Abstract
Natural products play a dominant role in the development of novel drug leads for the treatment and prevention of diseases. Toxicity is an expression of being poisonous, indicating the state of adverse effects led by the interaction between toxicants and cells. This interaction may vary depending on the chemical properties of the toxicants and the cell membrane. Hence, evaluation of toxic properties of a substance is crucial when considering for public health protection. Protease inhibitors (PIs) are low molecular mass proteins that are ubiquitous in nature. They are natural antagonists of protease, which are present in all life forms. The present study aims to determine the toxicity of purified PI from the fruits of Solanum aculeatissimum using an acute and subchronic oral toxicity test in animal models. No significant changes in the enzymes level were observed. No adverse changes were noticed in the experimental animal treated with purified PI.
Public Interest Statement
Protease inhibitors (PIs) are low molecular mass proteins that are ubiquitous in nature. They are natural antagonists of protease, which are present in all life forms. Solanum aculeatissimum Jacq., commonly known as African night shade, was used by the local people as fruit and vegetable during famine period. S. aculeatissimum is a shrubby perennial, armed with spines whose fruit is spherical, striped and turns yellow when ripe. The plant has been extensively used ethnically to cure disorders such as constipation, purgative, back pain, male impotence, snakebites, skin infections, cough and diarrhoea. Many potent PIs are not evaluated for their safety profiles. Indeed, many of the reported PIs have been known for its efficacy as drugs but not yet evaluated toxicologically. Hence, the main aim of the present study was to evaluate the toxic effects of protease inhibitors from Solanum aculeatissimum (SAPI) before it can be used for therapeutic applications that are of importance to the public.
Competing Interests
The authors declare no competing interests.
1. Introduction
Plants are the source of medication for preventive, curative, protective or promotive purposes (Grover & Yadav, Citation2004). Plant-derived foods help in the prevention of lifestyle-associated diseases. Several groups of constituents in plants have been identified as potentially health promoting in animal studies, including cholesterol-lowering factors, antioxidants, enzyme inducers and others (Dragsted et al., Citation2006). A thousand years ago, an extensive use of plants as medicines has been reported and was initially taken in the form of crude drugs and other herbal formulations (Aneela et al., Citation2011). A number of antinutrients have been shown to possess beneficial properties, for example: anticancer and antimicrobial. Such compounds are of increasing interest in the fields of biochemistry, medicine, pharmacology and nutrition (Santosh & Richard, Citation2005). Toxicology is the important aspect of pharmacology that deals with the adverse effects of bio active substances on living organisms prior to the use as drug or chemical in clinical use (Aneela et al., Citation2011). As per the OECD guidelines, in order to establish the safety and efficiency of a new drug, toxicological studies are very essential in animals like mice, rats, pigs, dogs, rabbits and monkeys under various conditions of the drug. Toxicological studies help make decisions whether a new drug should be adopted for clinical use or not. OECD (Organisation of Economic Co-operation and development) 401, 423 and 425 do not allow the use of drugs clinically without its clinical trial as well as toxicity studies. Depending on the duration of drug exposure to animals, toxicological studies may be of three types-: acute, subacute and chronic toxicological studies.
Solanum, the hyper and diverse genus, belongs to Solanaceae family. It is represented in Kerala by about 33 species including those domesticated for their leaves, fruit, vegetables or used as traditional medicine. S. aculeatissimum Jacq., commonly known as African night shade, is used by the local people as fruit and vegetable. S. aculeatissimum is a shrubby perennial plant, armed with spines, whose fruit is spherical, striped and marble green or creamy-white, turning dirty yellow when ripe. The plant is native to Southern Africa but is now widespread throughout Asia. The plant has been extensively used ethnically to cure various diseases such as constipation, purgative, back pain, male impotence, snakebites, toothache, headache, skin infections, cough and diarrhoea. Most of the medicinal attributes of the plant was due to the presence of steroidal glycoalkaloids (Patel, Singh, & Patel, Citation2013). Despite its medicinal potentiality, the plant is unrecognised and underutilised. Many potent protease inhibitors (PIs) have safety profile data. Indeed, many of the best PIs have been compromised in terms of efficacy; they had to be sacrificed to develop an efficient drug (Turk, Citation2006). Hence, the main aim of this study was to evaluate the toxic effects of PIs from S. aculeatissimum (SAPI) before it can be used for biological applications.
