Abstract
Glucagon-like peptide-1 (GLP-1) within the brain is a potent regulator of food intake and most studies have investigated the anorexic effects of central GLP-1. A range of brain regions have now been found to be involved in GLP-1 mediated anorexia, including some which are not traditionally associated with appetite regulation. However, a change in food intake can be indicative of not only reduced energy demand, but also changes in the organism’s motivation to eat following stressful stimuli. In fact, acute stress is well-known to reduce food intake. Recently, more research has focused on the role of GLP-1 in stress and the central GLP-1 system has been found to be activated in response to stressful stimuli. The source of GLP-1 within the brain, the preproglucagon (PPG) neurons, are ideally situated in the brainstem to receive and relay signals of stress and our recent data on the projection pattern of the PPG neurons to the spinal cord suggest a potential strong link with the sympathetic nervous system. We review here the role of central GLP-1 in the regulation of stress responses and discuss the potential involvement of the endogenous source of GLP-1 within the brain, the PPG neurons.
Public Interest Statement
Glucagon-like peptide-1 analogues are arguably the most promising new drugs in the fight against diabetes and obesity. In order to fully understand the therapeutic potential as well as the associated risks for these drugs, a thorough understanding of the effects of GLP-1 in the brain has to be obtained. This review focuses on one important aspect, the potential role of central GLP-1 in stress responses.
Competing Interests
The authors declare no competing interest.
1. GLP-1 is a regulator of homeostasis
Glucagon-like peptide-1 (GLP-1) is an incretin and neuropeptide best known for its role in glucose homeostasis and appetite regulation (Holst, Citation2007; Kreymann, Ghatei, Williams, & Bloom, Citation1987; Tang-Christensen et al., Citation1996; Turton et al., Citation1996; Wang et al., Citation1995). In the periphery, GLP-1 is released from L cells in the gut following ingestion of food (Vilsbøll et al., Citation2003). From the blood it reaches the pancreas where it acts on β-cells to enhance the secretion of insulin in response to glucose while inhibiting the release of glucagon from α-cells (de Heer, Rasmussen, Coy, & Holst, Citation2008; Holst, Citation2007; Ørskov, Holst, & Nielsen, Citation1988; Vilsbøll, Krarup, Madsbad, & Holst, Citation2003). In addition, peripheral GLP-1 has proliferative and protective effects on islet cells and inhibits gastric emptying (Egan, Bulotta, Hui, & Perfetti, Citation2003; Farilla et al., Citation2002; Nauck et al., Citation1997). Central GLP-1, here defined as GLP-1 acting within the central nervous system (CNS), is well-established as a potent regulator of food intake (Barrera et al., Citation2011; Larsen, Tang-Christensen, & Jessop, Citation1997; Turton et al., Citation1996; Williams, Baskin, & Schwartz, Citation2009). Within the brain, GLP-1 is produced in a subset of granule cells or short axon cells of the olfactory bulb, some pyramidal cells of the piriform cortex and a few neurons in the lumbar-sacral spinal cord (Larsen, Tang-Christensen, Holst, & Ørskov, Citation1997; Merchenthaler, Lane, & Shughrue, Citation1999; Thiebaud et al., Citation2016; Zheng, Cai, & Rinaman, Citation2015). However, the primary source of GLP-1 in the brain is in preproglucagon (PPG) neurons in the nucleus tractus solitarii (NTS) and the intermediate reticular nucleus in the lower brainstem (Merchenthaler et al., Citation1999). PPG neurons, also referred to as GLP-1 neurons, particularly in studies on rat, project throughout the brain to autonomic control centres and this projection pattern largely matches the expression of GLP-1 receptors in the brain (Larsen, Tang-Christensen, Holst, et al., Citation1997; Llewellyn-Smith, Gnanamanickam, Reimann, Gribble, & Trapp, Citation2013; Llewellyn-Smith, Reimann, Gribble, & Trapp, Citation2011; Merchenthaler et al., Citation1999; Trapp & Cork, Citation2015; Vrang, Hansen, Larsen, & Tang-Christensen, Citation2007). PPG neurons are activated in vitro and in vivo by a range of satiety hormones and peripheral signals relating to food intake and general homeostasis (Hisadome, Reimann, Gribble, & Trapp, Citation2010, Citation2011; Merchenthaler et al., Citation1999; Rinaman, Citation1999b; Trapp & Richards, Citation2013).
