Abstract
Under mild conditions and without any additional organic solvent, synthesis of carbamatoalkyl naphthols by the one-pot three-component reaction of β-naphthol with a wide range of aromatic aldehydes and methyl carbamate could be carried out in the presence of P2O5 supported on SiO2 (P2O5/SiO2). The results showed that the catalyst has high activity and the desired products were obtained in high yields in short reaction times. Other beneficial features of this protocol include inexpensive and easily obtained catalyst, simple work-up, and the recyclability and reusability of the catalyst for up to five consecutive runs.
Public Interest Statement
Synthesis of carbamatoalkyl naphthols which can be converted to important biologically active 1-aminomethyl-2-naphthol derivatives by carbamate hydrolysis is of great interest. Therefore, in this paper, a simple and efficient method for the synthesis of these compounds by the one-pot three-component reaction of β-naphthol, aromatic aldehydes, and methyl carbamate using P2O5/SiO2 as catalyst has been reported. The method was fast and the desired products were obtained within a few minutes in high yields under solvent-free conditions. Other advantages of this protocol include inexpensive and easily obtained catalyst, simple work-up, and the recyclability and reusability of the catalyst.
1. Introduction
Multi-component reactions (MCRs) have attracted much interest and are highly regarded in medicinal chemistry and discovery and synthesis of natural products because they are one‐pot processes that bring together three or more components and show high atom economy and high selectivity (Brauch, van Berkel, & Westermann, Citation2013; Dömling, Citation2006; Slobbe, Ruijter, & Orru, Citation2012; Thompson, Citation2000; Touré & Hall, Citation2009). They consist of two or more steps which are carried out without isolation of any intermediate. They also provide a rapid and efficient approach to organic synthesis (Davoodnia, Tavakoli-Nishaburi, & Tavakoli-Hoseini, Citation2011; Gholipour, Davoodnia, & Nakhaei-Moghaddam, Citation2015; Meerakrishna, Periyaraja, & Shanmugam, Citation2016). Still, great efforts are being made to develop new MCRs and improve known ones such as the synthesis of carbamatoalkyl naphthols. These compounds can be converted to important biologically active 1-aminomethyl-2-naphthol derivatives by carbamate hydrolysis. The hypotensive and bradycardiac effects of later compounds have been evaluated (Dingermann, Steinhilber, & Folkers, Citation2004; Shen, Tsai, & Chen, Citation1999). A literature survey revealed that a number of methods were known about the synthesis of carbamatoalkyl naphthols via the one-pot three‐component reaction of β-naphthol, an aldehyde, and a carbamate in the presence of a variety of catalysts such as cerium ammonium nitrate (CAN) (Wang, Liu, Song, & Zhao, Citation2013), Mg(HSO4)2 (Ghashang, Citation2014), Zwitterionic salt (Kundu, Majee, & Hajra, Citation2010), Tween® 20 (Yang, Jiang, Dong, & Fang, Citation2013), ionic liquids (Shaterian & Hosseinian, Citation2014; Zare, Yousofi, & Moosavi-Zare, Citation2012), PPA-SiO2 (Shaterian, Hosseinian, & Ghashang, Citation2009), CuCl2·2H2O (Song, Liu, Sun, & Cui, Citation2014), aluminum methanesulfonate (Al(MS)3·4H2O) (Song, Sun, Liu, & Cui, Citation2013), SnCl4·5H2O (Wang, Wang, Zhao, & Wan, Citation2013), and Mg(OOCCF3)2 (Mohammad Shafiee, Moloudi, & Ghashang, Citation2011). Though each of these methods has its own advantage, the discovery of new and efficient catalysts with high catalytic activity, short reaction times, recyclability, and simple reaction work-up for the preparation of carbamatoalkyl naphthols is of great interest.
