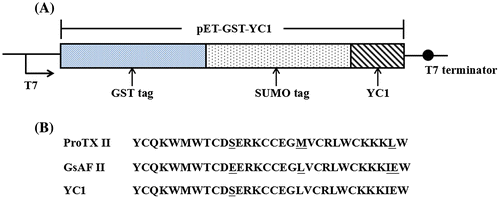
Abstract
The peptide toxin GsAF II (Kappa-theraphotoxin-Gr2c) is a 31-amino acid peptide, recently isolated from the venom of the tarantula spider Grammostola rosea. The peptide toxin ProTX II (β/ω-theraphotoxin-Tp2a), is a 30-amino acid peptide toxin recently isolated from the venom of the tarantula spider Thrixopelma pruriens. The GsAF II and ProTX II have similar sequence but have an impact on different activities. To find a method of obtaining toxin and to explore whether amino acid sequences affect activities or not, an amino acid mutant was constructed that the first two amino acids are tyr (Y) and cys (C). The YC1 sequence is as follows: YCQKWMWTCDSERKCCEGLVCRLWCKKKIEW. Then, we constructed the YC1 vector (pET-GST-YC1), which was transformed into the Escherichia coli strain SHuffleTM. rYC1 was expressed using auto-induction medium. After using a GST column, the expressed fusion protein was digested using SUMO protease (ULP1) to remove the GST-SUMO tag, and then RP-HPLC and ultrafiltration were used for further purification. rYC1 was further analyzed using SDS-PAGE. Then, the purified rYC1 was verified by MALDI-TOF/TOF mass spectrometry. Finally, the IC50 of rYC1 was determined to be 2.94 μM for the rabbit Nav1.3 (rNav1.3) and the activity is between the ProTX II and GsAF II. Finally, the described method is economical and convenient, and toxins obtained using this method can be used for the study of in channels, neurobiology, pharmacology, or other fields.
Public Interest Statement
The spider toxin is isolated from the venom of the spider. Some researchers found that the spider toxin may have therapeutic implications. But the extraction of the native toxin is too difficult to make it to be a common drug. In this paper, our group used gene engineering to obtain many purified and active toxins and it is convenient and cost-effective alternative method. This method provides a new idea for large-scale preparation of toxins or drugs.
Competing Interest
The authors declare no competing interest.
1. Introduction
The peptide toxin GsAF II is a 31-amino acid peptide, recently isolated from the venom of the tarantula spider Grammostola rosea. GsAF II modifies gating in voltage-gated Na+ channels, and it has the following amino acid sequence: YCQKWMWTCDEERKCCEGLVCRLWCKKKIEW (Ono, Kimura, & Kubo, Citation2011). The GsAF II is reported to block the following voltage-gated Na+ channels: Nav1.3, Nav1.4, Nav1.5, and Nav1.7, which have IC50 values of 24, 4, 44, and 1 μM, respectively. ProTX II, is a 30-amino acid peptide toxin recently isolated from the venom of the tarantula spider Thrixopelma pruriens and modifies gating in voltage-gated Na+ and Ca2+ channels (Bladen, Hamid, Souza, & Zamponi, Citation2014; Edgerton, Blumenthal, & Hanck, Citation2010; Richard et al., Citation2002; Salari, Vega, Milescu, & Milescu, Citation2016). The amino acid sequence of ProTX II is as follows: YCQKWMWTCDSERKCCEGMVCRLWCKKKLW (Smith, Alphy, Seibert, Seibert, & Blumenthal, Citation2005). ProTX II is reported to block the following voltage-gated Na+ channels: Nav1.3, Nav1.4, Nav1.5, and Nav1.7, with IC50 values of 0.102, 0.039, 0.079, and 0.0003 μM, respectively (Deuis et al., Citation2016; Edgerton, Blumenthal, & Hanck, Citation2011; Schmalhofer et al., Citation2008; Smith, Cummins, Alphy, & Blumenthal, Citation2007; Xiao, Blumenthal, Jackson, Liang, & Cummins, Citation2010). Thus, both GsAF II and ProTX II are potent inhibitors of the sodium channel subtypes tested (Nav1.3, Nav1.4, Nav1.5, and Nav1.7). GsAF II and ProTX II both have the six cysteine residues with spacing consistent with the ICK motif and also share some sequence homology (Kolmar, Citation2010).