It is clear that central GLP-1 reduces food intake. What is less clear is the physiological purpose and cause of this anorexic response. Importantly, a reduction in food intake can be a response to not only decreased energy demand, but also to changes in the emotional state that reduces the motivation to eat, or to visceral malaise leading to reduced appetite. Early studies addressed the role of taste aversion and nausea in the regulation of food intake. NTS GLP-1 neurons were activated by intraperitoneal injection of LiCl, a compound which is well known to cause malaise and taste aversion (Rinaman, Citation1999b). Furthermore, blockade of the GLP-1 receptor using the antagonist Exendin (9-39) reversed the LiCl-induced suppression of appetite in rat, suggesting a role for central GLP-1 in the response to malaise (Rinaman, Citation1999a). However, these results could not be reproduced in mouse (Lachey et al., Citation2005), suggesting that subtle, but important species differences may exist in the central GLP-1 system. In another early attempt to anatomically dissect different GLP-1 actions in the brain, van Dijk and Thiele (Citation1999) demonstrated that bilateral lesions in the PVN prevented the induction of satiety by GLP-1, but not the conditioned taste aversion observed with GLP-1. They also showed that lesions in the amygdala prevented GLP-1 induced taste aversion, but rats retained the GLP-1 induced reduction in food intake. These experiments clearly demonstrated the existence of two separate pathways for GLP-1 effects on satiety and malaise. As further evidence for a role of GLP-1 in the response to general malaise, there is now data supporting a link between inflammation and GLP-1 mediated reduction in food intake and the cytokine interleukin-6 (IL-6) has been shown to activate PPG neurons in the NTS (Anesten et al., Citation2016; Shirazi et al., Citation2013). More recent studies have revealed that GLP-1 receptor signalling in the mesolimbic system affects food intake by modulating reward pathways (Dickson et al., Citation2012; Mietlicki-Baase et al., Citation2013, Citation2014) and that activation of GLP-1 receptors in the hippocampus, a region not traditionally associated with appetite regulation, reduces food intake (During et al., Citation2003; Hsu, Hahn, Konanur, Lam, & Kanoski, Citation2015).
From all these emerging targets it is becoming evident that GLP-1 in the brain does not simply inhibit metabolically driven food intake, but that the reduced appetite may be part of a wider response to emotional stress or visceral malaise (Ghosal, Myers, & Herman, Citation2013; Kinzig et al., Citation2003; Kreisler & Rinaman, Citation2016; Maniscalco, Kreisler, & Rinaman, Citation2012; Maniscalco, Zheng, Gordon, & Rinaman, Citation2015; Rinaman, Citation1999b). In this review we discuss the evidence for the involvement of central GLP-1 in the regulation of stress and consider the potential role of the central source of GLP-1, the PPG neurons. Most evidence has been gathered in mouse and rat and since few anatomical and functional differences have been observed between species (including human and non-human primates), we assume here that most findings are relevant across species (Vrang & Grove, Citation2011; Zheng et al., Citation2015), though any paradigms where conflicting evidence exists between species, will be highlighted. We begin by briefly describing the organism’s response to stress. In the next section, we review the evidence for a role for GLP-1 in the regulation of stress responses and finally we discuss the potential role of the central source of GLP-1, the PPG neurons, in the stress response.
2. Stress activates two parallel coping systems, the hypothalamic-pituitary axis and the sympathetic nervous system
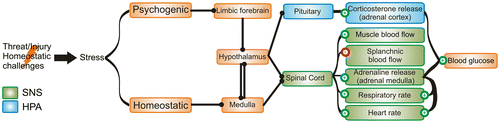
Stress is defined as the collection of physiological responses to homeostatic (physical) and psychological (perceived) challenges (Dayas, Buller, Crane, Xu, & Day, Citation2001; Sawchenko, Li, & Ericsson, Citation2000; Ulrich-Lai & Herman, Citation2009). Stress allows the organism to cope with aversive stimuli and appropriate stress responses are essential to the survival of the organism. On the other hand, inappropriate chronic stress responses can lead to long-term damaging disorders such as anxiety and depression. Neural control of stress responses is complex and involves forebrain, brainstem and spinal cord circuits that ultimately converge to activate two important effectors in the body’s response to stress: the sympathetic part of the autonomic nervous system (SNS) and the hypothalamus-pituitary-adrenal (HPA) axis (Ulrich-Lai & Herman, Citation2009) producing the so-called “fight-or-flight” response (Figure ). A perceived threat to homeostasis and the well-being of the organism leads to rapid activation of the SNS. Heart and blood pressure are increased via recruitment of catecholaminergic neurons in the rostral ventrolateral medulla (RVLM), the raphe pallidus and sympathetic preganglionic neurons in the spinal cord as well as higher order autonomic control sites in the hypothalamus and amygdala (Dampney, Citation1994; Dayas et al., Citation2001; Dimicco & Zaretsky, Citation2007). Increased sympathetic activity leads to release of adrenaline from the adrenal medulla and adrenaline in turn increases cardiac output and respiratory rate while redirecting blood flow to skeletal muscle and mobilising glucose from liver and skeletal muscle (Ulrich-Lai & Herman, Citation2009). The parallel recruitment of the HPA axis involves the activation of parvocellular neurons in the paraventricular nucleus of the hypothalamus (PVN). These neurons release corticotrophin-releasing hormone (CRH) onto adrenocorticotropic hormone (ACTH) expressing neurons in the anterior pituitary. From the pituitary, ACTH is released into the bloodstream through which it reaches the cortex of the adrenal gland. In the adrenal cortex ACTH elicits release of corticosterone, which works to mobilise glucose by stimulating gluconeogenesis securing the body’s demand for glucose during homeostatic challenges (Ulrich-Lai & Herman, Citation2009). Corticosterone provides negative feedback at the level of the pituitary and the hypothalamus to limit HPA activity (Ulrich-Lai & Herman, Citation2009).