Phosphorus pentoxide supported on silica gel (P2O5/SiO2) has received considerable attention as an efficient, heterogeneous, eco-friendly, highly reactive, stable, easy to handle, and non-toxic catalyst for various organic transformations including acetalization of carbonyl compounds (Mirjalili, Zolfigol, Bamoniri, Amrollahi, & Hazar, Citation2004), sulfonylation and nitration of aromatic compounds (Hajipour & Ruoho, Citation2005; Hajipour et al., Citation2005), N-acylation of sulfonamides (Massah et al., Citation2009), the Ritter and Schmidt Reactions (Eshghi & Hassankhani, Citation2006; Tamaddon, Khoobi, & Keshavarz, Citation2007), Fries and Beckmann rearrangement (Eshghi & Gordi, Citation2003; Eshghi, Rafie, Gordi, & Bohloli, Citation2003), the cross-aldol condensation (Hasaninejad, Zare, Balooty, Mehregan, & Shekouhy, Citation2010), and also the preparation of bis(indolyl)methanes (Hasaninejad, Zare, Sharghi, Niknam, & Shekouhy, Citation2007), highly substituted imidazoles (Shaterian, Ranjbar, & Azizi, Citation2011), 4,4′-epoxydicoumarins (Wu & Wang, Citation2011), Schiff bases (Naeimi, Sharghi, Salimi, & Rabiei, Citation2008), 1-substituted 1H-1,2,3,4-tetrazoles (Habibi, Nasrollahzadeh, Mehrabi, & Mostafaee, Citation2013), and β-enaminones (Mohammadizadeh, Hasaninejad, Bahramzadeh, & Sardari Khanjarlou, Citation2009), affording the corresponding products in excellent yields and high selectivity. Other applications of P2O5/SiO2 in organic synthesis have been reviewed by Eshghi and Hassankhani (Citation2012).
On the other hand, in recent years, considerable interest has been devoted to finding new methodologies for the synthesis of organic compounds in solvent-free condition (Davoodnia, Basafa, & Tavakoli-Hoseini, Citation2016; Kumar et al., Citation2016). The toxicity and volatile nature of many organic solvents have posed a serious threat to the environment. Thus, design of solvent-free catalytic reaction has received tremendous attention in recent times in the area of green synthesis (Bettanin, Botteselle, Godoi, & Braga, Citation2014).
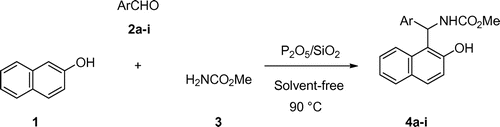
Considering the above facts and also in extension of our previous studies on the development of new environmental friendly methodologies in the synthesis of organic compounds using reusable catalysts (Davoodnia, Allameh, Fazli, & Tavakoli-Hoseini, Citation2011; Davoodnia, Khashi, & Tavakoli-Hoseini, Citation2013; Davoodnia, Zare-Bidaki, & Behmadi, Citation2012; Dehghan, Davoodnia, Bozorgmehr, & Bamoharram, Citation2016; Khashi, Davoodnia, & Prasada Rao Lingam, Citation2015; Moghaddas, Davoodnia, Heravi, & Tavakoli-Hoseini, Citation2012; Nakhaei & Davoodnia, Citation2014; Nakhaei, Yadegarian, & Davoodnia, Citation2016; Taghavi-Khorasani & Davoodnia, Citation2015), we report here the first application of P2O5/SiO2 as an efficient, low cost and reusable catalyst for the efficient solvent-free synthesis of carbamatoalkyl naphthols by the one-pot three-component reaction of β-naphthol (1) with aromatic aldehydes (2a-i) and methyl carbamate (3) (Scheme ).
2. Results and discussion
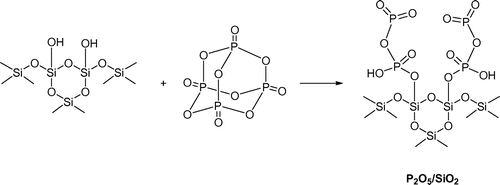
To begin our study P2O5/SiO2 was prepared according to the method reported by Eshghi (Eshghi, Rafei, & Karimi, Citation2001). Grinding of the mixture of P2O5 and SiO2 in dry conditions for 30 min gave the P2O5/SiO2 reagent as white powder. As shown in Scheme , the hydroxyl groups in silica gel can be phosphorylated to give the relatively stable P2O5/SiO2. This reagent that can act as an acid catalyst has less sensitivity to moisture than P2O5 (Eshghi & Hassankhani, Citation2012).
