Abstract
Natural products obtained from aromatic plants are considered to be one of the potential sources for the screening of antimicrobial, antioxidant, and free radical scavenging agents. Ocimum Lamiifolium (Damakese) is one of the well-known celebrated and most widely used home remedies. The shade dried leaves of Ocimum Lamiifolium were subjected to hydrodistillation in a Clevenger-type apparatus to extract its essential oil. The yield of hydrodistilled colorless, pungent odor oil was found to be 0.22% (v/w). The chemical composition of the oil was analyzed by GC-MS method. Ten constituents were identified representing 93.56% of the total oil. The main constituents of the oil were found to be Linalool (28.52%) and 1-octen-3-yl-npropionate (20.82%) followed by 3,7,11-trimethyl-(E,E)-2,6,10-dodecatrienal (12.14%). The yield of crude extract of Ocimum Lamiifolium was 0.28% (w/w). Phytochemical screening revealed the presence of terpenoids, flavonoids, tannin, saponins and steroids. The in vitro antibacterial activity of Ocimum Lamiifolium leaf ethanol extract and essential oil was studied by using disc diffusion method. The oil exhibited significant zone of inhibitions against to E. coli bacterial strains.
PUBLIC INTEREST STATEMENT
In this study, essential oil was extracted from leaves of Ocimum Lamiifolium (Damakese) and characterization was performed by GC-MS analysis. Phytochemical screening was also carried out for the crude extract obtained by Soxhlet extraction. Activity against to E. coli bacterial strains from the essential oil was further studied using disc diffusion method and good performance was observed. This study is very important to give attention finding new drug from this plant as the bacteria’s develop their resistance because of long-term usage of specific drug for long time.
Competing Interest
The authors declare no competing interests.
1. Introduction
The history of herbs and other plants to be applicable for medical purpose is decreasing when people became conscious of their environment. Traditionally, most medicinal plants are documented as medicine (Yinebeb, Citation2008). A report from the World Health Organization (WHO) estimates that 80% of the people in developing countries depend on traditional medicine for their primary health care, and around 85% of the medicines are plant extract. Accordingly, in Ethiopia also, up to 80% of the population rely on traditional medicine due to the cultural acceptability of doctors and local pharmacopeias, the affordability of the traditional Medicine and difficult access to modern health facilities. Generally, about 3.5–4 billion people in the world rely on plants as sources of drugs (Kebede, Kassaye, Amberbir, & Yunis, Citation2006; Schuster, Citation2001).
The increasing resistance of microorganisms such as bacteria, viruses and fungi to antibiotics and the shortage of coverage of the drugs usually used in the treatment of infectious diseases is becoming a major global health problem. This fascinated the researchers to search out alternative sources of natural product with large spectra of biological activities (Hafedh, Fethi, Mejdi, Emira, & Amina, Citation2010). Nowadays, the progress of natural products as valuable sources of new drug is increasing. Natural products and their derivatives still represent over 50% of all drugs in clinical use (Nazia, Ahmed, & Perween, Citation2006). Natural products produced by plants, fungi, bacteria, insects and animals have been isolated as biologically active pharmacophores. Furthermore, they are widely recognized in the pharmaceutical industry for their broad structural variety and wide range of applications. Chemically, they include the classes of organic compounds such as, terpenoids, polyketides, amino acids, peptides, proteins, carbohydrates, flavanoids, alkaloid, saponins, steroids, phenol compound, lipids, nucleic acid bases, ribonucleic acid (RNA), deoxyribonucleic acid (DNA), etc. (Omidbeygi, Barzegar, Hamidi, & Naghdibadi, Citation2007; Urinary Tract Infections in Adults, Citation2012). Plenty of species and herbs exert antibacterial influences due to their essential oil fractions. Study revealed that the essential oils plants like of oregano, thyme, sage, rosemary, clove, coriander, garlic, and onion show antimicrobial activity against both bacteria and molds. The antimicrobial activity of the plats varies depending on the composition, structure and functional groups of the oils (Janmoni & Latif, Citation2013,Tamalli, Bioprabhu, & Alghazal, Citation2013).