Figure 1. Stressors activate forebrain and brainstem regions to prepare for “fight-or-flight”.
3. GLP-1 activates both the HPA axis and the sympathetic nervous system
The majority of evidence for a role of GLP-1 in stress has been gathered using supraphysiological doses of GLP-1 or GLP-1 analogues (typically Exendin-4) to activate GLP-1 receptors both peripherally and centrally. These studies have shown that recruitment of central GLP-1 receptors potently activates the HPA axis in both humans and rodents with a resulting increase in both ACTH and corticosterone/cortisol concentrations in blood (Gil-Lozano et al., Citation2010; Kinzig et al., Citation2003). The adrenal cortex does not express GLP-1 receptors and isolated cells from the adrenal glands do not release corticosterone in response to GLP-1 ruling out the possibility that GLP-1 acts directly on the adrenal cortex (Dunphy, Taylor, & Fuller, Citation1998; Gil-Lozano et al., Citation2014). Importantly, central administration of Exendin-4 leads to an increase in corticosterone in rodents, suggesting an involvement of central GLP-1 receptors possibly expressed on CRH expressing neurons in the hypothalamus (Gil-Lozano et al., Citation2014; Larsen, Tang-Christensen, & Jessop, Citation1997; Sarkar, Fekete, Légrádi, & Lechan, Citation2003). In fact, central blockade of CRH receptors blocks Exendin-4-induced increases in ACTH and corticosterone, establishing a role for central GLP-1 receptors in HPA axis regulation (Gil-Lozano et al., Citation2014).
In a study investigating the involvement of central GLP-1 in both homeostatic and psychogenic stress, Kinzig et al. (Citation2003) found that injections of GLP-1 into the PVN increased blood ACTH and corticosterone concentrations. Targeting the amygdala increased anxiety-like behaviour with animals spending significantly less time in the open arms of an elevated plus maze after infusion of GLP-1. Different types of stressors, i.e. homeostatic vs. psychogenic, are known to activate distinct neural pathways and the data described above suggests that GLP-1 regulates both homeostatic and psychogenic stress responses through distinct neural pathways (Dayas et al., Citation2001; Kinzig et al., Citation2003).
Intriguingly, activation of central GLP-1 receptors not only stimulates the HPA axis, but also appears to increase sympathetic activity, the other important pathway for the physiological response to stress (Smits et al., Citation2016; Yamamoto et al., Citation2002). This is measured as an increase in heart rate in both rodents and humans following GLP-1 receptor activation (Gil-Lozano et al., Citation2014; Griffioen et al., Citation2011; Robinson, Holt, Rees, Randeva, & O’Hare, Citation2013; Smits et al., Citation2016; Yamamoto et al., Citation2002). Central GLP-1 receptor activation was found to stimulate both autonomic regulatory neurons, neurons in the spinal cord and cells in the adrenal medulla signifying clear recruitment of the sympathetic nervous system (Yamamoto et al., Citation2002). This suggests that central GLP-1 could activate both arms of the stress response, the HPA axis and the sympathetic nervous system.
4. NTS GLP-1 neurons are ideally situated to integrate signals of stress
Studies using supraphysiological activation of GLP-1 receptors provide little information about the physiological role of the endogenous GLP-1 system in stress regulation. Exogenous activation of central GLP-1 receptors clearly triggers release of ACTH and corticosterone, but the question remains, whether there is an endogenous source of GLP-1 eliciting these responses under physiological conditions? Further, which signals trigger release of GLP-1 from that source? GLP-1 released from L cells in the gut is rapidly degraded in the liver and bloodstream making it unlikely that GLP-1 reaches receptors in the brain in large quantities (Deacon, Citation2004; Hansen, Deacon, Ørskov, & Holst, Citation1999; Holst & Deacon, Citation2005; Kieffer, McIntosh, & Pederson, Citation1995; Vilsboll, Krarup, Deacon, Madsbad, & Holst, Citation2001). In contrast, the central source of GLP-1, the PPG neurons, are ideally situated in the NTS in the caudal brainstem to receive and process signals of stress from the rest of the body (Kreisler & Rinaman, Citation2016; Maniscalco et al., Citation2012; Merchenthaler et al., Citation1999; Rinaman, Citation1999b; Vrang, Phifer, Corkern, & Berthoud, Citation2003). The NTS is a well-established central site of integration of visceral afferent signals concerning general homeostasis, which are relayed to higher brain centres (Grill & Hayes, Citation2012). From the NTS, PPG neurons project to autonomic control sites throughout the brain including the PVN, the dorsomedial hypothalamus and the RVLM, which are all involved in the control of the HPA axis and/or sympathetic activity (Larsen, Tang-Christensen, Holst, et al., Citation1997; Llewellyn-Smith et al., Citation2011, Citation2013; Vrang et al., Citation2007). In the PVN, there is dense expression of GLP-1 receptors and GLP-1 immunoreactive axons make contact with parvocellular CRH producing neurons, supporting a role for PPG neurons in the regulation of CRH secretion from the hypothalamus (Cork et al., Citation2015; Larsen, Tang-Christensen, Holst, et al., Citation1997; Sarkar et al., Citation2003).