Different reaction parameters were optimized for the synthesis of compound 4c by the one-pot three-component reaction of β-naphthol (1) (1 mmol), 4-chlorobenzaldehyde (2c) (1 mmol), and methyl carbamate (3) (1.1 mmol) as a model reaction in the absence and presence of P2O5/SiO2 as catalyst. The results are summarized in Table . Only trace amounts of the product 4c was formed in the absence of the catalyst in refluxing H2O or EtOH and also under solvent-free conditions (Entries 1–3) indicating that the catalyst is necessary for the reaction. Several reactions were scrutinized using various solvents, such as H2O, MeOH, EtOH, CH3CN, CH2Cl2, and also under solvent-free conditions in the presence of P2O5/SiO2 as catalyst. As shown in Table , the trial reaction gives the best yield in the presence of 0.05 g of P2O5/SiO2 under solvent-free conditions and proceeds smoothly at 90°C to afford the desired product 4c in 2 min (entry 14). With tested solvents, the reaction gave low yields within comparable reaction times. Therefore, 0.05 g of the catalyst P2O5/SiO2 under solvent-free condition at 90°C were found to be the optimized conditions. All subsequent reactions were carried out in these optimized conditions.
Table 1. Optimization of reaction conditions for synthesis of compound 4c catalyzed by P2O5/SiO2Table Footnotea
Under the optimized reaction conditions, we investigated the scope and the limitations of the reaction employing a variety of aromatic aldehydes. The results are summarized in Table . Almost all reactions worked well and the desired compounds were obtained in high yields within short reaction time. Under the same conditions however, low yields of the products were obtained using aliphatic aldehydes.
Table 2. Synthesis of carbamatoalkyl naphthols (4a-i) using P2O5/SiO2Table Footnotea
Because of importance of recyclability and reusability of catalysts in organic reactions, the recovery and catalytic activity of recycled P2O5/SiO2 was explored. For this purpose, the synthesis of compound 4c was again studied under optimized conditions. The P2O5/SiO2 catalyst was readily recovered from the reaction mixture using the procedure outlined in the experimental section. The separated catalyst was washed with hot ethanol and then dried at 50°C under vacuum for 1 h before being reused in a similar reaction. The catalyst could be used at least four times with only a slight reduction in activity (94, 93, 92, 92, and 91% yields for first to fifth use, respectively) which clearly demonstrates the practical reusability of this catalyst. This reusability demonstrates the high stability and turnover of P2O5/SiO2 under the employed conditions. The stability of P2O5/SiO2 has been also confirmed in several papers reviewed by Eshghi and Hassankhani (Citation2012) and also two papers reported by Habibi et al. (Citation2013) and Eshghi, Rahimizadeh, Ghadamyari, and Shiri (Citation2012). While P2O5 is very sensitive to moisture, P2O5/SiO2 is stable in various reaction mixtures containing water, amines and alcohols.
3. Conclusion
In this paper, a simple, efficient, and eco-friendly method for the synthesis of carbamatoalkyl naphthols by the one-pot three-component reaction of β-naphthol with a wide range of aromatic aldehydes and methyl carbamate using P2O5/SiO2 as catalyst has been successfully developed. The method was fast and the desired products were obtained within a few minutes in high yields under solvent-free conditions at 90°C. The catalyst can be recycled after a simple work-up, and used at least five times without substantial reduction in its catalytic activity. The procedure is also advantageous in the sense that it is a solvent-free reaction and therefore operates under environmentally friendly conditions.
4. Experimental
All chemicals were available commercially and used without additional purification. Melting points were recorded on a Stuart SMP3 melting point apparatus. The IR spectra were obtained using a Tensor 27 Bruker spectrophotometer as KBr disks. The 1H NMR spectra were recorded with a Bruker 300 FT spectrometer.
4.1. Preparation of P2O5/SiO2
A mixture of P2O5 (3 g) and SiO2 (4 g, 230–400 mesh) was ground vigorously in a mortar for 30 min to give P2O5/SiO2 as a white powder (Eshghi et al., Citation2001).
4.2. General procedure for the synthesis of carbamatoalkyl naphthols (4a-i) catalyzed by P2O5/SiO2
A mixture of β-naphthol (1) (1 mmol), an aromatic aldehyde (2a-i) (1 mmol), methyl carbamate (3) (1.1 mmol), and P2O5/SiO2 (0.05 g) was heated in the oil bath at 90°C for 2–4 min and monitored by TLC. On completion of the transformation, the reaction mixture was cooled to room temperature and hot ethanol was added. The catalyst was collected by filtration, and the filtrate was cooled to room temperature. The crude product was collected and recrystallized from ethanol to give compounds 4a-i in high yields.