A urinary tract infection (UTI) is an infection in the urinary tract. Microorganisms are species those are too small to be seen without a microscope including fungi, viruses and bacteria that cause infections on our body if not cleaned properly. The most common cause for UTI is bacteria. Typically, bacteria that enter the urinary tract are rapidly removed by the body before they cause symptoms (Urinary Tract Infections in Infants and Children in Developing Countries in the Context of IMCI, World health organization, Citation2005). Nonetheless, sometimes bacteria overcome the body’s natural defenses and cause infection. A study of 53 cases of neonatal meningitis in Nigeria showed that 51% of organisms isolated from the cerebrospinal fluid were gram-negative bacteria (39% E. coli) possibly of urinary tract origin. Therefore, bacterium Escherichia coli (E. coli) is the main cause for the infections. UTIs are the second-most-common type of infection in the body, accounting for about 8.1 million visits to health-care providers each year (Sara, Citation2004).
UTI during pregnancy contributes significantly to maternal and perinatal morbidity. Abortion, small birth size, maternal anemia, hypertension, preterm labor, phlebitis, thrombosis and chronic pyelonephritis are related to UTI during pregnancy (Abdullah, Citation2009). All pregnant women should be screened for bacteriuria and subsequently treated with appropriate antibiotic therapy (Padma, Citation2004). UTI was also screened to belong to top ten diseases as a forth one in Mettu Karl hospital, Oromia regional state, Ethiopia, according to 2014 medical report of the hospital.
Ocimum Lamiifolium is a versatile aromatic genus (family Lamiaceae) well known for medicinal properties and also for economically important essential oils. The oils extracted from morphologically identical plants (one phenotype) show different physicochemical properties. Experiments on the essential oils of various Ocimum species have indicated that the oil possess biological activities. Of these, antimicrobial, antibacterial, antifungal properties are very important (Mouhssen, Citation2004). Traditionally in the present study area, Ocimumlamiifolium is used as a medicinal plant to treat infections of sores (expansions) and painful places observed around reproductive organs and urinary tracts of women giving birth. Therefore, the present study was conducted to identify the potentiality of Ocimum lamiifolium leaf essential oil as an antibacterial agent. The chemical composition of the leaf extract was also studied.
2. Experimental part
2.1. Materials and methods
2.1.1. Materials and chemicals
The plant Ocimum Lamiifolium was collected in December 2014 from Yayo forest, Ilu Aba Bora Zone, Oromiya region, Ethiopia. Botanical identity of samples was confirmed by the national herbarium, Department of Biology, Faculty of Science, Addis Ababa University where voucher specimens (collection no. HA 12006) have been deposited. Petridish (cell culture plates), micro-pipettes, rotary vapor, Clevenger-type hydrodistillation apparatus (equipped with heating mantle, 3 L round-bottomed flask, condenser (Jacketed coil) and 3 mL graduated receiver (Dean and Strack)), oven, autoclave (Pristage), analytical balance, incubator (Thermoelectric), refrigerator (Fisher Scientific 4791512) and GC-MS are the materials used. N-hexane, anhydrous sodium sulfate, methanol (0.02%), H2SO4, NaCl, distilled water, ampicillin (Reference Drugs),BaSO4, dehydrated barium chloride (BaCl2.H2O). E. coli. (microbial strains) and Muller Hinton agar (culture medium) are the chemicals used during the study.
2.2. Methods
2.2.1. Preparation of extracts
Air-drying of plant material has been performed in a shady place at room temperature for one week. The essential oil of the dried leave (460 g of air dried leaf mixed with 150 mL distilled water) was extracted by hydrodistillation using Cleavenger-type apparatus. The oil obtained was dried with anhydrous sodium sulfate (Na2SO4), and stored in a sealed amber-colored vial in refrigerator at +4ºC until further chemical and biological analysis (Cosentino et al., Citation1999; Hussain, Citation2009). The crude extract was prepared by using Soxhlet extraction method in the presence of ethanol solvent which was later separated by rotary vapor.