The YC1 sequence YCQKWMWTCDSERKCCEGLVCRLWCKKKIEW which is a mutation of the 11th amino acid in which the glutamic acid (E) of GsAF II is replaced with serine (S) in ProTX II. In the present study, we used single site of 11th amino acid to research this site’ affect. We used Escherichia coli to produce recombinant GsAF II mutant (YC1). The toxin has three pairs of disulfide bonds. A pET-GS-YC1 vector was constructed and transformed into the E. coli strain SHuffleTM. rYC1 was expressed using auto-induction medium, and purified with a GST column (Wu et al., Citation2016; Zhang et al., Citation2015). After the expressed fusion protein was digested using a SUMO protease (ULP1) to remove the GST-SUMO tag, reversed phase HPLC (RP-HPLC) and ultrafiltration were used for further purification (Eshaghi et al., Citation2015). The rYC1 protein was further analyzed using SDS-PAGE, then the purified rYC1 was verified by MALDI-TOF/TOF mass spectrometry. According to previous research the IC50 of rYC1 is 2.94 μM for rNav1.3, which is between ProTX II and GsAF II′ activity. The mutation of the 11th amino acid had an effect on the activity of rNav1.3. It developed a new method for toxin production and the method described herein is economical and convenient. The obtained toxins can be used in the study of ion channels, neurobiology, pharmacology, or other fields of study. In addition, this methodology may provide an effective method for searching for other mutants that affect other Voltage-gated sodium channels (VGSCs).
2. Materials and methods
2.1. Materials
E. coli strain DH5α competent cells were purchased from TaKaRa (Otsu, Shiga, Japan). The pET-43a (+) vector was purchased from Novagen (Madison, WI, USA). The SHuffleTM strain was obtained from NEB (Beverly, MA, USA). The cDNA for GsAF II and the GST tag were kept in Prof S. Liang’s laboratory. The KOD DNA polymerase was purchased from TOYOBO (Osaka, Japan). DNA sequencing and primer synthesis were performed by Sangon (Shanghai, China). All of the chemicals and reagents were purchased from Sigma (St. Louis, MO, USA). Glycine-SDS-PAGE gels (NuPAGE) and the Seeblue Plus2 pre-stained molecular weight marker were purchased from Invitrogen (Carlsbad, CA, USA).
2.2. Construction of the pET-GST-GsAF II vector
Restriction free (RF) cloning technology was used to construct the expression vector (Kozlov, Citation2007; Meng et al., Citation2011; Van & Löwe, Citation2006). The GST tag and SUMO sequences were previously cloned into pET-43a. The GsAF II gene was generated with a stop codon behind the SUMO sequence using the following primer pair: 5′-GCTCACCGTGAACAGATCGGTGGTTACTGCCAGAAATGGATGTG-3′(GsAF II-Forward) and 5′-GATGGTACCGTCGACGTCCTGCAGTTACCATTCGATTTTTTTTTTGC-3′(GsAF II-Reverse). Briefly, the underlined sequences of the forward primer and the reverse primer were complementary to the 5′ end and the 3′ end of the insertion or replacement point of the recipient vector pET-43a (+) with a T m value of 80°C, respectively. The italicized portions were complementary to the 5′ end and the 3′ end of GsAF II with T m value of 60°C, respectively. The resultant construct was verified by sequencing and named pET-GST. The resulting vector was named pET-GST-GsAF II.
2.3. Construction of the pET-GST-YC1 vector
The pET-GST-YC1 vector was constructed by combining the techniques of point mutagenesis and RF cloning. pET-GST-GsAF II was used as a template with the following primer pair: 5′-GGACCTGCGACTCTGAACGTAAATGCTGCGAAGGTCTGGTTTGCCGTCTGTGGTG-3′(YC1-Forward) and 5′-AGCATTTACGTTCAGAGTCGCAGGTCCACATCCATTTCTGGCA-GTAACCACC-3′(YC1-Reverse). The resulting vector was named pET-GST-YC1.
2.4. Expression of the recombinant YC1
The pET-GST-YC1 vector was transformed into E. coli SHuffleTM cells. Using the auto-induction method established by Studier FW, the GST-YC1 protein was expressed as described below (Studier, Citation2005). A single colony from a plate incubated overnight was picked and inoculated into 3 mL ZYM-505 medium containing 100 μg/mL ampicillin inoculated at 37°C in a shaker at 220 rpm. After eight hours, 1 mL of the medium was transferred into 500 mL of fresh ZYM-5051 medium (auto-induction medium and containing 100 μg/mL ampicillin). The culture was then incubated at 30°C in a shaker at 220 rpm. After 16 hours, the cells were harvested by centrifugation at 4,000 rpm at 4°C for 10 min, and the supernatant was removed and discarded.
