Abstract
Leguminous plants have been used for centuries by rural communities as food, medicine and for cosmetic applications. The aim of this study was to evaluate the influence of spray-drying temperature on physicochemical and functional properties of protein isolates from three leguminous species. We found that pH is a major parameter of proteins’ solubility. The highest solubility was observed at pH 2 and between pH 8–12. The isoelectric pH was 4.5. The protein content of protein isolates was between 78.97% for Vigna Unguiculata and 88.43% for Glycine max. Highest water absorption capacity was observed when spray-drying temperature was at 130°C. The smallest gelling concentration of isolates was between 18 and 20% for G. max; 14–16% for V. Unguiculata; and 8–12% for C. ensiformis. The coalescence phenomenon followed by clarification was observed on emulsions after 24 h with the help of a turbiscan. The granulometric distribution of emulsions was monomodal.
Public Interest Statement
Available and less costly, legumes constitute an important source of proteins for they are varied and greatly used in Africa, particularly in Cameroon. For their individual valorisation, it is necessary to have them in large quantities and as pure as possible. Proteins are currently commercialised in the form of concentrates and/or isolates. Many legumes have already been the object of numerous works, yet there still exist some varieties which are little known by the population, varieties as Mucuna pruriens and Canavalia ensiformis. Their dry grains have a protein content which could go to 32.4 and 35.0% of dry matter, respectively. It is for this reason that we study in our article some physicochemical and functional properties of protein isolates of three leguminous plants with respect to the spray-drying temperature.
1. Introduction
Leguminous plants have been used for centuries by rural communities as food, medicine and cosmetics. There have recently been new interests in these products specifically for use in food and non-food formulations. Leguminous plants are a great family of plants characterised by cloves which have several colours and shapes commonly called legumes. They gather almost the highest number of vegetable species with not less than 18,000 species and 650 genders (Citation1). Three major categories of leguminous plants can be distinguished from amongst the most known and cultivated in the world. These are beans (white beans, red, black, romans, mungo, soy, etc.), lentils (green, brown, black, red, etc.) and peas (broken, entire, meagre, etc.) (Citation2). In some areas, only soy, groundnut, bean, peas, beans and lentils are the most useful legumes to man. They are thus used as nutritional complements for the manufacture of livestock food due to their rich carbohydrate and fibre contents. Leguminous plants are also a good source of vitamins and minerals, but are globally poor in fats except for soy. Nevertheless, they have high protein contents which may go up to 40% depending on the variety; they also contain magnesium, iron, calcium and selenium, an antioxidant which helps fight against ageing (Citation3, 4).
Consumption of leguminous plants can be a good alternative to animal products as far as proteins are concerned. Elsewhere, there exist numerous species of leguminous plants which are less known and/or under exploited. This is principally the case of Canavalia ensiformis and Vigna Unguiculata. These plants are sources of food for certain tribes and ethnic groups of Asia and Africa (Citation5, 6). Unfortunately, C. ensiformis is less known and its grains are less consumed because it contains numerous anti-nutritional factors such as phytates, tannins, phenols, trypsin inhibitors, L-Dopa and canavanin (Citation7). Though V. unguiculata is consumed in most sub-Sahara African countries, its potential is still under exploited and post-harvest issues persist (Citation8). In fact, more than half of the harvest is lost because of devastating insects such as beetles, thysanopteran, heteropteran and lepidopteran, which are, respectively, prefloration insects and flower devourers, clove suckers and stock insects (Citation9). Most of these devastating insects can cause up to 70–100% yield loss beginning from flowering to storage (Citation10). Consequently, it is urgent to valorise the protein potential of these leguminous plants while keeping their essential properties.
Extraction is the most efficient way of separating and recovering proteins from non-protein components in these plants. The extraction of proteins therefore is not only aimed at valorising them. It is also a means of studying their properties with ease, thus obtaining a convenient product usable in food and non-food systems. Protein concentrates are used in the industry for their emulsifying and foaming properties. In order to obtain long-term stability in addition to great functionality and ease of use, numerous food products, liquid or solid, are dehydrated or transformed mechanically into powders. Criteria like process performance and the quality of the product should be therefore respected. Nowadays, the spray-drying technology is considered as one of the principal methods of powder production in various domains given its several advantages. The aim of this present work is therefore to study the effect of spray-drying technique on the protein properties of three Cameroonian leguminous plants.