2.2.2. Spectrometric analysis of Ocimum Lamiifolium leaves essential oils
The essential oil of Ocimum lamiifolium analysis was performed using a GC and Clarus 600 GC-MS fitted with Hp-5MS capillary column (30 m × 0.25 mm coated with 5% phenyl methyl siloxane film thickness 0.25 µm). The GC oven temperature was programmed from 50ºC to 250ºC at a rate of 5ºC/min. The injector and detector temperature was maintained at 250ºC. Helium was used as the carrier gas at a flow rate of 1 mL/min. The mass spectrometer was operated at electrum impact of 70 ev with ion source temperature of 230ºC. The identification of the compounds was based on the comparison of their retention times to n-hexane, compared to published data and spectra of authenticated compounds. Compounds were further identified and authenticated using their mass spectra compared to the Wiley version 7.0 N libraries. The results were also confirmed by the comparison of the elution order of the components with their relative retention indices on non-polar phases reported in the literature.
2.2.3. Test for antibacterial activity
2.2.3.1. Inoculum preparation
Inoculum was obtained from an overnight agar culture of the test organism, and was prepared by taking at least 3–5 well-isolated colonies of the same morphology from an agar plate culture. The top of each colony was touched with a sterile loop and the growth was transferred into a tube containing 4–5 mL of normal saline until it achieved the turbidity of the 0.5 McFarland standards (usually 2–6 hrs). This results in a suspension containing approximately 1 to 2 × 108CFU/mL. The turbidity of the actively growing broth culture has been adjusted with sterile broth to obtain turbidity comparable to that of the 0.5 McFarland standards.
2.2.3.2. Turbidity standard for inoculum preparation
To standardize the inoculum density for a susceptibility test, BaSO4 turbidity standard, equivalent to a 0.5 McFarland standards was used. A 0.5 McFarland standard has been prepared as described in NCCLS (NCCLS, 1997). One percent V/V solution of sulfuric acid was prepared by adding 1 mL of concentrated sulfuric acid to 99 mL of water and mixed well. A 1.175% W/V solution of barium chloride was prepared by dissolving 2.35 g of dehydrated barium chloride (BaCl2.H2O) in 200 mL of distilled water. To make the turbidity standard, 0.5 mL of the barium chloride solution was added to 1% 99.5 mL sulfuric acid solution and mixed well. A small volume of those turbid solutions were transferred to a screw-capped tube of the same type as used for preparing the control inoculate and stored in the dark at room temperature.
2.2.4. Antibacterial activity screening
The agar disc diffusion method was used for the determination of antibacterial activities of Ocimum Lamiifolium essential oil according to the method described (Ibrahim, Dada, & Adejare, Citation2010). Inoculate of the bacterial strains was prepared from overnight broth cultures and suspensions has been adjusted to 0.5 McFarland standard turbidity (corresponding to 107–108 CFU/mL). Then each inoculate of the respective bacteria was sterile in such a way as to ensure through coverage of the plates and uniform thick lawn of growth following incubation. Then a sterilized disc of 6 mm diameter was placed on agar plates using a sterile cork borer. Finally, 50 µL of the test oil was applied on the discs and the plates were incubated at 37ºC for approximately 24 hrs. Ampicillin has been used as positive reference standards to determine the sensitivity of a strain of each tested microbial species. Antimicrobial activity was evaluated by measuring the zone of inhibition against the test microorganisms (Cushnie & Lamb, Citation2005). In this study, all the tests assays were done in triplet for each treatment and control groups. The results were presented as Mean ± STD with the help of Microsoft Excel 2007 version.