2.5. Purification of GST-SUMO-YC1 fusion protein by GST affinity chromatography
The cell pellet was resuspended in 3 mL ice-cold Phosphate-buffered saline (PBS) buffer per 50 mL culture and centrifuged at 4,000 rpm at 4°C for 10 min. The supernatant was discarded and the pellet was suspended in 3 mL of ice-cold PBS buffer per 50 mL culture. The cells were lysed on ice by sonication for 10 min at 40% amplitude using a Branson SONIFIER S-250D (Emerson, USA). After centrifugation at 12,000 g at 4°C for 10 min, the supernatant was carefully transferred (soluble fraction) to a clean, pre-chilled tube. Aliquots of 10 μl from both soluble and insoluble fractions were used for SDS-PAGE analysis by adding an equal volume of 2 × SDS sample loading buffer. The samples were boiled for 5 min and used for SDS-PAGE to determine the yield and solubility of the GST-fusion protein. The supernatant was applied to a gravity GST column, and the eluent was desalinated using a 30 kDa ultrafiltration tube with distilled water.
2.6. Preparation of recombinant SUMO protease and removal of the GST-SUMO tag by ULP1
The preparation of the recombinant SUMO protease was performed as previously described and was stored at −20°C (Zhang et al., Citation2015). The purificated GST-SUMO-YC1 was digested with the recombinant SUMO protease (ULP1). SUMO protease digestion was performed for 16 h at 4°C to remove the GST-SUMO part of the fusion protein.
2.7. Purification of the recombinant YC1 (rYC1)
The digested mixtures were centrifuged at 12,000 rpm for 5 min to remove insoluble particles and were then filtered through a 0.45 μm filter before loading. The supernatant was then subjected to RP-HPLC purification using a C18 column (4.6 × 250 mm, 5 μm) with a linear acetonitrile gradient.
2.8. Identification of rYC1 by MALDI-TOF/TOF mass spectrometry
The molecular mass identification of rYC1 was performed on a Voyager-DE TM STR MALDI-TOF mass spectrometer (Applied Biosystems, Voyager-DE STR Biospectometry work station). Ionization was achieved by irradiation with a nitrogen laser (337 nm), with a 20 kV acceleration voltage. We used α-cyano-4-hydroxy-cinnamic acid as the matrix.
2.9. Cell culture and whole-cell patch-clamp experiments
HEK293T cells (ATCC) were grown under standard cell culture conditions (5% CO2 and 37°C) in Dulbecco’s Modified Eagle Medium (DMEM, Life Technology) supplemented with 10% fetal bovine serum. The Nav constructs were co-transfected into the HEK293T cells with a plasmid containing the β1 subunit and PEGFP-N1 using Lipofectamine 2000 (Life Technology) according to the manufacturer’s instructions.
The whole-cell-patch-clamp experiments were performed as described below (Wu et al., Citation2016). Briefly, purified rYC1 was dissolved in distilled water to a concentration of 1 mmole/L and stored at −20°C. Dilute the stock solution to the working concentrations with fresh bathing solution before use. Whole-cell patch-clamp assay recordings were performed at room temperature using an EPC-9 amplifier (HEKA, Lambrecht, Germany). Sodium currents were elicited at 210 mV from a holding potential of 280 mM. The recording and analysis of data were carried out using the Pulse + Pulsefit 8.0 (HEKA, Lambrecht, Germany) and the Sigmaplot 9.0 (Systat Software Inc.) programs, respectively.
3. Results
3.1. Construction of the pET-GST-YC1 vector
YC1 was cloned into the pET-GST vector, and the new vector was named pET-GST-YC1 (Figure ).