2. Material and methods
2.1. Material
The vegetable material used in this work was made of leguminous flours from C. ensiformis, Glycine max and V. unguiculata grains obtained from local markets of Ngaoundere town. Grains were sorted manually to remove impurities and infested grains. The other reagents used in this work were of analytical grade.
2.2. Preparation of C. ensiformis, G. max and V. unguiculata flours
The different flours were produced using the method of Kaptso (Citation11), which consists of steeping–drying–dehusking and drying. Grains were steeped separately in distilled water in a ratio of 1/5 (w/v) for 12 h at room temperature, then wrung and dried for 48 h in a ventilated electric dryer at 40°C. Dried grains were then dehusked with a manual abrasive, winnowed, ground and sieved with a 500-μm mesh sieve. Powders obtained were sealed in polyethylene bags and kept at 4°C for further analysis.
2.3. Proximate analysis
Water content, ash content, total protein content and water activity were determined using the standard methods (Citation12, 13).
2.4. Solubility of proteins with pH: determination of isoelectric pH
The effect of pH on the solubility of C. ensiformis, G. max and V. unguiculata proteins was determined with a modified method of Sathe et al. (Citation14). A solution of each flour was made with distilled water and the pH adjusted from 2 to 12 with HCl 1 M or NaOH 1 M. The obtained solutions were agitated for two hours at room temperature and then centrifuged at 4000 rpm for 1 h. The supernatants were collected and the proteins assayed using the BCA (Biccinchonic Acid) method. The quantity of soluble proteins was expressed in percentage of dry matter.
2.5. Protein extraction by precipitation at isoelectric pH
The extraction of soluble proteins of each leguminous flour was carried out using a modified method of Yemisi et al. (Citation15). Adequate quantities of flours were mixed with NaCl (0.1 M for G. max and V. unguiculata and 0.4 M for C. ensiformis) in a ratio of 1/5 (weight/volume). pH was then adjusted to 11 for G. max and V. unguiculata and 8.8 for C. ensiformis using a solution of NaOH or HCl at 1 M. The mixtures were then incubated at 28°C with agitation (600 rpm) using an electric Lab-Mix agitator 20 for 2 h, then centrifuged at 4,000 rpm for 1 h at 4°C. The supernatants were collected and re-extracted twice. The obtained supernatants were then precipitated at their isoelectric pH and spray-dried. Each experiment was carried out twice.
2.6. Spray-drying of protein extracts
Precipitates of protein extracts were dried by atomisation using a pilot plant’s Pignat atomiser. This device was equipped with a centrifuge scatter tool driven by an air turbine. The drying process was in co-current. The experimental test conditions were: three drying temperatures (130, 170 and 200°C), the rate of the fan was fixed at 70 m3/h, the compressed air pressure was at 2 bars and the rate of the pump was 16.6 mL per minute.
2.7. Physicochemical and functional properties
2.7.1. Water and oil absorption capacity
Water and oil absorption capacities were determined using the modified method of Adebowale et al. (Citation16). 0.5 g of sample was mixed with 10 mL of distilled water or 5 mL of sunflower oil (d = 0.865 ± 0.008), respectively, for water absorption capacity and oil absorption capacity. The mixture was then vigorously stirred at 250 rpm for 30 min with a Lab-Mix-20 magnetic stirrer and then centrifuged at 4,000 rpm for 30 min. The sediment was collected and the water and oil absorption capacity was calculated.
2.7.2. The least gelation concentration
The method used here was the one of Sathe et al. (Citation17). Suspensions (5 mL) of protein isolates from 2 to 20% (w/v) were mixed with distilled water in test tubes. They were then heated in a boiling water bath for 1 h, and allowed to cool at room temperature, then kept at 4°C for 2 h. The least gelation concentration was estimated visually as being the one where the gel didn’t flow after over turning the test tube.
2.7.3. Foaming capacity
The foaming power inside water was determined according to the method of Adebowale et al. (Citation16). A 2% (w/v) solution was made in a graduated cylinder, and submitted to intense mechanical agitation using a rotor–stator system device (Ultra-Turrax) for 5 min at 13,500 rpm. The foaming power (FP) in percentage was calculated with respect to the increase in volume due to the addition of gas. The foam stability (FS) corresponds to the time necessary for the volume of the foam to diminish after a certain time t.
