhazmat.2007.11.038
- Gup, R., Alpoguz, H. K., & Beduk, A. D. (2002). Synthesis and extraction properties of 1,2-bis(amidoxime) derivatives. Collection of Czechoslovak Chemical Communications, 67, 209–218.10.1135/cccc20020209
- Harit, T., Cherfi, M., Isaad, J., Riahi, A., & Malek, F. (2012). New generation of functionalized bipyrazolic tripods: Synthesis and study of their coordination properties towards metal cations. Tetrahedron, 68, 4037–4041.10.1016/j.tet.2012.03.052
- Hohenberg, P., & Kohn, W. (1964). Inhomogeneous electron gas. Physical Review, 136, B864–B871.
- Jamali, M. K., Kazi, T. G., Arain, M. B., Afridi, H. I., Jalbani, N., Kandhro, G. A., ... Baig, J. A. (2009). Speciation of heavy metals in untreated sewage sludge by using microwave assisted sequential extraction procedure. Journal of Hazardous Materials, 163, 1157–1164.10.1016/j.jhazmat.2008.07.071
- Kavitha, E., Sundaraganesan, N., & Sebastian, S. (2010). Molecular structure, vibrational spectroscopic and HOMO–LUMO studies of 4-nitroaniline by density functional method. Indian Journal of Pure and Applied Physics, 48, 20–30.
- Khazaeli, S., Nezamabadi, N., Rabani, M., & Panahi, H. A. (2013). A new functionalized resin and its application in flame atomic absorption spectrophotometric determination of trace amounts of heavy metal ions after solid phase extraction in water samples. Microchemical Journal, 106, 147–153.10.1016/j.microc.2012.06.002
- Khoutoul, M., Abrigach, F., Zarrouk, A., Benchat, N.-E., Lamsayah, M., & Touzani, R. (2015). New nitrogen-donor pyrazole ligands for excellent liquid–liquid extraction of Fe2+ ions from aqueous solution, with theoretical study. Research on Chemical Intermediates, 41, 3319–3334.
- Larsen, J. S., Zahran, M. A., Pedersen, E. B., & Nielsen, C. (1999). Synthesis of triazenopyrazole derivativesas potential inhibitors of HIV-1. Monatshefte für Chemie/Chemical Monthly, 130, 1167–1173.
- Lee, C., Yang, W., & Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B, 37, 785.10.1103/PhysRevB.37.785
- Malachowski, M. R., & Davidson, M. G. (1989). Novel mono- and binuclear Cu(II) complexes: Synthesis, characterization and catecholase activity. Inorganica Chimica Acta, 162, 199–204.10.1016/S0020-1693(00)83147-3
- Marzin, C., Budde, F., Steel, P., & Lerner, D. (1987). New ruthenium (II) complexes with pyridylpyrazole and pyridylpyrazoline ligands: Structural study by 1 H, 13 C and 99 Ru NMR. New Journal of Chemistry, 11, 33–41.
- Mitani, C., & Anthemidis, A. N. (2013). On-line liquid phase micro-extraction based on drop-in-plug sequential injection lab-at-valve platform for metal determination. Analytica Chimica Acta, 771, 50–55.10.1016/j.aca.2013.02.003
- Park, H.-J., Lee, K., Park, S.-J., Ahn, B., Lee, J.-C., Cho, H., & Lee, K.-I. (2005). Identification of antitumor activity of pyrazole oxime ethers. Bioorganic & Medicinal Chemistry Letters, 15, 3307–3312.10.1016/j.bmcl.2005.03.082
- Parr, R. G., Szentpály, L. v., & Liu S. (1999). Electrophilicity index. Journal of the American Chemical Society, 121, 1922–1924.10.1021/ja983494x
- Pearson, R. G. (1963). Hard and soft acids and bases. Journal of the American Chemical Society, 85, 3533–3539.10.1021/ja00905a001
- Pearson, R. G. (1966). Acids and bases. Science, 151, 172–177.
- Pilli, S. R., Banerjee, T., & Mohanty, K. (2015). HOMO–LUMO energy interactions between endocrine disrupting chemicals and ionic liquids using the density functional theory: Evaluation and comparison. Journal of Molecular Liquids, 207, 112–124.10.1016/j.molliq.2015.03.019
- Pimerova, E., & Voronina, E. (2001). Antimicrobial activity of pyrazoles and pyridazines obtained by interaction of 4-aryl-3-arylhydrazono-2, 4-dioxobutanoic acids and their esters with hydrazines. Pharmaceutical Chemistry Journal, 35, 18–20.