3.2. Expression of GST-YC1 in E. coli SHuffleTM
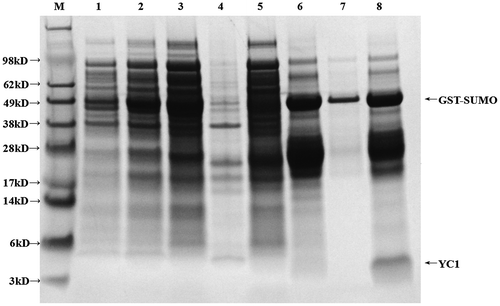
YC1 is similar to ProTX II and GsAF II in that they all have three pairs of disulfide bonds. Therefore, we utilized a SHuffleTM strain for protein expression as it is more conducive to the formation of disulfide bonds compared to other expression strains. The SUMO-YC1 fusion protein was expressed in SHuffleTM (Figure , lane 2), and no inclusion bodies were observed after sonication (Figure , lanes 3 and 4). Lastly, we observed a very bright fusion protein band after elution from the GST column (Figure , lane 6).
Figure 2. Expression of GST-YC1 in SHuffleTM.
3.3. Purification of GST-YC1 by affinity chromatography and ultrafiltration
The glycine-SDS-PAGE data are shown in Figure . After elution, the YC1 fusion protein was purified using a 30 kDa ultrafiltration tube (Figure , lane 7). YC1 was then subjected to ULP1 cleavage and purified by RP-HPLC (Figure , lane 8). YC1 was eluted in 17 min (Figure , asterisks). The obtained sample was ultrafiltrated with a 10 kDa ultrafiltration tube, and we collected the filtered liquid in the bottom of the ultrafiltration tube. The liquid was freeze dried to obtain purified rYC1 (Figure (A), lane 2). Obviously, the samples in the bottom of the ultrafiltration tube (Figure (A), lane 2) were richer and more pure than those in the ultrafiltration tube (Figure (A), lane 1).
Figure 3. RP-HPLC chromatography of rYC1. (A) Absorption of the eluted proteins was measured at 280 nm, and fractions corresponding to detected peaks were collected. (B) Absorption of the eluting solution was measured at 215 nm, and fractions corresponding to detected peaks were collected.
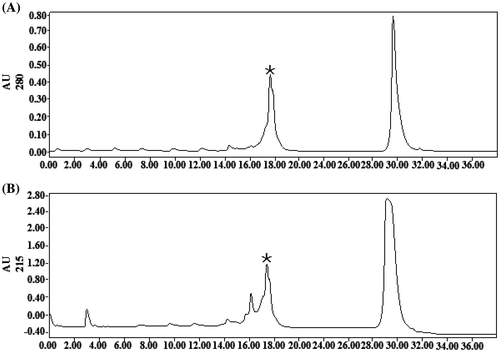
Figure 4. Identification of rYC1. (A) The results of the glycine-SDS-PAGE analysis. Lane 1, protein from eluent marked with asterisk in Figure ; lane 2, the protein after the ultrafiltration purification of eluent marked with asterisk (Figure ). (B) Mass spectra of the rYC1. The calculated theoretical molecular weight of rYC1 was 3,937.7 Da, and the measured molecular weight was 3,939.9 Da,
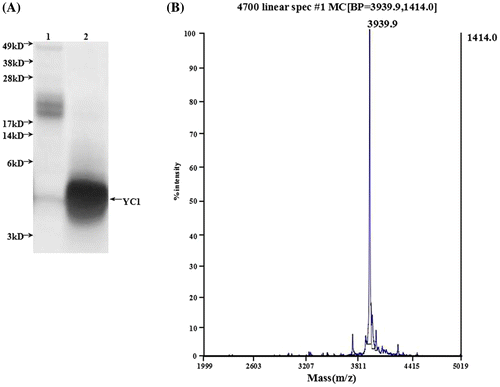
3.4. Identification of rYC1 by MALDI-TOF/TOF mass spectrometry
The molecular weight of the eluted fraction was further verified by mass spectrometry and determined to be 3,939.9 Da, which is identical to the theoretical value of YC1 (Figure (B)).
3.5. Identification and characterization of rYC1 in whole-cell patch-clamp experiments
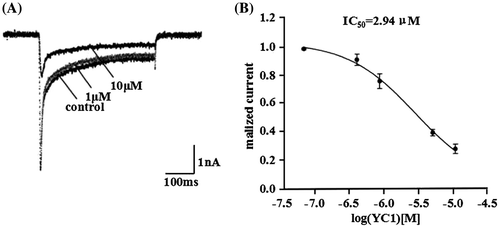
We observed that 1 μM rYC1 inhibited rNav1.3 current by approximately 20%, while 10 μM rYC1 inhibited rNav1.3 current by approximately 70% (Figure (A)). Figure (B) showed that the IC50 value for rYC1 was 2.94 μM for rNav1.3.