4.3. Selected spectral data
Methyl (3-chlorophenyl)(2-hydroxynaphthalen-1-yl)methylcarbamate (4b): IR (KBr disc): υ 3417 (NH), 3293 (OH), 1690 (C=O) cm−1; 1H NMR (300 MHz, DMSO-d6): δ 3.59 (s, 3H, OCH3), 6.87 (d, 1H, J = 5.7 Hz, CH), 7.16 (d, 1H, J = 6.9 Hz, arom-H), 7.20–7.33 (m, 6H, arom-H and NH), 7.42 (t, 1H, J = 7.2 Hz, arom-H), 7.81 (t, 2H, J = 8.4 Hz, arom-H), 7.92 (d, 1H, J = 8.4 Hz, arom-H), 10.24 (br, 1H, OH).
Methyl (4-chlorophenyl)(2-hydroxynaphthalen-1-yl)methylcarbamate (4c): IR (KBr disc): υ 3421 (NH), 3212 (OH), 1686 (C=O) cm−1; 1H NMR (300 MHz, DMSO-d6): δ 3.58 (s, 3H, OCH3), 6.83 (broadened doublet, 1H, CH), 7.18–7.36 (m, 6H, arom-H), 7.41 (t, 1H, J = 7.0 Hz, arom-H), 7.71 (br, 1H, NH), 7.76–7.85 (m, 2H, arom-H), 7.90 (d, 1H, J = 9.0 Hz, arom-H), 10.20 (br, 1H, OH).
Methyl (2,4-dichlorophenyl)(2-hydroxynaphthalen-1-yl)methylcarbamate (4d): IR (KBr disc): υ 3402 (NH), 3259 (OH), 1678 (C=O) cm−1; 1H NMR (300 MHz, DMSO-d6): δ 3.55 (s, 3H, OCH3), 6.82 (broadened doublet, 1H, CH), 7.14 (d, 1H, J = 9.0 Hz, arom-H), 7.29 (t, 1H, J = 7.2 Hz, arom-H), 7.37–7.57 (m, 4H, arom-H), 7.77 (d, 1H, J = 8.7 Hz, arom-H), 7.82 (d, 1H, J = 7.5 Hz, arom-H), 7.95 (br, 1H, NH), 8.01 (d, 1H, J = 8.7 Hz, arom-H), 9.99 (br, 1H, OH).
Methyl (4-bromophenyl)(2-hydroxynaphthalen-1-yl)methylcarbamate (4e): IR (KBr disc): υ 3417 (NH), 3288 (OH), 1688 (C=O) cm−1; 1H NMR (300 MHz, DMSO-d6): δ 3.58 (s, 3H, OCH3), 6.82 (broadened doublet, 1H, CH), 7.18 (d, 2H, J = 8.4 Hz, arom-H), 7.23 (d, 1H, J = 9.0 Hz, arom-H), 7.45 (t, 1H, J = 8.4 Hz, arom-H), 7.37–7.50 (m, 3H, arom-H), 7.70–7.85 (m, 3H, arom-H and NH), 7.90 (d, 1H, J = 7.8 Hz, arom-H), 10.12 (br, 1H, OH).
Methyl (4-fluorophenyl)(2-hydroxynaphthalen-1-yl)methylcarbamate (4f): IR (KBr disc): υ 3422 (NH), 3224 (OH), 1685 (C=O) cm−1; 1H NMR (300 MHz, DMSO-d6): δ 3.58 (s, 3H, OCH3), 6.85 (d, 1H, J = 9.0 Hz, CH), 7.09 (t, 2H, J = 8.1 Hz, arom-H), 7.20–7.45 (m, 5H, arom-H), 7.70–7.85 (m, 3H, arom-H and NH), 7.92 (d, 1H, J = 8.1 Hz, arom-H), 10.23 (br, 1H, OH).