NTS neurons are generally thought to regulate sympathetic activity indirectly through ascending projections to either the RVLM or hypothalamic nuclei. Recent findings in our laboratory have demonstrated that PPG neurons in the brainstem not only send ascending projections to autonomic control sites mainly in the hypothalamus, but also directly innervate sympathetic preganglionic neurons in the spinal cord (Llewellyn-Smith et al., Citation2015). These data highlight the possibility that the central GLP-1 system may integrate incoming stress signals and relay them via both ascending projections to CRH neurons in the PVN to elicit HPA activity and descending projections to preganglionic sympathetic neurons in the intermediolateral column (IML) and central autonomic area (CAA) of the spinal cord to increase sympathetic outflow.
In a thorough dissection of HPA-GLP-1 crosstalk, Lee et al. (Citation2016) explored the neural pathways underlying GLP-1 receptor initiated increases in corticosterone in rat. Systemic (intraperitoneal; i.p.) exendin-4 activated catecholaminergic (CA), non-PPG neurons in the NTS and RVLM. Most of these neurons were found to project to the parvocellular and magnocellular PVN. Selective ablation of this CA-PVN connection using DBH-saporin prevented the i.p. exendin-4 induced increase in blood concentrations of corticosterone. This demonstrated that activation of the HPA axis by systemic exendin-4 is dependent on CA input to the PVN. On first sight this seems surprising given that PPG neurons project heavily to the PVN and make contacts to CRH neurons as discussed above. However, keeping in mind that PPG neurons do not express GLP-1 receptors, these findings might just indicate that the peripheral and central GLP-1 systems are more separate than widely thought. It also emphasises that still more studies are needed that explore which exact peripheral signals activate PPG neurons and which do not.
5. The central GLP-1 system is activated in response to both homeostatic and psychogenic stress
An early study suggesting a link between central GLP-1 and stress was conducted by Rinaman (Citation1999b). Interoceptive stress was induced through intraperitoneal injection of LiCl. LiCl is considered a nauseogenic agent and is known to reduce food intake while increasing the concentration of stress hormones ACTH and corticosterone in the blood (Kinzig, Hargrave, & Honors, Citation2008). LiCl was found to activate GLP-1 neurons in the NTS which were found to project to the HPA-regulating parvocellular region of the PVN (Rinaman, Citation1999b). Kinzig et al. later demonstrated that the LiCl-induced increase in stress hormones is dependent on central GLP-1 signalling (Kinzig et al., Citation2003). Third ventricular infusion of a GLP-1 receptor antagonist abolished the increase in both ACTH and corticosterone following systemic LiCl injections. The discovery of close appositions from GLP-1 immunoreactive axon terminals on CRH producing PVN neurons in rat further substantiate these findings (Tauchi, Zhang, D’Alessio, Stern, & Herman, Citation2008). Similarly, axons of mouse PPG neurons have close appositions on CRH producing PVN neurons (personal communication, Ida Llewellyn-Smith). These data suggest that homeostatic stress following a toxic challenge activates the central GLP-1 system to recruit systemic stress pathways.
Similarly, acute psychogenic stress induced by physical restraint reduces food intake and activates the HPA axis (Kinzig et al., Citation2008). Maniscalco et al. recently demonstrated that the number of cFOS positive GLP-1 neurons increases following 30mins restraint stress or 5mins exposure on an elevated platform, suggesting that GLP-1 neurons are activated by psychogenic stress (Maniscalco et al., Citation2015). Furthermore, they found that 30 mins restraint stress reduced food intake at the onset of dark phase and that this hypophagic response was blocked by central infusion of GLP-1 receptor antagonist, suggesting that psychogenic stress recruits the central GLP-1 system to reduce food intake (Maniscalco et al., Citation2015).