4. Discussion
Since ancient times polypeptide compounds and small organic molecules isolated from natural venoms have been used in folk medicine worldwide. Numerous extracts, ointments, and decoctions have been prepared from toxic spiders, poisonous plants, venomous serpents, lizards, arthropods, and marine residents. The most interesting compounds that show excellent stability and specificity of action are being patented locally or worldwide. Spider venom is a source of many biologically active peptides that may have therapeutic implications. Researchers have studied spider toxins for their potential to target ion channels and receptors (Klint et al., Citation2012; Saez et al., Citation2010).
But the extraction of the native toxin is too difficult to make it to be a common drug. In general, there are three methods for researchers to obtain spider toxins. The first is to obtain toxins directly from the spider; however, this method produces relatively little toxin. The second way is to purchase spider toxin from a company, and this method is too costly. Spider toxin can cost as much as $432.30 per milligram when purchased from Sigma (United States). A third way is chemical synthesis, although this method may require complex refolding steps and result in a low refolding rate. In this paper, prokaryotic expression as a means to obtain biological polypeptides was found to be a convenient and cost-effective alternative to these other techniques.
The GsAF II mutant rYC1 was expressed using a prokaryotic expression system. GST column, RP-HPLC, and ultrafiltration were used to further purify rYC1. The resulting protein was assayed using glycine-SDS-PAGE and mass spectrometry to identify the isolated peptide as rYC1. In addition, the mass spectrometry assays showed that rYC1 has three disulfide bonds. The purified rYC1 has an IC50 value of 2.94 μM for rNav1.3, an activity that is between that of ProTX II and GsAF II. Thus, the 11th amino acid, which was altered in the mutant, was observed to have an effect on the activity of rNav1.3.
Compared with other reported procedures for the expression of disulfide bond-rich peptides in the periplasm of E. coli, four significant improvements are presented in this work. First, the SHuffleTM expression system was found to be good for the formation of disulfide bonds and GST, SUMO was used in this system to lead the periplasmic expression of rYC1. The tags are essential for the functional expression of disulfide bond-rich toxins and useful for fusion protein solubility. . Second, instead of IPTG induction, an auto-induction medium was used in these procedures. Auto-induction with lactose is more convenient and simpler than IPTG induction. Third, the use of RP-HPLC and ultrafiltration steps can save time and obtain highly purified rYC1. Fourth, we provide an effective method for searching other mutants that had an effect on other VGSCs. It developed a simple method for active toxin production. We suppose that other disulfide bond-rich peptides can also be produced by this efficient prokaryotic expression strategy.
Funding
This work was supported by the Research Program of the National University of Defense Technology [grant number JC2006-02-01] to Prof D.Y. Zhang and Lateral Research Funds [grant number 1501020414029 to Dr. Meng].
Author contributors
HW, EM, and DYZ conceived and designed the experiments. HW and WYL performed the experiments. HW, LW, and LYZ analyzed the data. EM and DYZ contributed reagents/materials/analysis tools. HW and EM wrote the paper.
Additional information
Notes on contributors
Hui Wu
Hui Wu is presently perusing PhD in biomedical engineering from School of Research Center of Biological Information, College of Science, National University of Defense. Wu is presently working on protein expression and purification. Protein expression and purification are the key progress in the study and can obtain many purified and active peptide. It is more convenient and cost-effective alternative than traditional methods. Wu et al. use this method to obtain the purified and large-scale preparation of toxins.
References
- Bladen, C., Hamid, J., Souza, I., & Zamponi, G. W. (2014). Block of T-type calcium channels by protoxins I and II. Molecular Brain, 7, 1–8.
- Deuis, J., Wingerd, J., Winter, Z., Durek, T., Sousa, S., Zimmermann, K., … Vetter, I. (2016). Analgesic effects of GpTx-1, PF-04856264 and CNV1014802 in a mouse model of Nav1.7-mediated pain. Toxins, 8, 1–19.
- Edgerton, G., Blumenthal, K., & Hanck, D. (2010). Inhibition of the activation pathway of the T-type calcium channel CaV3.1 by ProTxII. Toxicon Official Journal of the International Society on Toxinology, 56, 624–636.10.1016/j.toxicon.2010.06.009
- Edgerton, G., Blumenthal, K., & Hanck, D. (2011). Evidence for multiple effects of ProTxII on activation gating in NaV1.5. Toxicon Official Journal of the International Society on Toxinology, 52, 489–500.