2.2.5. Phytochemical screening
2.2.5.1. Preparation of reagents
Dilute ammonia solutions with 5N were prepared in 100 mL volumetric flask. 0.1% of ferric chloride was also geared up in the same compartment. By fraction of 1:100 mercuric chloride in water and sufficient KI Mayer’s reagent was equipped. Wagner’s reagent also prepared in 100 mL H2O by pleasing1 g iodine and 5g KI.
2.2.5.2. Test for anthraquinones
0.25 g of the extract was boiled with 5 mL of sulfuric acid (H2SO4) and filtered while hot. The filtrate was then shaken with 2.5 mL of chloroform until brown-color solution appeared. The chloroform layer was pipette into another test tube and 0.5 mL of dilute ammonia solution was added.
2.2.5.3. Test for terpenoids (Salkowski test)
0.25 g of the extract was added to 2 mL of chloroform. Concentrated H2SO4 (1.5 mL) was added carefully. A reddish-brown coloration of the interface indicates the presence of terpenoids.
2.2.5.4. Test for flavonoids
Two methods were applied for flavonoids test. For the First method, dilute ammonia (5 mL) were used to add to a portion of the extract (3 g), then concentrated sulfuric acid (1 mL) was added. Yellow colorations that disappear on standing indicate the presence of flavonoids. During the second method, a portion of the extract was heated with 10 mL of ethyl acetate over a steam bath for 3 min. The mixture was then filtered and 4 mL of the filtrates were shaken with 1 mL of dilute ammonia solution.
2.2.5.5. Test for saponins
0.5 g of extract was added to 5 mL of distilled water in a test tube. The solution then shaken vigorously and observed for a stable persistent froth. The frothing was mixed with 3 drops of olive oil and shaken robustly after which it was observed for the formation of an emulsion.
2.2.5.6. Test for tannins
About 0.5 g of the extract was boiled in 10 mL of water in a test tube and then filtered. A few drops of 0.1% ferric chloride was added and observed for brownish-green or blue-black colorations (Bounihi, Hajjaj, Alnamer, Cherrah, & Zellou, Citation2013).
2.2.5.7. Test for alkaloids
Small portion of solvent-free extract was stirred separately with a few drops of dilute hydrochloric acid, and filtered. The filtrates were tested with different alkaloidal reagents.
2.2.5.8. Mayer’s test
The filtrate was treated with Meyer’s reagent (potassium mercuric iodide), then creamy ppt. was formed.
2.2.5.9. Wagner’s test
The filtrate was treated with few drops of Wagner’s reagent (iodine in potassium iodide solution) (Yesil et al., Citation2007).
2.2.5.10. Test for steroids: (Liebermann Burchard reaction).
1 g of extract were added to 10 mL of chloroform and then filtered. 2 mL of acetic anhydride and concentrated H2SO4 was added to the 2 mL of the extract. Formation of blue, greenish coloration indicates the presence of steroids.
3. Result and discussion
3.1. Chemical composition
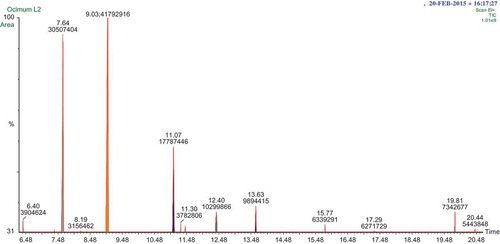
The air dried leaves were subjected to hydrodistillation in order to extract the volatile oil. The yield of the colorless essential oil from Ocimum lamiifolium leaves extracted by hydrodistillation was 0.22% (v/w) in relation to the dry weight of the plant. Ten compounds which constitute 93.56% of the total oil were identified by using GC and GC-MS analysis and the chromatogram is depicted in Figure . Most of the isolated compounds from Ocimum lamiifolium leaf essential oil are presented in Table .