Finally, in a study focusing on the role of GLP-1 in cocaine addiction, GLP-1 neurons were found to be activated by an injection of corticosterone into the fourth ventricle (Schmidt et al., Citation2016). Fourth ventricle corticosterone reduced cocaine self-administration and this reduction was blocked by GLP-1 receptor antagonism in the ventral tegmental area (Schmidt et al., Citation2016). These data suggest that not only does central GLP-1 activate the HPA axis, but corticosterone in turn activates the central GLP-1 system.
6. Conclusions
It is clear that the central GLP-1 system plays a role in the regulation of food intake. However, increasing numbers of studies report effects of GLP-1 in brain regions not classically associated with appetite control and it is becoming increasingly clear that central GLP-1 may be responsible for much wider homeostatic control. In particular, the anorexic effects of GLP-1 may in some cases be secondary to responses to homeostatic and psychogenic stress.
We have discussed here evidence for a role of central GLP-1 in the regulation of the body’s stress response. It is clear that overactivation of brain GLP-1 receptors enhances secretion of stress hormones and activity of the SNS. In contrast, the role of the central source of GLP-1, the PPG neurons, is less explored and the neural pathways underlying the GLP-1 mediated modulation of stress are largely unknown. The evidence discussed here suggests a model in which peripheral signals of homeostatic and psychogenic stress activate PPG neurons (Figure ). The PPG neurons are ideally situated in the NTS to integrate signals of stress and relay that signal on to parvocellular neurons in the PVN. Furthermore, in this model psychogenic or homeostatic stress would lead to release of GLP-1 from PPG neurons. Downstream activation of GLP-1 receptors then increases sympathetic activity via direct and indirect pathways. Directly, PPG neurons modulate the activity of sympathetic preganglionic neurons in the spinal cord. Indirectly, GLP-1 from PPG neurons activates presympathetic RVLM and PVN neurons which in turn project to spinal sympathetic preganglionic neurons.
Figure 2. PPG pathways to activate both HPA axis and sympathetic nervous system in the control of stress responses.
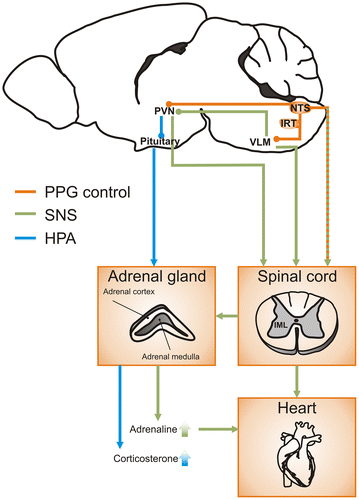
The evidence discussed here suggests that central GLP-1 does not simply regulate food intake in response to changes in energy demand, but that the PPG neurons are activated by stress and that central GLP-1 modulates acute stress, homeostatic or psychogenic, by increasing corticosterone, mobilising glucose and increasing heart rate, allowing the organism to cope with potential threats.
Additional information
Funding
Notes on contributors
Marie K. Holt
Marie K Holt is pursuing her PhD in Stefan Trapp’s group at University College London, UK. The focus of her research is autonomic and metabolic neuroscience with a special interest in the brain GLP-1 system. Her research aims to further our understanding of the physiological role of GLP-1 in the brain and in particular the source of GLP-1 in the brain, the preproglucagon (PPG) neurons. The main focus is on the role of the PPG neurons in both food intake and cardiovascular control. These studies are performed both in vitro and in vivo on a cellular level and in the context of the whole animal using a combination of viral gene transfer and transgenic mouse strains.
References
- Anesten, F., Holt, M. K., Schéle, E., Pálsdóttir, V., Reimann, F., Gribble, F. M., … Jansson, J. O. (2016). Preproglucagon neurons in the hindbrain have IL-6 receptor-α and show Ca 2+ influx in response to IL-6. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 311, R115–R123.10.1152/ajpregu.00383.2015
- Barrera, J. G., Jones, K. R., Herman, J. P., D’Alessio, D. A., Woods, S. C., & Seeley, R. J. (2011). Hyperphagia and increased fat accumulation in two models of chronic cns glucagon-like peptide-1 loss of function. Journal of Neuroscience, 31, 3904–3913.10.1523/JNEUROSCI.2212-10.2011
- Cork, S. C., Richards, J. E., Holt, M. K., Gribble, F. M., Reimann, F., & Trapp, S. (2015). Distribution and characterisation of glucagon-like peptide-1 receptor expressing cells in the mouse brain. Molecular Metabolism, 4, 718–731.10.1016/j.molmet.2015.07.008
- Dampney, R. A. (1994). Functional organization of central pathways regulating the cardiovascular system. Physiological Reviews, 74, 323–364.