1.1. Results and discussion
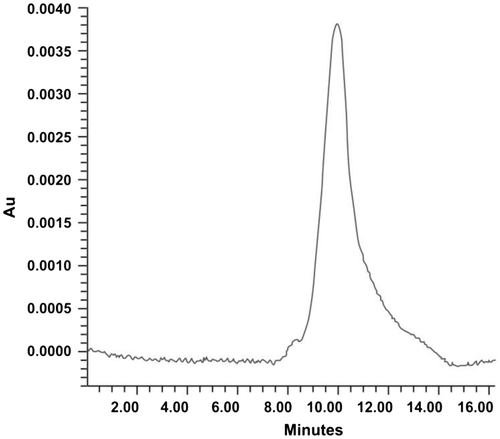
PI activities from different plant parts of S. aculeatissimum were evaluated. Fruits displayed the maximum PI activity compared to leaves, stems and seeds, i.e. 54% for trypsin and 48% chymotrypsin inhibitory activity. SAPI was concentrated with (NH4)2SO4 precipitation with varying concentrations ranging from 0 to 20, 20 to 40, 40 to 60, 60 to 80 and 80 to 90% saturation. The (NH4)2SO4 precipitation resulted in 1.41- and 1.51-fold of purification compared to the crude extract (Table ). Pooled active fractions from 0.18 to 0.24 M NaCl (fractions: 9–12) were dialysed and showed 93.2 TlU (trypsin inhibitory unit) and 90.2 ClU (chymotrypsin inhibitory unit) for trypsin–chymotrypsin inhibitory activities, respectively. The purity of PI was further checked by RP-HPLC with retention time of 10 min in 50 mM Tris–HCl buffer, pH 8.0, coinciding with the protein peak (Figure ). Thus, purified SAPI yielded specific activity of 502 TIU and 433.7 ClU U/mg, with low protein content of 0.95 mg. Overall, the specific activity increased about 92.6- and 82. 9-folds with 9.8 and 8.77% yield with respect to trypsin and chymotrypsin, respectively (Table ).
Table 1. Purification profile of S. aculeatissimum PI
1.2. Molecular mass
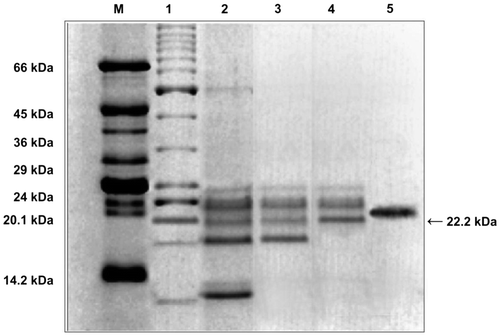
SDS-PAGE electrophoretic separation of SAPI showed a single prominent band of 22.2 kDa mass (Figure ). Size elution chromatography also revealed the same mass.
The benefits for the treatment or prevention of disease or infection that may occur from either dietary or topical administration are not quite scientifically evaluated. S. aculeatissimum has been cited in the scientific literature as having antibiotic, antitrypanosomal, hypotensive, antispasmodic, antiulcer, anti-inflammatory, hypocholesterolemic and hypoglycemic properties and even reduction of pathogens. However, a second scientific judgement is required to access the efficacy of traditional cures.
1.3. Toxicity results
The body weights of the animals were recorded in Tables and . All animals exhibited normal increase in body mass with a marginal variation between control and treated groups. No behavioural changes or death were observed at the end of the treatment period. Similarly, no significant differences in food intake and weight gain were observed between control and treated groups during this period. The results of the biochemical parameters of the experiment are summarised in Tables . Tables and represents the liver function test in the serum of rats. No significant changes in the level of serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) alkaline phosphatase (ALP), total protein, albumin, globulin and bilirubin were observed with that of the control. Similar results were observed for renal function test also (Table ). All the results were compared with a positive control Kunitz Soy PI, which also showed similar patterns of haematological and biochemical parameters with the control.