2.7.4. Emulsifying properties
2.7.4.1. Preparation of emulsions
The protein solutions were prepared at 5% (w/v) concentrations with distilled water at room temperature (Citation18). These solutions were then agitated at 500 rpm for 2 h so as to solubilise the powder. Sunflower oil (d = 0.865 ± 0.008) was used to make a 20% (w/v) oil-in-water emulsion while mixing the appropriate volume of oil with the protein solution for 10 min using Ultra turax device equipped with a rotor/stator system at 13,500 rpm.
2.7.4.2. Characterisation of emulsions
The physicochemical characterisation of different emulsions was done on the same day of their preparation. Their stability was studied using the Turbiscan. Measures were recorded each hour for 24 h. The size of emulsion droplets was measured by laser diffraction using a Mastersizer Malvern in humid conditions (Hydro 2000S, Malvern Instruments SA, Orsay, France) at 25°C. Distribution curves obtained were represented in volumic fraction (%) and correspond to the mean of three independent measures.
3. Results and discussion
3.1. Proteins’ solubility: Isoelectric pH
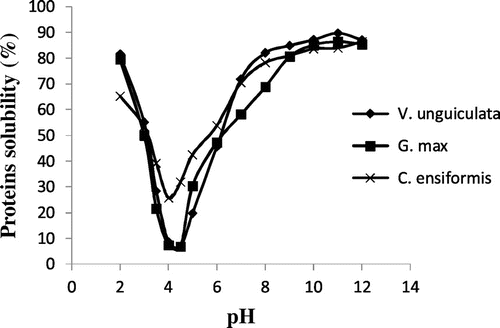
Figure shows the solubility curve of different proteins. It can be observed from this figure that the amount of soluble proteins decreases rapidly from 2 to 4. From pH 4, different protein solubility increases with increase in pH. The highest protein solubility of the three leguminous plants was observed at pH: 2, 8 and 12. The isoelectric pH was 4.5. The tendency of those curves is similar to those obtained by Adebowale et al. (Citation16) and Yemesi et al. (Citation15) on Mucuna protein species and by Abdel et al. (Citation19) on protein isolates of Vigna subterranean flours. These show that the solubility of proteins is influenced by the pH of the medium in which they are found. They are also in accordance with those obtained by Carbonaro et al. (Citation20) who show that the solubility of proteins from leguminous plants gets higher as we move away from their isoelectric pH. This can be explained by the fact that at isoelectric pH, proteins have a null net charge and thus no repulsive interactions, and the solubility is lower because protein–protein interactions are favoured (Citation21). When we move away from isoelectric pH, net charges are induced and protein–water interactions are favoured, rendering the proteins soluble. pH thus plays a major role in the solubility of proteins. Solubility will however also depend on other factors such as the method of extraction and the ionic strength of the medium.
3.2. Physicochemical composition of protein extracts
The protein content varies from 79.15% for V. Unguiculata atomised at 170°C to 88.43% for G. max atomised at 200°C on dry base (Table ). These values are higher than the one obtained by Boye et al. (Citation22) which is 79.1% but closer to the one obtained by Bhatty and Christison (Citation23) which is 86.7% on protein concentrates of lentils and peas. Nevertheless, no matter the spray-drying temperature, the protein content obtained is higher than that of Ngatchic et al. (Citation24) which was 63.81% on protein concentrates of C. ensiformis. Ash and water contents on their part vary, respectively, around 4% for C. ensiformis and V. Unguiculata; 3% for G. max; 3% for C. ensiformis; and 4% for V. Unguiculata and G. max (Table ). These values are similar to those obtained by many authors (Citation25–27). Given the water activity variation of protein isolates with spray-drying temperature being globally low (lesser than 0.6), micro-organisms will not grow easily. This will lead to a longer preservation time. Differences in the composition of protein extracts may be linked to the treatment conditions (extraction method, duration, temperature, material and drying method) during the extraction process and the cultivation or harvest seasons (Citation28).