- Eshaghi, S., Hedrén, M., Nasser, M., Hammarberg, T., Thornell, A., & Nordlund, P. (2005). An efficient strategy for high-throughput expression screening of recombinant integral membrane proteins. Protein Science, 14, 676–683.10.1110/ps.041127005
- Klint, J., Senff, S., Rupasinghe, D., Er, S., Nicholson, G., & King, G. (2012). Spider-venom peptides that target voltage-gated sodium channels: Pharmacological tools and potential therapeutic leads. Toxicon, 60, 478–491.10.1016/j.toxicon.2012.04.337
- Kolmar, H. (2010). Biological diversity and therapeutic potential of natural and engineered cystine knot miniproteins. Current Opinion in Pharmacology, 41, 608–614.
- Kozlov, S. (2007). Polypeptide toxins from animal venoms. Recent Patents on DNA & Gene Sequences, 1, 200–206.10.2174/187221507782360254
- Meng, E., Cai, T., Li, W., Zhang, H., Liu, Y., Peng, K., … Zhang, D. (2011). Functional expression of spider neurotoxic peptide huwentoxin-I in E. coli. PLoS ONE, 6, 1–6.
- Ono, S., Kimura, T., & Kubo, T. (2011). Characterization of voltage-dependent calcium channel blocking peptides from the venom of the tarantula Grammostola rosea. Toxicon Official Journal of the International Society on Toxinology, 58, 265–276.10.1016/j.toxicon.2011.06.006
- Richard, E., Vivien, A., Richard, L., Jeremy, C., Chou, J., Ge, D., … Mchardy, M. (2002). Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry, 41, 14734–14747.
- Saez, N., Senff, S., Jensen, J., Sing, Er., Herzig, V., Rash, L., & King, G. (2010). Spider-venom peptides as therapeutics. Toxins, 2, 2851–2871.
- Salari, A., Vega, B., Milescu, L., & Milescu, L. (2016). Molecular interactions between tarantula toxins and low-voltage-activated calcium channels. Scientific Reports, 6, 1–12.
- Schmalhofer, W., Calhoun, J., Burrows, R., Bailey, T., Kohler, M., Kaczorowski, G., … Priest, B. (2008). ProTx-II, a selective inhibitor of Nav1.7 sodium channels, blocks action potential propagation in nociceptors. Molecular Pharmacology, 74, 1476–1484.10.1124/mol.108.047670
- Smith, J., Alphy, S., Seibert, A., Seibert, A., & Blumenthal, M. (2005). Differential phospholipid binding by site 3 and site 4 toxins. Journal of Biological Chemistry, 280, 11127–11133.10.1074/jbc.M412552200
- Smith, J., Cummins, T., Alphy, S., & Blumenthal, K. (2007). Molecular interactions of the gating modifier toxin ProTx-II with Nav1.5: Implied existence of a novel toxin binding site coupled to activation. Journal of Biological Chemistry, 282, 12687–12697.10.1074/jbc.M610462200
- Studier, F. (2005). Protein production by auto-induction in high density shaking cultures. Protein Expression & Purification, 41, 207–234.10.1016/j.pep.2005.01.016
- Van, D., & Löwe, J. (2006). RF cloning: A restriction-free method for inserting target genes into plasmids. Journal of Biochemical & Biophysical Methods, 67, 67–74.
- Wu, H., Chen, B., Jiang, H., Wu, L., Zhu, L., Meng, E., & Zhang, D. (2016). Heterologous expression and purification of neurotoxic hainantoxin-III in E. coli. Preparative Biochemistry & Biotechnology, 47, 158–162.
- Xiao, Y., Blumenthal, K., Jackson, J., Liang, S., & Cummins, T. (2010). The tarantula toxins ProTx-II and Huwentoxin-IV differentially interact with human Nav1.7 voltage sensors to inhibit channel activation and inactivation. Molecular Pharmacology, 78, 1124–1134.10.1124/mol.110.066332
- Zhang, H., Huang, P., Meng, E., Li, W., Zhou, L., Zhu, L., … Zhang, D. (2015). An efficient strategy for heterologous expression and purification of active peptide hainantoxin-IV. PLoS ONE, 10, 1–13.