Table 1. Chemical components of Ocimum lamiifolium leaf essential oil
The chromatogram of the chemicals listed in Table is depicted in Figure . The main constituents are Linalool (28.52%) and 1-octen-3-yl-n-propionate (20.82%) followed by 3,7,11-trimethyl-(E,E)-2,6,10-dodecatrienal (12.14%). The data obtained in current study are in good agreement with previously reported works (Al-Hajj et al., Citation2014; Phukan, Bawari, & Sengupta, Citation2015; Runyoro, Ngassapa, Vagionas, Gaikou, & Chinou, Citation2010). As it can be seen in Table , the oil is highly dominated by oxygenated terpene such as diterpenoid (36.57%), sesquiterpenoid (14.72%). The remaining are diterpene (6.75%), sespoterpene (5.01%) and other oxygenated compounds (23.48%). The chromatogram with the mass spectra of each compound is presented in Appendix (Fig. S1).
3.2. Phytochemical screening of crud extract
The yield of crud extract of Ocimum Lamiifolium was 0.28% (w/w). Phytochemical screening revealed the presence of terpenoids, flavonoids, tannin, saponins and steroids (Table ). The phytochemical components are shown in Table .
Table 2. Phytochemical components of ethanol extract
The present study investigated the phytochemical constituent of ethanol extracts of Ocimum Lamiifolium leaf for the presence of seven phytochemicals like terpenoids, flavonoids, saponins, tannins, steroids and alkaloids. Out of these phytochemicals, terpenoids, flavonoids, saponins, tannins and steroids were found in the extract. As opposed to the methanol extract of Ocimum lamiifolium sample collected from Adama, Oromiya Region, Ethiopia as the absence of flavonoids reported (Seid & Ayisha, Citation2015). The results of the various phytochemical tests indicated that plants are rich in various biologically active compounds which could serve as potential source of the crude drugs. All of which have been reported to exhibit physiological activities in man, animals and microorganisms, suggests that the plant may be used as a potent plant drug. Some phytochemicals are used in the pharmaceutical industry for the production of various drugs.
The phenolic compounds are one of the largest and most universal groups of plant metabolites. They possess biological properties such as anti-apoptosis, antiaging, anti-carcinogenic, anti-inflammation, anti-atherosclerosis, cardiovascular protection and improvement of endothelial function, as well as inhibition of angiogenesis and cell proliferation activities (Mann, Citation2012). Flavonoids and tannins are phenolic compounds that are likely to be responsible for the free radical scavenging effect (Telci, Bayram, Yılmaz, & Avcı, Citation2006). Moreover, flavonoids have been reported to possess anti-oxidants, anti-inflammatory and hypoglycemic activities, and are used as antimicrobial, anti-ancer, antiviral, anti-allergic, anti-platelet, antitumor and anti-allergic remedies (Adel, Néji, Mohamed, & Radhouane, Citation2011). In general. tannins have the tendency to inactivate and kill microorganisms those cause disease (Prashant, Bimlesh, Mandeep, Gurpreet, & Harleen, Citation2011).
The folkloric use of medicinal plants in the treatment of Mitch and anti-inflammatory activity might be due to presence of flavonoids and tannin. Terpenes or terpenoids are active against bacteria, fungi, viruses and protozoa. It has been reported that 60% of essential oil derivatives examined to be inhibitory to fungi while 30% inhibited bacteria (Yadav & Agarwala, Citation2011). Terpenes such as monoterpenes, sesquiterpenes and triterpenes, and sterols had been reported to exhibit various biological activities in animals and microorganisms like anti-inflammatory, antimicrobial and hormonal activities. Some tannin had been reported as antiviral and antitumor agents as well as diuretics. Saponins and cardiac glycosides have also been reported as antifungal as well as cardio tonics. Some steroidal compounds have been reported to exhibit antidiabetic and antibacterial properties (Anwar et al., Citation2011; Sankar, Citation2012).
3.3. Antibacterial activity
The Ocimum Lamiifolium leaf extract showed comparable antibacterial activity relative to our positive control and the zone of inhibition is presented in Table at various concentrations.