Methyl (2-hydroxynaphthalen-1-yl)(4-nitrophenyl)methylcarbamate (4h): IR (KBr disc): υ 3422 (NH), 3268 (OH), 1683 (C=O), 1517 and 1345 (NO2) cm−1; 1H NMR (300 MHz, DMSO-d6): δ 3.61 (s, 3H, OCH3), 6.96 (d, 1H, J = 7.5 Hz, CH), 7.23 (d, 1H, J = 9.0 Hz, arom-H), 7.30 (t, 1H, J = 7.5 Hz, arom-H), 7.36–7.52 (m, 3H, arom-H), 7.80–7.95 (m, 4H, arom-H and NH), 8.16 (d, 2H, J = 8.7 Hz, arom-H), 10.26 (br, 1H, OH).
Funding
We gratefully acknowledge financial support from the Islamic Azad University, Mashhad Branch, Iran.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/23312009.2017.1317582.
Supplementary_material.docx
Download MS Word (486.8 KB)Additional information
Notes on contributors
Abolghasem Davoodnia
Abolghasem Davoodnia was born in 1971, Mashhad, Iran. He studied chemistry at Tehran University, Tehran, Iran, where he received BSc in 1994. He received his MSc degree in organic chemistry in 1997 from Ferdowsi University of Mashhad, Mashhad, Iran, under the supervision of Professor Majid M. Heravi and completed his PhD in organic chemistry in 2002 under the supervision of Prof. Mehdi Bakavoli at the same university. Currently, he is working as a professor at the Chemistry Department, Mashhad Branch, Islamic Azad University, Mashhad, Iran. He has published over 140 peer-reviewed articles in ISI journals. His current research interest is on heterocyclic chemistry, catalysis and new synthetic methodologies.
References
- Bettanin, L., Botteselle, G. V., Godoi, M., & Braga, A. L. (2014). Green synthesis of 1,3-diynes from terminal acetylenes under solvent-free conditions. Green Chemistry Letters and Reviews, 7, 105–112. doi:10.1080/17518253.2014.895868
- Brauch, S., van Berkel, S. S., & Westermann, B. (2013). Higher-order multicomponent reactions: Beyond four reactants. Chemical Society Reviews, 42, 4948–4962. doi:10.1039/c3cs35505e
- Davoodnia, A., Allameh, S., Fazli, S., & Tavakoli-Hoseini, N. (2011). One-pot synthesis of 2-amino-3-cyano-4-arylsubstituted tetrahydrobenzo[b]pyrans catalysed by silica gel-supported polyphosphoric acid (PPA-SiO2) as an efficient and reusable catalyst. Chemical Papers, 65, 714–720. doi:10.2478/s11696-011-0064-8
- Davoodnia, A., Basafa, S., & Tavakoli-Hoseini, N. (2016). Neat synthesis of octahydroxanthene-1,8-diones, catalyzed by silicotungstic acid as an efficient reusable inorganic catalyst. Russian Journal of General Chemistry, 86, 1132–1136. doi:10.1134/S107036321605025X
- Davoodnia, A., Khashi, M., & Tavakoli-Hoseini, N. (2013). Tetrabutylammonium hexatungstate [TBA]2[W6O19]: Novel and reusable heterogeneous catalyst for rapid solvent-free synthesis of polyhydroquinolines via unsymmetrical Hantzsch reaction. Chinese Journal of Catalysis, 34, 1173–1178. doi:10.1016/S1872-2067(12)60547-6
- Davoodnia, A., Tavakoli-Nishaburi, A., & Tavakoli-Hoseini, N. (2011). Carbon-based solid acid catalyzed one-pot mannich reaction: A facile synthesis of β-amino carbonyl compounds. Bulletin of the Korean Chemical Society, 32, 635–638. doi:10.5012/bkcs.2011.32.2.635
- Davoodnia, A., Zare-Bidaki, A., & Behmadi, H. (2012). A rapid and green method for solvent-free synthesis of 1,8-dioxodecahydroacridines using tetrabutylammonium hexatungstate as a reusable heterogeneous catalyst. Chinese Journal of Catalysis, 33, 1797–1801. doi:10.1016/S1872-2067(11)60449-X
- Dehghan, M., Davoodnia, A., Bozorgmehr, M. R., & Bamoharram, F. F. (2016). Synthesis, characterization and application of two novel sulfonic acid functionalized ionic liquids as efficient catalysts in the synthesis of 1,8-dioxo-octahydroxanthenes. Heterocyclic Letters, 6, 251–257.
- Dingermann, T., Steinhilber, D., & Folkers, G. (2004). In molecular biology in medicinal chemistry. Weinheim: Wiley-VCH.