Table 2a. Change in body weight of mice during acute toxicity studies (7 days)
Table 2b. Change in body weight of Wistar rats during subchronic toxicity studies
Table 3. Haematological results from rats in the subchronic toxicity study
Table 4a. Serum biochemical results (LFT) from rats in the subchronic toxicity study
Table 4b. Serum biochemical results (LFT) from rats in the subchronic toxicity study
Table 5. Serum biochemical results (RFT) from rats in the subchronic toxicity study
Oral administration of the SAPI with doses from 500 to 1,000 g/kg did not produce significant changes in behaviour, growth and metabolic characteristics. Hepatic function was well preserved by the administration of SAPI in rats indicated by the serum enzyme levels and was comparable with the control group. Moreover, the extracts at higher doses did not show any conspicuous toxicity, indicating its potential use as a safe herbal drug and also compromised the medicinal use of this plant in tribal or folk medicine. Sathya, Kokilavani, and Ananta (Citation2012) showed that Acalypha indica Linn extract at different levels tested did not produce considerable change in the levels of the different parameters and was comparable with the results of SAPI.
There is no significant change in haematological parameters like haemoglobin, total WBC, neutrophil, lymphocytes and eosinophils in the extracts of the treated animals. Haematological changes such as anaemia are often accompanied with bone marrow toxicity (Hamid, Jaouad, Zafar, & Lyoussia, Citation2008; Ruby, Raj Kapoor, & Mohammad, Citation2011). Anaemia, that results after administration of drugs, can be a result of lysis of blood cells (Onyeyilli, Iwuoha, & Akinniyi, Citation1998). However, no such anaemia is observed after chronic treatment with the extracts, suggesting that there is no lysis of blood cells. The observed values of blood parameters within the normal range show that the drug is non-toxic in nature.
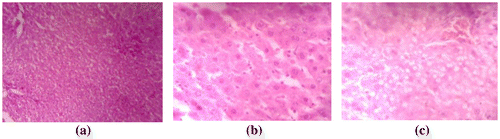
No marked changes in the histomorphology of the liver tissues were observed when rats are fed with different concentrations of SAPI (Figure (a)–(c)). The observation of the current study reveals that the oral administration of SAPI did not cause any mortality nor altered the biochemical and histopathological indices in subchronic studies. This indicates that the plant extract is not harmful at the level tested and can be safely used as a drug.
Herbal medicines are ethnic traditional medicines and are dietary supplements. They should be assured of their safety or efficacy through animal studies. Toxicological research involving laboratory animals is necessary to ensure and enhance human and animal health and protection of the environment. Changes in body weight may be used as a marker of toxic effects of phytochemicals (Raza, Al-Shabanah, El-Hadiyah, & Al-Majed, Citation2002; Teo et al., Citation2002). Interestingly, in the present study, no adverse changes were noticed in the experimental animals treated with purified PI from S. aculeatissimum. Further, no change was seen in the assay results of biochemical parameters in comparison to the control group including transaminases (AST and ALT), which suggests the ideal indicators of liver function. Changes in the AST and ALT levels may indicate alteration of cellular permeability or cellular injury and necrosis (Kaneko, Harvey, & Bruss, Citation1997). The analysis of such biochemical parameters is essential in relation to herbal products’ toxicity studies (Stedman, Citation2002; Teschke, Gaus, & Loew, Citation2003). Ikebe et al. (Citation2000) reported the ameliorative effect of α-PI against hepatic ischaemia reperfusion via liver enzymes and antioxidant machinaries. PIs have the potential to scavenge reactive nitrogen species and thereby contribute to its potent cytoprotective activity. Similarly, Yan et al. (Citation2013) proved the role of cathepsin β inhibitor related with hepatoprotective feature. According to Marwa, Ghada, Abd-El Mobd’ea, and Rehim (Citation2013), administration