Table 3. Inhibition zone of ethanol extract and hydrodistilled essential oil
The solvent chloroform did not inhibit the growth of bacteria at the concentration used. According to the this study, E. coli bacterial strain was almost linearly inhibited with concentration; i.e. the inhibition zone is almost directly proportional to concentration of the sample essential oil and ethanol crude extract. Both aqueous and ethanol extracts are active against E. coli with an MIC of less than 0.05 mg/mL ethanol extract and 0.1% aqueous extract. These results are in parallel with the previous report by Kifle and coworkers (Kifle, Seyoum, Asres, & Mazumder, Citation2007). Thus, the traditional usage of this plant extracts to prevent infection related to urinary track and reproductive organ is scientifically supported in this study. This may be due to the higher content of oxygenated and alcoholic compounds including terpenoids that are active against bacteria, fungi, viruses and protozoa (Cuéllarcuéllar & Okori, Citation2010). In agreement with the antimicrobial activities of essential oil constituents, it has been reported that EOs containing aldehydes or phenols, such as cinnamaldehyde, citral, carvacrol or eugenol as major components showed the highest antibacterial activity, followed by EOs containing terpene alcohols (Pandey, Citation2006). The fact that both ethanol and aqueous extracts of some plants are showing similar efficacy against some species of bacteria could be due to extraction ability of active ingredients responsible for antibacterial activity by the two extraction systems (Imael, Nestor, & Rodolfo, Citation2012, Srivastava, Ateeque, Neetu, Kagarwal, & Symasunder, Citation2001).
4. Conclusion
The present study showed the tendency of the Ocimum lamiifolium leaf to treat bacterial infections due to the presence of bioactive compounds in the extract. It is confirmed that the extract of Ocimum lamiifolium leaves have powerful antimicrobial activity which could be used in traditional system of medicines. The phytochemical analysis of Ocimum lamiifolium species extracts has led to the identification of terpenoids, flavonoids, tannin, saponins and steroids. Furthermore, both methanol and ethanol extract and hydrodistilled essential oil were found to exhibit a good antibacterial activity against E. coli in vitro assays. Generally, the result of the current study was used to indorse the traditional practice of these medicinal plants for treatments of some microbial infection and other diseases. Further research is necessary for determining the practical applicability of Ocimum lamiifolium leaf essential oil as antifungal, antimalarial, etc. In addition to its application for medical purpose, its side effect to cell (cytotoxicity test) should be studied which is very important to improve its activity and stability.
Contribution of the Authors
All authors have equal contribution to the work.
1440894_Supplementary_material.doc
Download MS Word (213.5 KB)Acknowledgments
The authors are grateful for financing provided by Mettu University.
Supplementary material
Supplemental material for this article can be accessed here https://doi.org/10.1080/23312009.2018.1440894.
Additional information
Funding
Notes on contributors
Leta Deressa Tolesa
Nigus Aweke Sahalie, Lijalem Hadush Abrha, and Leta Deressa Tolesa were born in Ethiopia. They received their BSc in applied chemistry from Jimma University, Axum University and Ambo University, respectively, and their MSc in Organic chemistry from Mekele University (for the first and second authors) and Bahir Dar University, respectively. They then worked as lecturer of Organic chemistry at Mettu University. Then, they joined National Taiwan University of Science and Technology (NTUST), Department of Chemical Engineering as PhD student. All authors are currently PhD student at NTUST, Taiwan.
References
- (Abdullah, I. H. (2009). Characterization and biological activities of essential oils of some species of lamiaceae. ( A thesis for doctor of philosophy in chemistry). University of agriculture, Faisalabad, Pakistan, 1–218.
- Adel, K., Néji, G., Mohamed, D., & Radhouane, G. (2011). Chemical composition and in vitro antioxidant properties of essential oil of Ricinus communis L. Journal of Medicinal Plants Research, 5(8), 1466–1470.