- Dömling, A. (2006). Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chemical Preview, 106, 17–89. doi:10.1021/cr0505728
- Eshghi, H., & Gordi, Z. (2003). An easy method for the generation of amides from ketones by a beckmann type rearrangement mediated by microwave. Synthetic Communications, 33, 2971–2978. doi:10.1081/SCC-120022469
- Eshghi, H., & Hassankhani, A. (2006). P2O5/SiO2-catalyzed one-pot synthesis of amides from ketones via schmidt reaction under microwave irradiation in dry media. Synthetic Communications, 36, 2211–2216. doi:10.1080/00397910600638978
- Eshghi, H., & Hassankhani, A. (2012). Phosphorus pentoxide supported on silica gel and alumina (P2O5/SiO2, P2O5/Al2O3) as useful catalysts in organic synthesis. Journal of the Iranian Chemical Society, 9, 467–482. doi:10.1007/s13738-011-0057-0
- Eshghi, H., Rafei, M., & Karimi, M. H. (2001). Document P2O5/SiO2 as an efficient reagent for esterification of phenols in dry media. Synthetic Communications, 31, 771–774. doi:10.1081/SCC-100103268
- Eshghi, H., Rafie, M., Gordi, Z., & Bohloli, M. (2003). Improvement of selectivity in the Fries rearrangement and direct acylation reactions by means of P2O5/SiO2 under microwave irradiation in solvent-free media. Journal of Chemical Research, 763–764. doi:10.3184/030823503772913674
- Eshghi, H., Rahimizadeh, M., Ghadamyari, Z., & Shiri, A. (2012). P2O5/SiO2 as an efficient and mild catalyst for trimethylsilylation of alcohols using hexamethyldisilazane. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 42, 1435–1439. doi:10.1080/15533174.2012.682686
- Ghashang, M. (2014). Preparation of methyl/benzyl(2-hydroxynaphthalen-1-yl)(aryl)methylcarbamate derivatives using magnesium hydrogen sulfate. Research on Chemical Intermediates, 40, 1357–1364. doi:10.1007/s11164-013-1044-0
- Gholipour, S., Davoodnia, A., & Nakhaei-Moghaddam, M. (2015). Synthesis, characterization, and antibacterial evaluation of new alkyl 2-amino-4-aryl-4H-chromene-3-carboxylates. Chemistry of Heterocyclic Compounds, 51, 808–813. doi:10.1007/s10593-015-1779-1
- Habibi, D., Nasrollahzadeh, M., Mehrabi, L., & Mostafaee, S. (2013). P2O5-SiO2 as an efficient heterogeneous catalyst for the solvent-free synthesis of 1-substituted 1H-1,2,3,4-tetrazoles under conventional and ultrasound irradiation conditions. Monatshefte für Chemie - Chemical Monthly, 144, 725–728. doi:10.1007/s00706-012-0871-9
- Hajipour, A. R., & Ruoho, A. E. (2005). Nitric acid in the presence of P2O5 supported on silica gel—a useful reagent for nitration of aromatic compounds under solvent-free conditions. Tetrahedron Letters, 46, 8307–8310. doi:10.1016/j.tetlet.2005.09.178
- Hajipour, A. R., Zarei, A., Khazdooz, L., Pourmousavi, S. A., Mirjalili, B. F., & Ruoho, A. E. (2005). Direct sulfonylation of aromatic rings with aryl or alkyl sulfonic acid using supported P2O5/Al2O3. Phosphorus, Sulfur and Silicon and the Related Elements, 180, 2029–2034. doi:10.1080/104265090902796
- Hasaninejad, A., Zare, A., Balooty, L., Mehregan, H., & Shekouhy, M. (2010). Solvent-free, cross-aldol condensation reaction using silica-supported, phosphorus-containing reagents leading to α, α′-Bis(arylidene) cycloalkanones. Synthetic Communications, 40, 3488–3495. doi:10.1080/00397910903457282
- Hasaninejad, A., Zare, A., Sharghi, H., Niknam, K., & Shekouhy, M. (2007). P2O5/SiO2 as an efficient, mild, and heterogeneous catalytic system for the condensation of indoles with carbonyl compounds under solvent-free conditions. Arkivoc, 14, 39–50.