of S. nigrum ethanolic extract decreased the lambda cyhalothrin-induced elevated enzyme levels in animal models, suggesting its role in liver protection mechanism. The non-toxic nature of ethanol extract of Solanum incanum is evident from the acute oral toxicity studies in albino mice (Indhumathi, Mohandas, & Shibi, Citation2014). In addition, the integrity of hepatic function was analysed by liver histopathological analysis. The present results did not show any visible sign of histological disorder on these organs that could be associated to hepatotoxicity reactions. In addition, the evaluation of renal function is equally important since there were reports related with the various renal syndromes after the use of medicinal herbals such as Fanconi’s syndrome, tubular necrosis, papillary necrosis, chronic interstitial nephritis, acute interstitial nephritis, hypokalemia or hyperkalemia, hypertension, nephrolithiasis, urinary retention and tumours of the urinary tract (Isnard Bagnis, Deray, Baumelou, Le Quintrec, & Vanherweghem, Citation2004). On the other hand, herbal products have been used to prevent nefrotoxicity induced by gentamicin. Current results such as variation in creatinine and accumulation of urea were not noticed, which are good indicators for renal function impairment (Palani, Senthilkumaran, & Govindasamy, Citation1999). These results were also corroborated by kidney histopathological analysis, which indicated chronic administration devoid of nefrotoxicity. Jebasingh, Jackson, Venkataraman, and Emerald (Citation2012) justified the use of medicinal plant Cyperus rotundus in terms of physiochemical and toxicological aspects. Mohd Fuat and Aidoo (Citation2011) evaluated toxicity of Eurycoma longifolia used by local people for curing many ailments. Phani and Ravi (Citation2015) compared toxicity of Vitex leucoxylon, Vitex negundo and Vitex trifolia using similar parameters. Acute and subchronic oral toxicity studies of hydro-methanolic extracts of Grewia crenata leaves were evaluated in rats by Ukwuani, Abubakar, Hassan, and Agaie (Citation2012), who got similar results. However, Sini, Sinha, and Rajasekaran (Citation2010) reported toxicity effects of aqueous leaf extract of Capparis grandiflora in terms of excitement, tremors, restlessness, loss of appetite and general weakness, initially. But no muscular numbness of the hind and forelegs, salivation or diarrhoea was observed. Meanwhile, present results showed no such abnormalities in the rats. Further, even the acute administration was safe, and thereby providing a support to the application of PI in indigenous system of medicine. However, further long-term toxicological studies are needed in order to establish it as a drug.
2. Experimental
2.1. Plant material and purification of SAPI
S. aculeatissimum Jacq. fruits were obtained from Munnar hills of Western Ghats, Kerala. PI was isolated and purified from the fruits of S. aculeatissimum via four sequential step procedures, i.e. salt precipitation to Sepharose affinity chromatography (Meenu Krishnan & Murugan, Citation2015).
2.2. PI activity assay and SDS-PAGE
SAPI activity was determined by estimating the residual hydrolytic activity of trypsin and chymotrypsin towards the substrates BAPNA (N-benzoyl-L-arginine-p-nitroanilide) and BTPNA (N-benzoyl-L-tyrosyl-p-nitroanilide), respectively, at pH 8.0 after pre-incubation with inhibitor (Prasad et al. Citation2010). Molecular mass and purity of PI were evaluated by SDS-PAGE (Laemmli, Citation1970). The molecular mass was further confirmed by size elution chromatography using Sephacryl S-200 (Murugan, Citation2003).
2.3. Animals
Albino mice and Wistar rats of either sex were used for acute and subchronic studies. They were housed in a standard environmental condition and fed with rodent standard diets and water. Animal care and handling conformed to accepted guidelines of Organisation of Economic Co-operation and development (OECD, Citation2002). Ethical approval was obtained from the institutional Ethical Committee for Teaching and Research.