Table 1. Physicochemical characteristics of concentrates/protein isolates
3.3. Oil and water absorption capacity
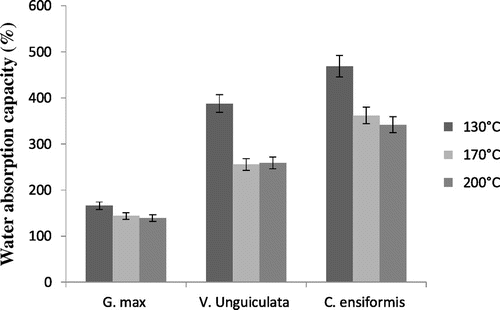
Water absorption capacity ranges between 140% for G. max spray-dried at 200°C and 470% for C. ensiformis spray-dried at 130°C (Figure ). Proteic concentrates of C. ensiformis show a higher water absorption capacity when compared to V. Unguiculata and G. max. In addition, no matter the leguminous plant, the highest water absorption capacity at the spray-drying temperature of 130°C was 168% for G. Max; 390% for V. Unguiculata; and 470% for C. ensiformis. The poor water absorption capacity of protein isolates of G. max compared to the two other leguminous plants can be explained by the presence of fats in the isolates. In fact, the absence of fats in proteins exposes water acceptor groups of the proteins in a hydrophilic environment, leading to an increase in water absorption capacity contrary to proteins whose units groups are blocked in a lipophilic environment. Similar observations were made by Adebowale et al. (Citation16) on proteins of six Mucuna species.
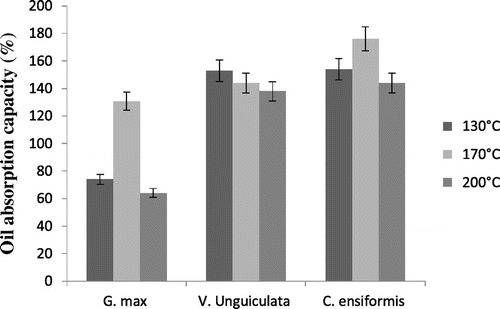
Oil absorption capacity as far as it is concerned extends from 64% for spray-dried G. max at 200°C, which is the least value, to 176% for spray-dried C. ensiformis at 170°C, which is the highest value (Figure ). The three species of leguminous plants have a better oil absorption capacity at 170°C. However, just as water absorption capacity, G. max has the smallest oil absorption capacity no matter the spray-drying temperature. This can be explained by the fact that lipophilic groups of G. max proteins initially contain more oil than the other two. These values are largely greater than those obtained by some authors like Adebowale et al. (Citation16, Citation29). The capacity of the spray-dried protein isolates to absorb water shows their ability to conserve oil and water, which will certainly influence their behaviour in food and non-food systems.
3.4. The least gelation concentration
The least gelation concentration of the protein isolates of each leguminous plant was not significantly different with the spray-drying temperature (Table ). However, it varies with the variety. Thus, no matter the spray-drying temperature, it varies between 18 and 20% for G. max, between 14 and 16% for V. Unguiculata and between 8 and 12% for C. ensiformis. On the one hand, values obtained for G. max and V. Unguiculata are similar to those of Udensi and Okoronkwo (Citation29) on fermented and germinated protein isolates of Mucuna cochinchinensis and on the other hand to those obtained by Adebowale et al. (Citation16) on proteins of six species of Mucuna. Likewise, values obtained for C. ensiformis are close to those obtained by Lawal and Adebowale (Citation30) which range from 4 to 8% on starch of C. ensiformis. The gelling property is an important parameter which can influence the texture of creams or gels during a formulation.
Table 2. The least gelation concentration
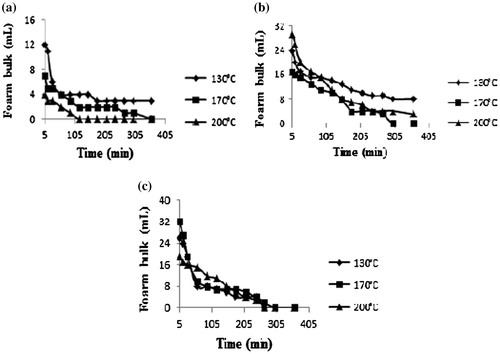
3.5. Foaming properties
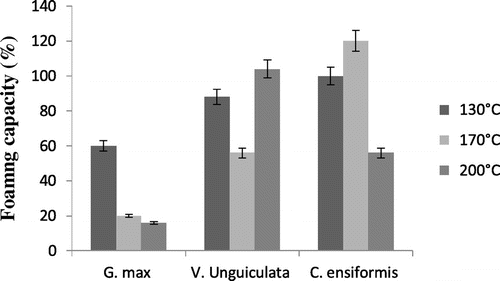
The foaming capacity of the three protein isolates varied with the variety of the leguminous plant (Figure ). In general, protein isolates of G. max presented the smallest foaming capacity no matter the spray-drying temperature, whereas those of V. unguiculata spray-dried at 200°C and C. ensiformis spray-dried at 170 and 200°C presented a foaming capacity greater than 100. These values are greater than those obtained by Okorie and Bello (Citation31) on the protein isolates of soy.