- Dayas, C. V., Buller, K. M., Crane, J. W., Xu, Y., & Day, T. A. (2001). Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. European Journal of Neuroscience, 14, 1143–1152.10.1046/j.0953-816x.2001.01733.x
- Deacon, C. F. (2004). Circulation and degradation of GIP and GLP-1. Hormone and Metabolic Research, 36, 761–765.10.1055/s-2004-826160
- de Heer, J., Rasmussen, C., Coy, D. H., & Holst, J. J. (2008). Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia, 51, 2263–2270.10.1007/s00125-008-1149-y
- Dickson, S. L., Shirazi, R. H., Hansson, C., Bergquist, F., Nissbrandt, H., & Skibicka, K. P. (2012). The glucagon-like peptide 1 (glp-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic glp-1 receptors. Journal of Neuroscience, 32, 4812–4820.10.1523/JNEUROSCI.6326-11.2012
- Dimicco, J. A., & Zaretsky, D. V. (2007). The dorsomedial hypothalamus: A new player in thermoregulation. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 292, R47–63.
- Dunphy, J. L., Taylor, R. G., & Fuller, P. J. (1998). Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Molecular and Cellular Endocrinology, 141, 179–186.10.1016/S0303-7207(98)00096-3
- During, M. J., Cao, L., Zuzga, D. S., Francis, J. S., Fitzsimons, H. L., Jiao, X., … Haile, C. N. (2003). Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nature Medicine, 9, 1173–1179.10.1038/nm919
- Egan, J. M., Bulotta, A., Hui, H., & Perfetti, R. (2003). GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes/Metabolism Research and Reviews, 19, 115–123.10.1002/(ISSN)1520-7560
- Farilla, L., Hui, H., Bertolotto, C., Kang, E., Bulotta, A., Di Mario, U., & Perfetti, R. (2002). Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in zucker diabetic rats. Endocrinology, 143, 4397–4408.10.1210/en.2002-220405
- Ghosal, S., Myers, B., & Herman, J. P. (2013). Role of central glucagon-like peptide-1 in stress regulation. Physiology & Behavior, 122, 201–207.10.1016/j.physbeh.2013.04.003
- Gil-Lozano, M., Pérez-Tilve, D., Alvarez-Crespo, M., Martís, A., Fernandez, A. M., Catalina, P. A. F., … Mallo, F. (2010). GLP-1(7-36)-amide and exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology, 151, 2629–2640.10.1210/en.2009-0915
- Gil-Lozano, M., Romaní-Pérez, M., Outeiriño-Iglesias, V., Vigo, E., González-Matías, L. C., Brubaker, P. L., & Mallo, F. (2014). Corticotropin-releasing hormone and the sympathoadrenal system are major mediators in the effects of peripherally administered exendin-4 on the hypothalamic-pituitary-adrenal axis of male rats. Endocrinology, 155, 2511–2523.10.1210/en.2013-1718
- Griffioen, K. J., Wan, R., Okun, E., Wang, X., Lovett-Barr, M. R., Li, Y., … Mattson, M. P. (2011). GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovascular Research, 89, 72–78.10.1093/cvr/cvq271
- Grill, H. J., & Hayes, M. R. (2012). Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metabolism, 16, 296–309.10.1016/j.cmet.2012.06.015
- Hansen, L., Deacon, C. F., Ørskov, C., & Holst, J. J. (1999). Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology, 140, 5356–5363.
- Hisadome, K., Reimann, F., Gribble, F. M., & Trapp, S. (2010). Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: Electrical properties of glucagon-like peptide 1 neurons. Diabetes, 59, 1890–1898.10.2337/db10-0128
- Hisadome, K., Reimann, F., Gribble, F. M., & Trapp, S. (2011). CCK stimulation of GLP-1 neurons involves α1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes, 60, 2701–2709.10.2337/db11-0489
- Holst, J. J. (2007). The physiology of glucagon-like peptide 1. Physiological Reviews, 87, 1409–1439.10.1152/physrev.00034.2006
- Holst, J. J., & Deacon, C. F. (2005). Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia, 48, 612–615.10.1007/s00125-005-1705-7
- Hsu, T. M., Hahn, J. D., Konanur, V. R., Lam, A., & Kanoski, S. E. (2015). Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology, 40, 327–337.10.1038/npp.2014.175
- Kieffer, T. J., McIntosh, C. H., & Pederson, R. A. (1995). Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology, 136, 3585–3596.
- Kinzig, K. P., D’Alessio, D. A., Herman, J. P., Sakai, R. R., Vahl, T. P., Figueiredo, H. F., … Seeley, R. J. (2003). CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. The Journal of Neuroscience, 23, 6163–6170.