2.4. Acute oral toxicity test
The acute oral toxicity of the purified SAPI was evaluated in rats using the procedures described by Miller and Tainter (Citation1944). During acute toxicity studies, a total of 36 animals were divided into 6 dosage groups with 6 animals per dose. The control group was given normal diet. Gavage dosing was performed using a curved, ball-tipped incubation needle affixed to a 5-ml syringe. All solutions were prepared just prior to dosing and were kept chilled and tightly capped. Body weight, food and water consumption were monitored daily. Animals fast approximately 12 h prior to dosing. Following administration of a single dose of SAPI in the animals, they were observed for behavioural changes and general toxicity signs. Results were recorded for the first 30 min and at hourly intervals for the next 24 h, and thereafter for a total of 7 days. Body weight was recorded from Day 0 (before dosing) to Day 7.
2.5. Subchronic oral toxicity test
The dose LD50 was 500 mg/kg and due to the absence of mortality during acute study, a high dose of 1,000 mg/kg was selected for the subchronic toxicity (4 weeks) studies. Animals were divided into three groups of four animals each. Group 1 received normal saline and served as control. Groups 2 and 3 received SAPI doses of 500–1,000 mg/kg body wt, respectively. Mortality, body weight, food consumption as well as observation for general toxicity signs of the animals were evaluated daily for 30 days.
2.6. Haematological and biochemical parameters
Blood samples were collected through retro orbital plexus puncture into ethylene diamine tetra acetic acid (EDTA) tubes. The blood samples were analysed for red blood cells (RBC), white blood cells (WBC) and haemoglobin (Hb). Biochemical parameters studied include blood glucose level, urea, creatinine, triglycerides, cholesterol and total bilirubin, albumin, globulin, total protein, SGOT, SGPT and ALP.
2.7. Histopathology
The liver of all the animals was fixed in 10% buffered formalin in labelled bottles, and processed routinely for histological examination. Tissues embedded in paraffin wax were sectioned 5-μm thick, stained with haematoxylin and eosin, mounted on glass slides and then examined under a standard light microscope (Gupta, Bhalla, Khurana, & Singh, Citation2009).
3. Statistics
Statistical analysis was carried out using GraphPad Instat. Differences among the tested extract were analysed using one-way ANOVA. Values were expressed as mean ± SD and differences between groups were considered to be significant if p < 0.05.
4. Conclusion
In conclusion, PI from S. aculeatissimum may be considered safe with the oral doses tested since it did not cause any death or adverse behavioural changes in the acute toxicity assay on mice and also in chronic studies on rats. However, to confirm this hypothesis, clinical and hepatoprotective evaluation must be undertaken. Herbal plants would continue to provide new lead molecules for the development of drugs against various ailments. At the same time, there is need for more clinical research studies to support safety and efficacy of these herbal products.
Acknowledgement
We are grateful to NR Pillai, Department of Pharmacology & Toxicology, R&D Department, Pankajakasthuri Herbals India Pvt Ltd., Kattakada (Reg. No. PRC/Expt.10/2015-16 dated 04.07.15, Form No. 25/03/03-AWD, GOI) for providing the infrastructural facilities at the institute to fulfil this work. We also acknowledge Department of Science and Technology, Government of India for providing INSPIRE fellowship (IF130468) connected with the PhD work.
Additional information
Funding
Notes on contributors
V.G. Meenu Krishnan
V.G. Meenu Krishnan is an INSPIRE fellowship (IF130468) who secured her master’s degree in Botany (First Rank) from University of Kerala. She is presently doing her research in the topic of protease inhibitor from wild Solanum sps. under the guidance of K. Murugan.
K. Murugan
K Murugan is presently working as the principal, University College, Trivandrum, Kerala. He did his PhD in “Histomorphological and biochemical adaptations of Basin mangroves of Ayiramthengu” from the University of Kerala. His area of research includes Plant Biochemistry and Molecular Biology. Studies in the field of protease inhibitors, anthocyanin isolation and phytochemical evaluation and stress responses in lower plant groups are the major ongoing works under his guidance.