The volume of the foams from V. unguiculata and C. ensiformis was still high above 10 mL after 60 min, which expresses their stability for a relatively long time (Figure ). This good foaming property of protein isolates of V. unguiculata and C. ensiformis shows that they can be used as foaming agents in food creams or in cosmetics such as shampoos, shower gels and foaming baths.
3.6. Properties of emulsions
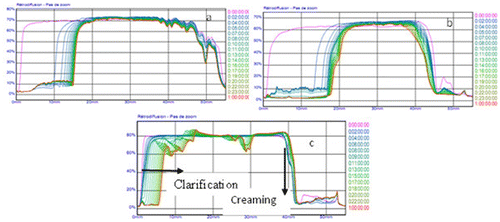
3.6.1. Emulsions’ stability
No matter the spray-drying temperature and the variety of leguminous plant, the emulsion destabilised with time and the destabilising phenomenon observed was sedimentation (Figure ). The emulsion made with protein isolate of G. max atomised at 170°C sedimented less rapidly than those of V. Unguiculata and C. ensiformis when the sample’s particle size is less than 10 mm. Above 10 mm, coalescence could be observed till 40 min, after which the emulsion clarified.
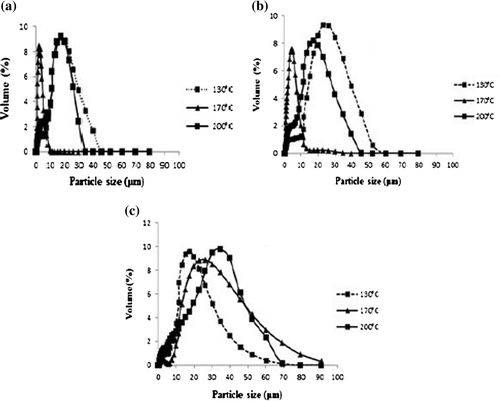
3.6.2. Particles size distribution of emulsions
Figure shows the particle size distribution of the protein isolate emulsions. It can be observed in general that all the granulometric curves are monomodals, and that the emulsions obtained with protein isolates spray-dried at 170°C had the smallest size compared to those spray-dried at 130 and 200°C, which provides it stabilisation with time; since the lesser the particles size, the better the stability of the emulsion (Citation32). These results reflected the ones obtained with the Turbiscan.
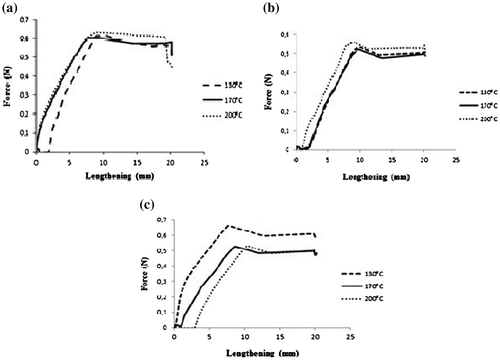
3.6.3. Emulsion textures
The textural profile of emulsions, obtained using penetration tests, shows a positive area which varies with the hardness and elasticity of the emulsion (Figure ). The curve trends are similar to those obtained by Zhihua Pang et al. (Citation33) on gels of gelatine with and without milk proteins where we observed that the hardness increased rapidly with the elasticity till a maximum before decreasing in spite of the increase in the elasticity. In general, no matter the spray-drying temperature and the type of leguminous plant, the elasticity of the emulsion was between 8 and 10 min. Emulsions made with protein isolates of V. Unguiculata showed a hardness of up to 0.55 N for a spray-drying temperature of 200°C, 0.53 N at both 130°C and 170°C. Elsewhere, the highest hardness (0.66 N) was obtained with protein isolates of G. max at 130°C. Points of the curves where the hardness changes concavity are points of emulsion droplets’ breakage. This emulsion textural profile is important for the flow of cosmetic products when conserved in a bottle.
4. Conclusion
The influence of spray-drying temperature on physicochemical and functional properties of protein concentrates of three leguminous plants was evaluated. This study shows that no matter the spray-drying temperature, the protein content of the protein isolates is high compared to other methods of concentration/purification of protein extracts. Also, great water and oil absorption capacities of protein isolates obtained by this method show their ability to keep water and oil if they are used in food and non-food systems. The good foaming property of protein isolates of V. unguiculata and C. ensiformis gives them the possibility of being used as foaming agents in cosmetic products like shampoos, shower gels and foaming baths. However, the coalescence phenomenon of emulsions obtained in this study was observed in less than 24 h with an adequate textural profile for the flow of products during their conservation.