- Khashi, M., Davoodnia, A., & Prasada Rao Lingam, V. S. (2015). DMAP catalyzed synthesis of some new pyrrolo[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines. Research on Chemical Intermediates, 41, 5731–5742. doi:10.1007/s11164-014-1697-3
- Kumar, S., Aggarwal, R., Kumar, V., Sadana, R., Patel, B., Kaushik, P., & Kaushik, D. (2016). Solvent-free synthesis of bacillamide analogues as novel cytotoxic and anti-inflammatory agents. European Journal of Medicinal Chemistry, 123, 718–726. doi:10.1016/j.ejmech.2016.07.033
- Kundu, D., Majee, A., & Hajra, A. (2010). Zwitterionic-type molten salt: An efficient mild organocatalyst for synthesis of 2-amidoalkyl and 2-carbamatoalkyl naphthols. Catalysis Communications, 11, 1157–1159. doi:10.1016/j.catcom.2010.06.001
- Massah, A. R., Dabagh, M., Shahidi, S., Javaherian Naghash, H., Momeni, A. R., & Aliyan, H. (2009). P2O5/SiO2 as an efficient and recyclable catalyst for N-Acylation of sulfonamides under heterogeneous and solvent-free conditions. Journal of the Iranian Chemical Society, 6, 405–411. doi:10.1007/BF03245851
- Meerakrishna, R. S., Periyaraja, S., & Shanmugam, P. (2016). Copper-Catalyzed Multicomponent Synthesis of Fluorescent 2′-Phenyl-1′H-spiro[fluorene-9,4′-naphtho[2,3-h]quinoline]-7′,12′-dione Derivatives. European Journal of Organic Chemistry, 2016, 4516–4525. doi:10.1002/ejoc.201600647
- Mirjalili, B. F., Zolfigol, M. A., Bamoniri, A., Amrollahi, M. A., & Hazar, A. (2004). An efficient procedure for acetalization of carbonyl compounds with P2O5/SiO2. Phosphorus, Sulfur and Silicon and the Related Elements, 179, 1397–1401. doi:10.1080/10426500490463583
- Moghaddas, M., Davoodnia, A., Heravi, M. M., & Tavakoli-Hoseini, N. (2012). Sulfonated carbon catalyzed biginelli reaction for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and -thiones. Chinese Journal of Catalysis, 33, 706–710. doi:10.1016/S1872-2067(11)60377-X
- Mohammadizadeh, M. R., Hasaninejad, A., Bahramzadeh, M., & Sardari Khanjarlou, Z. (2009). P2O5/SiO2 as a new, efficient, and reusable catalyst for preparation of β-enaminones under solvent-free conditions. Synthetic Communications, 39, 1152–1165. doi:10.1080/00397910802513052
- Naeimi, H., Sharghi, H., Salimi, F., & Rabiei, Kh. (2008). Facile and efficient method for preparation of schiff bases catalyzed by P2O5/SiO2 under free solvent conditions. Heteroatom Chemistry, 19, 43–47. doi:10.1002/hc.20383
- Nakhaei, A., & Davoodnia, A. (2014). Application of a Keplerate type giant nanoporous isopolyoxomolybdate as a reusable catalyst for the synthesis of 1,2,4,5-tetrasubstituted imidazoles. Chinese Journal of Catalysis, 35, 1761–1767. doi:10.1016/S1872-2067(14)60174-1
- Nakhaei, A., Yadegarian, S., & Davoodnia, A. (2016). Efficient and rapid hantzsch synthesis of1,4-dihydropyridines using a nano isopolyoxomolybdate as a reusable catalyst under solvent-free condition. Heterocyclic Letters, 6, 429–439.