References
- Aneela, S., de, S., Lakshmi, K. K., Choudhury, N. S. K., Das, S. L., & Sagar, K. V. (2011). Acute Oral toxicity studies of pongamia pinnata and annona squamosa on albino wister rats. International Journal of Research in Pharmacy and Chemistry, 1, 820–824.
- Dragsted, L. O., Krath, B., Ravn-Haren, G., Vogel, U. B., Vinggaard, A. M., Jensen, P. B., ... Pedersen, A. (2006). Biological effects of fruit and vegetables. Proceedings of the Nutrition Society, 65, 61–67.10.1079/PNS2005480
- Grover, J. K., & Yadav, S. P. (2004). Pharmacological actions and potential uses of Momordica charantia. A review. Journal of Ethnopharmacology, 93, 123–132.10.1016/j.jep.2004.03.035
- Gupta, E., Bhalla, P., Khurana, N., & Singh, T. (2009). Histopathology for the diagnosis of infectious diseases. Indian Journal of Medical Microbiology, 27, 100–106.10.4103/0255-0857.49423
- Hamid, R., Jaouad, E. H., Zafar, H. I., & Lyoussia, B. (2008). Acute and subchronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents. Journal of Ethnopharmacology, 118, 378–386.
- Ikebe, N., Akaike, T., Miyamoto, Y., Kazuyuki, H., Jun, Y., Ogawa, M., & Maeda, H. (2000). Protective effect of S-Nitrosylated a1-protease inhibitor on hepatic ischemia-reperfusion injury. The Journal of Pharmacology and Experimental Therapeutics, 295, 904–912.
- Indhumathi, T., Mohandas, S., & Shibi, A. (2014). Acute toxicity study of ethanolic extract of Solanum incanum. L fruit. Asian Journal of Pharmaceutical and Clinical Research, 7, 98–100.
- Isnard Bagnis, C., Deray, G., Baumelou, A., Le Quintrec, M., & Vanherweghem, J. L. (2004). Herbs and the kidney. American Journal of Kidney Diseases, 44, 1–11.10.1053/j.ajkd.2004.02.009
- Jebasingh, D., Jackson, D. D., Venkataraman, S., Emerald, B. S. (2012). Physiochemical and toxicological studies of the medicinal plant Cyperus rotundus L. (Cyperaceae). International Journal of Applied Research in Natural Product, 5, 1–8.
- Kaneko, J. J., Harvey, J. W., & Bruss, M. L. (1997). Clinical biochemistry of domestic animals (5th ed. p. 932). San Diego, CA: Academic Press.
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.10.1038/227680a0
- Marwa, M. A. E., Ghada, Z. A., Abd-El Mobd’ea, E., & Rehim, A. E. (2013). Effect of Solanum nigrum Linn against lambda cyhalothrin induced toxicity in rats. International Journal of Pharmacy and Biological Sciences, 5, 55–62.
- Meenu Krishnan, V. G., & Murugan, K. (2015). Purification, characterization and kinetics of protease inhibitor from fruits of Solanum aculeatissimum Jacq. Food Science and Human Wellness, 4, 97–107.10.1016/j.fshw.2015.06.003
- Miller, L. C., & Tainter, M. L. (1944). Estimation of the ED50 and its error by means of logarithmic-probit graph paper. Experimental Biology and Medicine, 57, 261–264.10.3181/00379727-57-14776
- Mohd Fuat, A. R., & Aidoo, K. E. (2011). Toxicity studies of Eurycoma longifolia (jack) based remedial products Asian. Asian Journal of Pharmaceutical and Clinical Research, 4, 23–27.
- Murugan, K. (2003). Histomorphological and biochemical adaptations of basin mangroves with reference to ayiremthengu flora (Thesis). University of Kerala, Thiruvananthapuram.
- OECD. (2002). OECD guidelines for the testing of chemicals/Section 4: Health effects test No. 423: Acute oral toxicity—Acute toxic class method. Paris: Organization For Economic Cooperation And Development.