Funding
The authors received no direct funding for this research.
Acknowledgement
Our gratitude goes to the EBInnov laboratory of EBI (Ecole de Biologie Industrielle, Cergy Pontoise, France) and Biophysics, Food Biochemistry and Nutrition laboratory of the National School of Agro-Industrial Sciences of Ngaoundere University where this work was conducted. We thank all the staff of theses laboratories who relented no effort for the realisation of this work.
Additional information
Notes on contributors
Kom Blaise
Our research activities are based on extraction, splitting, purification, conservation and the study of the implementation of active principles: applicable to proteins of some leguminous grains.
The work which we present in this article is aimed at valorising the proteins of some under/badly exploited leguminous protein isolates obtained after extraction and spray-drying in powder form. They will thus be more easily conserved and used compared to those in liquid form. Gifted with physicochemical and functional properties, these isolates could be used in food and non-food systems.
References
- Duc, G. Valeur alimentaire et usages des graines de légumineuses, Le courrier de l’environnement de l’INRA. Station de Génétique et d’Amélioration des plantes domaine d’Epoisses, 2010.
- FAO. Les graines de légumineuses dans l’alimentation humaine. FAO: Rome, 1982; 152 pp.
- Souci, S.W.; Fachmann, W.; Kraut H. La composition des aliments. Tableau des valeurs nutritives, 7ème éd.; MedPharm, Taylor and Françis, 2008.
- WHO/FAO/UNU. Expert consultation on protein and amino-acid requirements in Human Nutrition, Protein and amino-acid requirements in human nutrition; Report of a WHO/FAO/UNU. WHO, technical report series n°935, 2007.
- Ukachukwu, S.N.; Obioha, F.C. Chemical evaluation of Mucuna cochinchinensisas alternative protein feedstuff. J. Appl. Chem. Agric. Res. 1997, 4, 33–38.
- Iyayi, E.A.; Egharevba, J.I. Biochemical evaluation of seeds of an underutilized legume (Mucuna utilis). Nigerian J. Animal Production 1998, 25, 40–45.
- Okonkwo, J.C.; Udedibie, A.B.I. Preliminary observations on the yield performance of jackbean (Canavalia ensiformis) and sword bean (Canavalia gladiata) in the Guinea Savanna of Nigeria, 27th Annual Conference of Agric. Soc. of Nigeria, Minna, Nigeria, 1991.
- Murdock, L.L.; Seck, D.; Ntoukam, G.; Kitch, L. Preservation of cowpea grain in sub-Saharan Africa—Bean/Cowpea CRSP contributions. Field Crop Res. 2003, 82 (2–3), 169–178.10.1016/S0378-4290(03)00036-4
- Singh, S.R.; Allen, D.J. Les insectes nuisibles et maladies en niébé; Manuel N°2. IITA, 1979, 113 p.
- Gueye, M.T.; Dogo, S.; Wathelet, J.P.; Lognay, G. Lutte contre les ravageurs des stocks de céréales et de légumineuses au Sénégal et en Afrique occidentale: synthèse bibliographique. Biotechnol. Agron. Soc. Environ. 2010, 1370–6233, 1780–4507.
- Kaptso, K.G. Potentiel technologique des farines de niébé (Vigna unguiculata) et de voandzou (Vigna subterranea) pour la préparation du koki (gâteau de pate cuite à la vapeur). Thesis, ENSAI, Université de Ngaoundéré, Cameroon, 2008.
- AOAC. Official methods of analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, 1990.
- AFNOR. Aliments des animaux. Détermination de la teneur en eau, 1982; NF V18-109 5 pp.
- Sathe, S.K.; Deshpande, S.S.; Salunkhe, D.K. Functional properties of winged bean (Psophocarpus tetragonolobus, L) proteins. J. Food Sci. 1982, 47 (2), 503–509.10.1111/jfds.1982.47.issue-2
- Yemisi, A.A.; Kayode, O. Emulsifying properties of Mucuna flour and protein isolates. J. Food Technol. 2008, 6 (2), 66–79.