- Kinzig, K. P., Hargrave, S. L., & Honors, M. A. (2008). Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiology & Behavior, 95, 108–113.10.1016/j.physbeh.2008.04.026
- Kreisler, A. D., & Rinaman, L. (2016). Hindbrain glucagon-like peptide-1 neurons track intake volume and contribute to injection stress-induced hypophagia in meal-entrained rats. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 310, R906–R916.10.1152/ajpregu.00243.2015
- Kreymann, B., Ghatei, M.A., Williams, G., & Bloom, S. R. (1987). Glucagon-like peptide-1 7-36: A physiological incretin in man. The Lancet, 330, 1300–1304.10.1016/S0140-6736(87)91194-9
- Lachey, J. L., D’Alessio, D. A., Rinaman, L., Elmquist, J. K., Drucker, D. J., & Seeley, R. J. (2005). The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: Differential effects in rats and mice. Endocrinology, 146, 458–462.10.1210/en.2004-0419
- Larsen, P. J., Tang-Christensen, M., Holst, J. J., & Ørskov, C. (1997). Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience, 77, 257–270.10.1016/S0306-4522(96)00434-4
- Larsen, P. J., Tang-Christensen, M., & Jessop, D. S. (1997). Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology, 138, 4445–4455.
- Lee, S. J., Diener, K., Kaufman, S., Krieger, J.-P., Pettersen, K. G., Jejelava, N., … Langhans, W. (2016). Limiting glucocorticoid secretion increases the anorexigenic property of Exendin-4. Molecular Metabolism, 5, 552–565.10.1016/j.molmet.2016.04.008
- Llewellyn-Smith, I. J., Gnanamanickam, G. J., Reimann, F., Gribble, F. M., & Trapp, S. (2013). Preproglucagon (PPG) neurons innervate neurochemicallyidentified autonomic neurons in the mouse brainstem. Neuroscience, 229, 130–143.10.1016/j.neuroscience.2012.09.071
- Llewellyn-Smith, I. J., Marina, N., Manton, R. N., Reimann, F., Gribble, F. M., & Trapp, S. (2015). Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons. Neuroscience, 284, 872–887.10.1016/j.neuroscience.2014.10.043
- Llewellyn-Smith, I. J., Reimann, F., Gribble, F. M., & Trapp, S. (2011). Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience, 180, 111–121.10.1016/j.neuroscience.2011.02.023
- Maniscalco, J. W., Kreisler, A. D., & Rinaman, L. (2012). Satiation and stress-induced hypophagia: Examining the role of hindbrain neurons expressing prolactin-releasing peptide or glucagon-like peptide 1. Frontiers in Neuroscience, 6, 199.
- Maniscalco, J. W., Zheng, H., Gordon, P. J., & Rinaman, L. (2015). Negative energy balance blocks neural and behavioral responses to acute stress by "silencing" central glucagon-like peptide 1 signaling in rats. Journal of Neuroscience, 35, 10701–10714.10.1523/JNEUROSCI.3464-14.2015
- Merchenthaler, I., Lane, M., & Shughrue, P. (1999). Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of Comparative Neurology, 403, 261–280.10.1002/(ISSN)1096-9861
- Mietlicki-Baase, E. G., Ortinski, P. I., Reiner, D. J., Sinon, C. G., McCutcheon, J. E., Pierce, R. C., … Hayes, M. R. (2014). Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic ampa/kainate signaling. Journal of Neuroscience, 34, 6985–6992.10.1523/JNEUROSCI.0115-14.2014
- Mietlicki-Baase, E. G., Ortinski, P. I., Rupprecht, L. E., Olivos, D. R., Alhadeff, A. L., Pierce, R. C., & Hayes, M. R. (2013). The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. AJP: Endocrinology and Metabolism, 305, E1367–E1374.10.1152/ajpendo.00413.2013
- Nauck, M. A., Niedereichholz, U., Ettler, R., Holst, J. J., Ørskov, C., Ritzel, R., & Schmiegel, W. H. (1997). Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. American Journal of Physiology, 273, E981–988.
- Ørskov, C., Holst, J. J., & Nielsen, O. V. (1988). Effect of truncated glucagon-like peptide-1 [proglucagon-(78–107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology, 123, 2009–2013.10.1210/endo-123-4-2009
- Rinaman, L. (1999a). A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. American Journal of Physiology, 277, R1537–1540.
- Rinaman, L. (1999b). Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. American Journal of Physiology, 277, R582–590.