- Onyeyilli, P. A., Iwuoha, C. L., & Akinniyi, J. A. (1998). Chronic toxicity study of Fiscus platyphylla blume in rats. West African Journal of Pharmacology and Drug Research, 14, 27–30.
- Palani, V., Senthilkumaran, R. K., & Govindasamy, S. (1999). Biochemical evaluation of antitumor effect of Muthu Marunthu (a herbal formulation) on experimental fibrosarcoma in rats. Journal of Ethnopharmacology, 65, 257–265.10.1016/S0378-8741(98)00159-7
- Patel, K., Singh, R. B., & Patel, D. K. (2013). Medicinal significance, pharmacological activities, and analytical aspects of solasodine: A concise report of current scientific literature. Journal of Acute Disease, 2, 92–98.10.1016/S2221-6189(13)60106-7
- Phani, K., & Ravi, K. A. (2015). Toxicity studies of combined extracts of Vitex leucoxylon, Vitex negundo and Vitex trifolia. Journal of Chemical and Pharmaceutical Sciences, 7, 54–58.
- Prasad, E. R., Dutta-Gupta, A., & Padmasree, K. (2010). Purification and characterization of a Bowman-Birk proteinase inhibitor from the seeds of black gram (Vigna mungo). Phytochemistry, 71, 363–372.
- Raza, M., Al-Shabanah, O. A., El-Hadiyah, T. M., & Al-Majed, A. A. (2002). Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Scientia Pharmaceutica, 70, 135–145.
- Ruby, K. K., Raj Kapoor, B., & Mohammad, A. (2011). Acute and sub acute toxicity of methanol extract of Elytraria acaulis Lindau. in rats. Pharmacologyonline , 3, 229–242.
- Santosh, K., & Richard, K. (2005). Antinutritional factors in food legumes and effects of processing. The Role of Food, Agriculture, Forestry and Fisheries in Human Nutrition, 4, 35–40.
- Sathya, M., Kokilavani, R., & Ananta, T. K. (2012). Acute and subacute toxicity studies of ethanolic extract of Acalypha Indica Linn in male Wistar rats. Asian Journal of Pharmaceutical and Clinical Research, 5, 97–100.
- Sini, K. R., Sinha, B. N., & Rajasekaran, A. (2010). Acute toxicity studies of aqueous leaf extract of Capparis grandiflora Wall Ex Hook. f & Thomson. Journal of Chemical and Pharmaceutical Research, 2, 112–117.
- Stedman, C. (2002). Herbal hepatotoxicity. Seminars in Liver Disease, 22, 195–206.10.1055/s-2002-30104
- Teo, S., Stirling, D., Thomas, S., Hoberman, A., Kiorpes, A., & Khetani, V. (2002). A 90-day oral gavage toxicity study of d-methylphenidate and d,l-methylphenidate in Sprague–Dawley rats. Toxicology, 179, 183–196.10.1016/S0300-483X(02)00338-4
- Teschke, R., Gaus, W., & Loew, D. (2003). Kava extracts: Safety and risks including rare hepatotoxicity. Phytomedicine, 10, 440–446.10.1078/0944-7113-00314
- Turk, B. (2006). Targeting proteases: Successes, failures and future prospects. Nature Reviews: Drug Discovery, 5, 785–799.
- Ukwuani, A. N., Abubakar, M. G., Hassan, S. W., & Agaie, B. M. (2012). Toxicological studies of hydromethanolic leaves extract of Grewia crenata. International Journal of Pharmaceutical Sciences and Drug Research, 4, 245–249.
- Yan, B. Z., Chen, L. Y., Kang, L., Wang, X. R., Bi, M. R., Wang, W., & Yang, B. S. (2013). Hepatoprotective effects of cathepsin B inhibitor on acute hepatic failure induced by lipopolysaccharide/D-galactosamine in mice. Hepatobiliary & Pancreatic Diseases International, 12, 81–86.