- Shafiee, M. R. M., Moloudi, R., & Ghashang, M. (2011). Document Preparation of methyl (2-hydroxynaphthalen-1-yl)(aryl)methyl/ benzylcarbamate derivatives using magnesium (II) 2,2,2-trifluoroacetate as an efficient catalyst. Journal of Chemical Research, 622–625. doi:10.3184/174751911X13182405888457
- Shaterian, H. R., & Hosseinian, A. (2014). Efficient synthesis of 1-carbamatoalkyl-2-naphthols using Brønsted acidic ionic liquid as reusable catalyst. Research on Chemical Intermediates, 40, 3011–3019. doi:10.1007/s11164-013-1147-7
- Shaterian, H. R., Hosseinian, A., & Ghashang, M. (2009). PPA-SiO2 catalyzed multi-component synthesis of n-[α-(β-hydroxy-α-naphthyl)(benzyl)] o-alkyl carbamate derivatives. Chinese Journal of Chemistry, 27, 821–824. doi:10.1002/cjoc.200990137
- Shaterian, H. R., Ranjbar, M., & Azizi, K. (2011). Efficient multi-component synthesis of highly substituted imidazoles utilizing P2O5/SiO2 as a reusable catalyst. Chinese Journal of Chemistry, 29, 1635–1645. doi:10.1002/cjoc.201180293
- Shen, A. Y., Tsai, C. T., & Chen, C. L. (1999). Synthesis and cardiovascular evaluation of N-substituted 1- aminomethyl-2-naphthols. European Journal of Medicinal Chemistry, 34, 877–882. doi:10.1016/S0223-5234(99)00204-4
- Slobbe, P., Ruijter, E., & Orru, R. V. A. (2012). Recent applications of multicomponent reactions in medicinal chemistry. MedChemComm, 3, 1189–1218. doi:10.1039/c2md20089a
- Song, Z. G., Sun, X. H., Liu, L. L., & Cui, Y. (2013). Efficient one-pot synthesis of 1-carbamatoalkyl-2-naphthols using aluminum methanesulfonate as a reusable catalyst. Research on Chemical Intermediates, 39, 2123–2131. doi:10.1007/s11164-012-0744-1
- Song, Z., Liu, L., Sun, X., & Cui, Y. (2014). Copper chloride-catalyzed efficient three-component one-pot synthesis of carbamatoalkyl naphthols under solvent-free conditions. Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 53B, 740–745.
- Taghavi-Khorasani, F., & Davoodnia, A. (2015). A fast and green method for synthesis of tetrahydrobenzo[a]xanthene-11-ones using Ce(SO4)2·4H2O as a novel, reusable, heterogeneous catalyst. Research on Chemical Intermediates, 41, 2415–2425. doi:10.1007/s11164-013-1356-0
- Tamaddon, F., Khoobi, M., & Keshavarz, E. (2007). (P2O5/SiO2): A useful heterogeneous alternative for the Ritter reaction. Tetrahedron Letters, 48, 3643–3646. doi:10.1016/j.tetlet.2007.03.134
- Thompson, L. A. (2000). Recent applications of polymer-supported reagents and scavengers in combinatorial, parallel, or multistep synthesis. Current Opinion in Chemical Biology, 4, 324–337. doi:10.1016/S1367-5931(00)00096-X
- Touré, B. B., & Hall, D. G. (2009). Natural product synthesis using multicomponent reaction strategies. Chemical Reviews, 109, 4439–4486. doi:10.1021/cr800296p
- Wang, M., Liu, Y., Song, Z., & Zhao, S. (2013). A convenient three-component synthesis of carbamatoalkyl naphthols catalyzed by cerium ammonium nitrate. Bulletin of the Chemical Society of Ethiopia, 27, 421–426. doi:10.4314/bcse.v27i3.11
- Wang, M., Wang, Q. L., Zhao, S., & Wan, X. (2013). Solvent-free one-pot synthesis of 1-carbamatoalkyl-2-naphthols by a tin tetrachloride catalyzed multicomponent reaction. Monatshefte für Chemie - Chemical Monthly, 144, 975–980. doi:10.1007/s00706-013-0927-5
- Wu, L., & Wang, X. (2011). P2O5/SiO2 as a new, efficient and reusable catalyst for preparation of 4,4’-epoxydicoumarins under solvent-free conditions. E-Journal of Chemistry, 8, 1626–1631. doi:10.1155/2011/849795
- Yang, J. M., Jiang, C. N., Dong, H., & Fang, D. (2013). Synthesis of 1-carbamatoalkyl-2-naphthols in Tween® 20 aqueous micelles. Journal of Chemical Research, 279–281. doi:10.3184/174751913X13647554585207
- Zare, A., Yousofi, T., & Moosavi-Zare, A. R. (2012). Ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate: A novel and highly efficient catalyst for the preparation of 1-carbamatoalkyl-2-naphthols and 1-amidoalkyl-2-naphthols. RSC Advances, 2, 7988–7991. doi:10.1039/c2ra20679j