- Robinson, L. E., Holt, T. A., Rees, K., Randeva, H. S., & O’Hare, J. P. (2013). Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: Systematic review and meta-analysis. BMJ Open, 3. doi:10.1136/bmjopen-2012-001986
- Sarkar, S., Fekete, C., Légrádi, G., & Lechan, R. M. (2003). Glucagon like peptide-1 (7-36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Research, 985, 163–168.10.1016/S0006-8993(03)03117-2
- Sawchenko, P. E., Li, H. Y., & Ericsson, A. (2000). Circuits and mechanisms governing hypothalamic responses to stress: A tale of two paradigms. Progress in Brain Research, 122, 61–78.10.1016/S0079-6123(08)62131-7
- Schmidt, H. D., Mietlicki-Baase, E. G., Ige, K. Y., Maurer, J. J., Reiner, D. J., Zimmer, D. J., … Hayes, M. R. (2016). Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology, 41, 1917–1928.10.1038/npp.2015.362
- Shirazi, R., Palsdottir, V., Collander, J., Anesten, F., Vogel, H., Langlet, F., … Skibicka, K. P. (2013). Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proceedings of the National Academy of Sciences, 110, 16199–16204.10.1073/pnas.1306799110
- Smits, M. M., Muskiet, M. H., Tonneijck, L., Hoekstra, T., Kramer, M. H., Diamant, M., & van Raalte, D. H. (2016). Exenatide acutely increases heart rate in parallel with augmented sympathetic nervous system activation in healthy overweight males. British Journal of Clinical Pharmacology, 81, 613–620.10.1111/bcp.v81.4
- Tang-Christensen, M., Larsen, P. J., Goke, R., Fink-Jensen, A., Jessop, D. S., Moller, M., & Sheikh, S. P. (1996). Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. American Journal of Physiology, 271, R848–856.
- Tauchi, M., Zhang, R., D’Alessio, D. A., Stern, J. E., & Herman, J. P. (2008). Distribution of glucagon-like peptide-1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. Journal of Chemical Neuroanatomy, 36, 144–149.10.1016/j.jchemneu.2008.07.009
- Thiebaud, N., Llewellyn-Smith, I. J., Gribble, F., Reimann, F., Trapp, S., & Fadool, D. A. (2016). The incretin hormone glucagon-like peptide 1 increases mitral cell excitability by decreasing conductance of a voltage-dependent potassium channel. The Journal of Physiology, 594, 2607–2628.10.1113/tjp.2016.594.issue-10
- Trapp, S., & Cork, S. C. (2015). PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 309, R795–R804.10.1152/ajpregu.00333.2015
- Trapp, S., & Richards, J. E. (2013). The gut hormone glucagon-like peptide-1 produced in brain: Is this physiologically relevant? Current Opinion in Pharmacology, 13, 964–969.10.1016/j.coph.2013.09.006
- Turton, M. D., O’Shea, D., Gunn, I., Beak, S. A., Edwards, C. M., Meeran, K., … Bloom, S. R. (1996). A role for glucagon-like peptide-1 in the central regulation of feeding. Nature, 379, 69–72.10.1038/379069a0
- Ulrich-Lai, Y. M., & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10, 397–409.10.1038/nrn2647
- van Dijk, G., & Thiele, T. E. (1999). Glucagon-like peptide-1 (7–36) amide: A central regulator of satiety and interoceptive stress. Neuropeptides, 33, 406–414.10.1054/npep.1999.0053
- Vilsboll, T., Krarup, T., Deacon, C. F., Madsbad, S., & Holst, J. J. (2001). Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes, 50, 609–613.10.2337/diabetes.50.3.609
- Vilsbøll, T., Krarup, T., Madsbad, S., & Holst, J. J. (2003). Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regulatory Peptides, 114, 115–121.10.1016/S0167-0115(03)00111-3
- Vilsbøll, T., Krarup, T., Sonne, J., Madsbad, S., Vølund, A., Juul, A. G., & Holst, J. J. (2003). Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. The Journal of Clinical Endocrinology & Metabolism, 88, 2706–2713.10.1210/jc.2002-021873
- Vrang, N., & Grove, K. (2011). The brainstem preproglucagon system in a non-human primate (Macaca mulatta). Brain Research, 1397, 28–37.10.1016/j.brainres.2011.05.002
- Vrang, N., Hansen, M., Larsen, P. J., & Tang-Christensen, M. (2007). Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Research, 1149, 118–126.10.1016/j.brainres.2007.02.043
- Vrang, N., Phifer, C. B., Corkern, M. M., & Berthoud, H. R. (2003). Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 285, R470–R478.10.1152/ajpregu.00732.2002
- Wang, Z., Wang, R. M., Owji, A. A., Smith, D. M., Ghatei, M. A., & Bloom, S. R. (1995). Glucagon-like peptide-1 is a physiological incretin in rat. Journal of Clinical Investigation, 95, 417–421.10.1172/JCI117671
- Williams, D. L., Baskin, D. G., & Schwartz, M. W. (2009). Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology, 150, 1680–1687.10.1210/en.2008-1045
- Yamamoto, H., Lee, C. E., Marcus, J. N., Williams, T. D., Overton, J. M., Lopez, M. E., … Elmquist, J. K. (2002). Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. Journal of Clinical Investigation, 110, 43–52.10.1172/JCI0215595
- Zheng, H., Cai, L., & Rinaman, L. (2015). Distribution of glucagon-like peptide 1-immunopositive neurons in human caudal medulla. Brain Structure and Function, 220, 1213–1219.10.1007/s00429-014-0714-z