- Radi, S., Salhi, S., & Radi, A. (2010). Synthesis and preliminary biological activity of some new pyrazole derivatives as acyclonucleoside analogues. Letters in Drug Design & Discovery, 7, 27–30.10.2174/157018010789869307
- Ramesh, A., Mohan, K. R., Seshaiah, K., & Jeyakumar, N. (2001). Determination of trace elements by inductively coupled plasma-atomic emission spectrometry (ICP-AES) after preconcentration on a support impregnated with piperidine dithiocarbamate. Analytical Letters, 34, 219–229.10.1081/AL-100001574
- Rapposelli, S., Lapucci, A., Minutolo, F., Orlandini, E., Ortore, G., Pinza, M., & Balsamo, A. (2004). Synthesis and COX-2 inhibitory properties of N-phenyl- and N-benzyl-substituted amides of 2-(4-methylsulfonylphenyl)cyclopent-1-ene-1-carboxylic acid and of their pyrazole, thiophene and isoxazole analogs. Il Farmaco, 59, 25–31.10.1016/j.farmac.2003.09.003
- Rout, A., & Binnemans, K. (2014). Separation of rare earths from transition metals by liquid–liquid extraction from a molten salt hydrate to an ionic liquid phase. Dalton Transactions, 43, 3186–3195.10.1039/C3DT52541D
- Samal, S., Das, R., Dey, R., & Acharya, S. (2000). Chelating resins VI: Chelating resins of formaldehyde condensed phenolic Schiff bases derived from 4, 4′-diaminodiphenyl ether with hydroxybenzaldehydes—synthesis, characterization, and metal ion adsorption studies. Journal of Applied Polymer Science, 77, 967–981.10.1002/(ISSN)1097-4628
- Sechi, M., Sannia, L., Carta, F., Palomba, M., Dallocchio, R., Dessi, A., ... Neamati, N. (2005). Design of novel bioisosteres of -diketo acid inhibitors of HIV-1 Integrase. Antiviral Chemistry and Chemotherapy, 16, 41–61.10.1177/095632020501600105
- Slattery, S., Bare, W., Jameson, D., & Goldsby, K. (1999). Redox regulation in ruthenium complexes containing β-diketonate ligands and 2,6-bis(N-pyrazolyl)pyridine and its methyl-substituted derivatives. Journal of the Chemical Society, Dalton Transactions, 8, 1347–1352.10.1039/a805088k
- Tarrago, G., Zidane, I., Marzin, C., & Tep, A. (1988). Synthesis and ionophore properties of a series of new tetrapyrazolic macrocycles. Tetrahedron, 44, 91–100.10.1016/S0040-4020(01)85096-1
- Tewari, A. K., & Mishra, A. (2001). Synthesis and anti-inflammatory activities of N4,N5-disubstituted-3-methyl-1H-pyrazolo[3,4-c]pyridazines. Bioorganic & Medicinal Chemistry, 9, 715–718.10.1016/S0968-0896(00)00285-6
- Tokalioǧlu, Ş., Kartal, Ş., & Elçi, L. (2000). Determination of heavy metals and their speciation in lake sediments by flame atomic absorption spectrometry after a four-stage sequential extraction procedure. Analytica Chimica Acta, 413, 33–40.10.1016/S0003-2670(00)00726-1
- Touzani, R., Ramdani, A., Ben-Hadda, T., El Kadiri, S., Maury, O., Bozec, H. L., & Dixneuf, P. H. (2001). Efficient synthesis of new nitrogen donor containing tripods under microwave irradiation and without solvent. Synthetic Communications, 31, 1315–1321.10.1081/SCC-100104040
- Wolinski, K., Hinton, J. F., & Pulay, P. (1990). Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. Journal of the American Chemical Society, 112, 8251–8260.10.1021/ja00179a005
- Yilmaz, M., & Deligöz, H. (1996). Selective extraction of Fe 3+ cation by Calixarene-based cyclic ligands. Separation Science and Technology, 31, 2395–2402.10.1080/01496399608001055
- Yurtsever Sarica, D., & Türker, A. R. (2007). Method validation for the determination of lead in raw cow’s milk electrothermal atomic absorption spectrometry. Annali di Chimica, 97, 983–993.10.1002/(ISSN)1612-8877
- Zerrouki, A., Touzani, R., & El Kadiri, S. (2011). Synthesis of new derivatized pyrazole based ligands and their catecholase activity studies. Arabian Journal of Chemistry, 4, 459–464.10.1016/j.arabjc.2010.07.013

![Figure 5. log D vs. log [L] for the extraction of Fe2+ using L1–L4.](/cms/asset/933bbdc9-226c-4dad-973c-6a033badd3a1/oach_a_1230359_f0005_oc.gif)
![Figure 6. log D vs. log [L] for the extraction of Fe2+ using L2–L4.](/cms/asset/8fb32205-518d-4fa1-a791-935d36bc5595/oach_a_1230359_f0006_oc.gif)