- Al-Hajj, N. Q. M., Wang, H., Chaoyang, M., Zaixiang, L., & Thabit, R. (2014). GC-MS analysis of chemical compounds from some yemeni medicinal plants. International Journal of Pharma Research & Review, 3(5), 46–51.
- Anwar, F., Sulman, M., Hussain, A., Saari, N., Iqba, S., & Rashid, B. (2011). Physicochemical composition of hydrodistilled essential oil from coriander (Coriandrum sativum L.) seeds cultivated in Pakistan. Journal of Medicinal Plant Research, 5(15), 3537–3544.
- Bounihi, A., Hajjaj, G., Alnamer, R., Cherrah, Y., & Zellou, A. (2013). In Vivo potential anti-inflammatory activity of Melissa officinalis L. Essential oil. Advances in Pharmacological Sciences, 2013, 1–7. doi:10.1155/2013/101759
- Cosentino, S. C., Tuberoso, I. G., Pisano, B., Satta, M., Mascia, V., Arzedi, E., & Palmas, F. (1999). Invitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Letters in Applied Microbiology, 29, 130–135. doi:10.1046/j.1472-765X.1999.00605.x
- Cuéllarcuéllar, A., & Okori, O. D. (2010). Preliminary phytochemical and antimicrobial evaluation of the fresh and dried whole plant extracts from Commelina benghalensis. Revista Colombiana de Ciencia Animal, 2(1), 104–115.
- Cushnie, T., & Lamb, A. (2005). Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 26, 343–356. doi:10.1016/j.ijantimicag.2005.09.002
- Hafedh, H., Fethi, B., Mejdi, S., Emira, N., & Amina, B. (2010). Effect of Menthalogifolia L. ssp essential oil on the morphology of four pathogenic bacteria visualized by atomic force microscopy. African Journal of Microbiology Research, 4, 1122–1127.
- Hussain, A. (2009). Characterization and biological activities of essential oils of some species of Lamiaceae. Journal of Pharmaceutical Chemistry, 7, 14–52.
- Ibrahim, T., Dada, B., & Adejare, R. (2010). Comparative Phytochemical properties of crude ethanol extracts and physicochemical characteristics of essential oils of Myristical fragrans (nutmeg) seeds and Zingiber officinate (ginger) roots. Electronic Journal of Environmental, Agricultural and Food Chemistry, 9, 1110–1116.
- Imael, H., Nestor, B., & Rodolfo, H. J. (2012). Essential oils in combination and their antimicrobial properties. Molecules, 17, 3989–4006. doi:10.3390/molecules17043989
- Janmoni, K. M., & Latif, K. (2013). Commercial potentialities of essential oil of Ocimum members growing in North East India. International Journal of Pharmacy & Life Sciences, 4(4), 2559–2567.
- Kebede, D., Kassaye, A., Amberbir, B., & Yunis, M. (2006). A historical overview of traditional medicine practices and policy in Ethiopia. Ethiopian Journal of Health Development, 2, 1–2.
- Kifle, A., Seyoum, K., Asres, A., & Mazumder, F. (2007). Composition, antimicrobial and free-radical scavenging properties of the essential oil of Damakese (Ocimum lamiifolium): A popular home remedy in Ethiopia. International Journal of Essential Oil Therapeutics, 1, 110–116.
- Mann, A. (2012). Phytochemical constituents and antimicrobial and grain protectant activities of clove basil (Ocimum gratissimum L.) grown in Nigeria. International Journal of Plant Research, 2(1), 51–58. doi:10.5923/j.plant.20120201.08
- Mouhssen, L. (2004). Methods to study the phytochemistry and bioactivity of essential oil. Phytotherapy Research, 18(6), 435–448. doi:10.1002/ptr.1465
- Nazia, M., Ahmed, C., & Perween, T. (2006). Antimicrobial activity of Cinnamonum cassia against diverse microbial flora with its nutritional and medicinal impacts. Pakistan Journal of Botany, 38(1), 169–174.