- Adebowale, Y.A.; AdeyemiI, A.; Oshodi, A.A. Functional and physicochemical properties of flours of six Mucuna species. Afr. J. Biotechnol. 2005, 4 (12), 1461–1468.
- Sathe, S.K.; Deshpande, S.S.; Salunkhe, D.K. Functional properties of black gram (Phaseolus mango, L) proteins. Lebensm-wiss. U. Technol. 1981, 16, 69–74.
- Bernard, C.; Regnault, S.; Gendreau, S.; Charbonneau, S.; Relkin, P. Enhancement of emulsifying properties of whey proteins by controlling spray-drying parameters. Food Hydrocoll. 2010, 25, 758–763.
- Abdel, R.S.M.; Eltayeb, A.O.; Ali, A.A.; Abou-Arab; Ferial, M.A. Chemical composition and functional properties of flour and protein isolate extracted from Bambara groundnut (Vigna subterranean). Afr. J. Food Sci. 2011, 5 (2), 82–90.
- Carbonaro, M.; Cappelloni, M.; Nicoli, S.; Lucarini, M.; Carnovale, E. Solubility−digestibility relationship of legume proteins. J. Agric. Food Chem. 1997, 45 (9), 3387–3394.10.1021/jf970070y
- Singh, N.; Kaur, M.; Sandhu, K.S. Physicochemical and functional properties of freeze-dried and oven dried corn gluten meals. Drying Technol. 2005, 23, 1–14.
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43 (2), 537–546.10.1016/j.foodres.2009.07.021
- Bhatty, R.S.; Christison, G.I. Composition and nutritional quality of pea (Pisum sativum L.), faba bean (Vicia faba L. spp. minor) and lentil (Lens culinaris Medik) meals, protein concentrates and isolates. Plant Foods Human Nutr. 1984, 34 (1), 41–51.10.1007/BF01095071
- Ngatchic, M.T.J.; Njintang, Y.N.; Oben, J.E.; Mbofung, C.M.F. Protein quality and antigrowth effect of protein isolate of Mucuna (Mucuna Pruriens) and Canavalia (Canavalia ensiformis) seeds. Scholars Acad. J. Biosci. 2013, 1 (5), 183–191.
- Betancur-Ancona, D.; Peraza-Mercado, G.; Moguel-Ordoneza, Y.; Fuertes-Blancob, S. Physicochemical characterization of lima bean (Phaseolus lunatus) and Jack bean (Canavalia ensiformis) fibrous residues. Food Chem. 2004, 84 (2), 287–295.10.1016/S0308-8146(03)00213-9
- Chau, C.F.; Cheung, P.C.K.; Wong, Y.S. Functional properties of protein concentrates from three Chinese Indigenous legume seeds. J. Agric. Food Chem. 1997, 45 (7), 2500–2503.10.1021/jf970047c
- Wiltoon, P.; Larry, R.; Beuchat, K.; Dixon, P. Functional properties of cowpea (Vigna unguiculata) flour as affected by soaking, boiling and fungal fermentation. J. Food Sci. 1997, 45, 480–486.
- Russin, T.; Arcand, Y.; Boye, J.I. Raw material particle size effect on yield and purity of soy protein isolates. J. Food Process. Pres. 2007, 31 (3), 308–319.10.1111/jfpp.2007.31.issue-3
- Udensi, E.A.; Okoronkwo, K.A. Effects of fermentation and germination on the physicochemical properties of Mucuna cochinchinensis protein isolate. Afr. J. Biotechnol. 2006, 5 (10), 896–900.
- Lawal, O.S.; Adebowale, K.O. An assessment of changes in thermal and physicochemical parameters of jack bean (Canavalia ensiformis) starch following hydrothermal modifications. Eur. Food Res. Technol. 2005, 221, 631–638.10.1007/s00217-005-0032-z
- Okerie, B.O.; Bello, A.B. Physicochemical and functional Properties of Winged bean flour and isolate compared with Soy isolate. J. Food Sci. 1988, 53, 450–454.
- Madzlan, K.; Steve, W.; Cui, H.; Douglas, G. Emulsifying properties of soy whey protein isolateefenugreek gum conjugates in oil-in-water emulsion model system. Food Hydrocoll. 2013, 30, 691–697.
- Zhihua, P.; Deeth, H.; Sopade, P.; Sharma, R.; Bansal, N. Rheology, texture and microstructure of gelatin gels with and without milk proteins. Food Hydrocoll. 2014, 35, 484–493.