- Omidbeygi, M., Barzegar, M., Hamidi, Z., & Naghdibadi, H. (2007). Antifungal activity of thyme, summer savory and clove essential oils against Aspergillusflavus in liquid medium and tomato paste. Food Control, 18, 1518–1523. doi:10.1016/j.foodcont.2006.12.003
- Padma, S. V. (2004). Essential oils and fragrances from natural sources. Ecological and analytical testing in IIT, Kanpur. Resonance, 9, 30–41.
- Pandey, B. P. (2006). A textbook of botany: Angiosperms, taxonomy, anatomy, embryology and economic botany. Ram Nagar, New Delhi: S. Chand and Co., Ltd.
- Phukan, P., Bawari, M., & Sengupta, M. (2015). Promising neuroprotective plants from north-east India. International Journal of Pharmacy & Pharmaceutical Sciences, 7(3), 28–39.
- Prashant, T., Bimlesh, K., Mandeep, K., Gurpreet, K., & Harleen, K. (2011). Phytochemical screening and extraction: A review. International Pharmaceutic Science, 1, 103–105.
- Runyoro, D., Ngassapa, O., Vagionas, K., Gaikou, N., & Chinou, I. (2010). Chemical composition and antimicrobial activity of the essential oils of four Ocimum species growing in Tanzania. Food Chemistry, 119, 311–316. doi:10.1016/j.foodchem.2009.06.028
- Sankar, N. S. (2012). Phytochemical analysis and antibacterial potential of Moringa oleifera. International Journal of Science Innovations and Discoveries, 2(4), 401–407.
- Sara, A. B. (2004). Antibacterial activity of essential oils: Potential applications in food. International Journal of Food Microbiology, 94(3), 223–253. doi:10.1016/j.ijfoodmicro.2004.03.022
- Schuster, B. (2001). Demonstrating the validity of natural products as anti-infective drugs. The Journal of Alternative and Complementary Medicine, 7, 73–82. doi:10.1089/107555301753393832
- Seid, M., & Ayisha, A. (2015). Extraction and phytochemical determination of some selected traditional medicinal plants for antimicrobial susceptibility test, in Adama, Ethiopia. International Journal of Science, Engineering and Technology, 3(5), 1290–1297.
- Srivastava, S. K., Ateeque, A., Neetu, J., Kagarwal, K., & Symasunder, K. (2001). Essential Oil composition of callistemon citrus leaves from the Lower Region of Himalayas. Journal of Essential Oil Research, 13, 359–361. doi:10.1080/10412905.2001.9712233
- Tamalli, M., Bioprabhu, S., & Alghazal, M. A. (2013). Urinary tract infection during pregnancy at Al-khoms, Libya. International Journal of Medicine and Medical Sciences, 3(5): 455–459. ISSN. 2167-040.
- Telci, I., Bayram, E., Yılmaz, G., & Avcı, B. (2006). Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biochemical Systematics and Ecology, 34, 489–497. doi:10.1016/j.bse.2006.01.009
- Urinary Tract Infections in Adults. (2012). National kidney and urologic diseases information clearinghouse. National Institutes of Health. U.S. Department of Health and Human Services.
- Urinary Tract Infections in Infants and Children in Developing Countries in the Context of IMCI, World health organization. (2005). Department of Child and Adolescent Health and Development. WHO/FCH/CAH/05.11.
- Yadav, R., & Agarwala, M. (2011). Phytochemical analysis of some medicinal plants. Journal of Phytology, 3(12), 10–14.
- Yesil, C. O., Hames, K. E., Bedir, E., Vardar, F., Ozek, T., & Baser, K. C. (2007). Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chemistry, 100, 553–559. doi:10.1016/j.foodchem.2005.10.011
- (Yinebeb, T. (2008). In vitro efficacy study of some selected medicinal plants against leishmania spp. A thesis for the Degree of Master of Science in Medicinal Chemistry submitted to the School of Graduate Studies of the Addis Ababa University