Abstract
The objective of this study was to assess the time limits within which proliferative cells can be recovered in bovine skin stored separately at 4 and 25°C after animal death. In the first experiment, skin explants (n = 110; 2–3 mm2) from 11 animals stored at 4°C were cultured weekly up to 7 weeks in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 50 units/mL of penicillin, 50 μg/mL of streptomycin, and 2.5 μg/mL of fungizone. The presence/absence of fibroblast-like cell outgrowth around explants was scored. Out of 640 explants cultured, 567 (87%) adhered to dish surface, of which 333 (58.73%) exhibited outgrowth including 16.67% explants from 49 days postmortem tissues. Similarly, in the second experiment, when the tissues were stored at 25 ± 2°C prior to culturing on alternate days up to 17 days, 204 (48%) explants exhibited outgrowth that included 19.15% from 15 dpm tissues. The number of explants exhibiting outgrowth was inversely proportional to postmortem time interval in both temperatures studied. Secondary cultures established from outgrowth for selected time points showed stable chromosomes, normal GFP gene expression, and comparable growth morphology to fresh tissue-derived cells. The cells lasted in culture for more than 20 passages. These results suggest that live and usable cells can be recovered from bovine skin tissues up to about 2 weeks postmortem, if skin is stored at 25°C, and about three times more (>6 weeks), if stored at 4°C.
Public Interest Statement
Death is an ultimate destination of life. Or is it? When we die, what happen to the individual cells, of which, we are made up of? Are they all dead as the blood circulation stops and the oxygen, which is our life line, is no more transported to the cells? Or are they still alive for some time, and if yes, for how long, are the questions that always puzzled scientists. This research paper addresses some of these questions and demonstrates that individual cells in mammalian tissues are alive for much longer time than was previously thought. This study further demonstrates that these live cells retain normal characters and can be stored frozen for long time. This research highlights the huge potential of utilization of postmortem tissues, for the recovery of stem cells, for cellular therapies in human and veterinary medicine and preservation of germplasm to meet the climatic challenges to feed the expanding world by cloning food animals.
Competing Interests
The authors declare no competing interest.
1. Introduction
Angus is a beef-producing breed of cattle, which is said to have been developed from native cattle of Aberdeen shire and Angus in Scotland and thus is known as Aberdeen Angus in most parts of the world (Briggs & Briggs, Citation1980). They are naturally polled (hornless) animals and are found in black or red colors. They were introduced in United States in 1873 by George Grant. He brought four Angus bulls without cows from Scotland for crossbreeding with native cattle herds. Subsequently, many more animals of both sexes were imported by US farmers, due to their desirable qualities. Black Angus is the most common breed today. There are 320,362 heads of Angus cattle registered in 2015 in the United States (http://www.angus.org/pub/faqs.aspx).
Cloning of animals including Angus by nuclear transfer is an important milestone in agricultural biotechnology. Almost every livestock species have now been cloned (https://en.wikipedia.org/wiki/List_of_animals_that_have_been_cloned). Cloning requires fusion of desired somatic cells (nuclear donor) with an enucleated oocyte cytoplasm. Therefore, the preservation of somatic cells/tissues from elite animals has been suggested, so as to utilize these resources globally. This will help to meet the climatic and/or other challenges in future by cloning of these animals to supply the global demand for protein. Additionally, cloning of animals with desirable meat qualities after postmortem carcass evaluation is gaining importance among producers. Recent studies show effective preservation of postmortem tissues and production of live animals from these tissues by SCNT aka cloning (Ogura, Inoue, & Wakayama, Citation2013). However, for the success of a cloning experiment, nuclear integrity of donor cells is a requirement (Hoshino et al., Citation2009). In vitro culture of cells is one of the methods to ensure nuclear integrity (Mastromonaco, Perrault, Betts, & King, Citation2006). In vitro culture of cells from both live and dead animal tissues preserved at subzero temperatures has been reported (Erker et al., Citation2010; Hoshino et al., Citation2009; Palmer et al., Citation2001; Viel, McManus, Cady, Evans, & Brewer, Citation2001; Wakayama et al., Citation2008) and the cells have been used to clone the animals even after many years of their death (Hoshino et al., Citation2009; Wakayama et al., Citation2008). However, in all these cases, the tissues were preserved within few hours of animal death. Delays in preservation may compromise cloning efficiency and quality of cloned offspring. There are only limited studies to show as how long the live and culturable cells can be recovered from postmortem tissues in mammalian species and, how different these cells are, compared to fresh tissue-derived cells. Literature survey shows that neural stem/progenitor cells were cultured from postmortem rat brains, stored at 4°C for a week (Xu, Kimura, Matsumoto, & Ide, Citation2003). Fibroblasts were recovered in postmortem skin tissues stored at 4°C up to 14 days in rabbits and pigs (Silvestre, Saeed, Cervera, Escribá, & Garcı́a-Ximénez, Citation2003), 12 days in goat, and sheep (Silvestre, Sánchez, & Gómez, Citation2004), and up to 41 days in goats (Okonkwo & Singh, Citation2015). Muscle stem cells have been shown to survive up to 17 days postmortem in human beings and up to 16 days postmortem in mice (Latil et al., Citation2012). To our knowledge, there are only two studies in bovine; in first study, fibroblasts were cultured up to 12 days from skin stored at 4°C (Silvestre et al., Citation2004), and in another muscle and cartilage, cells were cultured up to 9 days of postmortem tissue storage at 4°C (Caputcu, Akkoc, Cetinkaya, & Arat, Citation2012). Here, we show in vitro culture of fibroblast-like cells up to 49 days in bovine steer skin tissues, if the tissue is stored at 4°C, and up to 15 days, if stored at 25 ± 2°C after the animal death. We further show that these cells are cytogenetically stable with normal karyotype, have comparable growth profile, can be recultured postcryopreservation, and transfected to express GFP gene from a plasmid, suggesting their potential utility in postmortem cryopreservation for animal cloning and/or cell therapy.
2. Materials and methods
2.1. Sample preparation and explant culture
The tissue samples were procured from the USDA-inspected university slaughter house. The animals were not slaughtered for these experiments but were routine food animals for human consumption, and thus, there were no ethical violations. Animal ears of slaughtered animals were excised from the head and brought to the laboratory. Ear skin was cleaned first with tape water and then with 70% alcohol swabs, wrapped in clean sterile paper towels, and stored in plastic bags in the laboratory, either at room temperature (25 ± 2°C) or in refrigerator set at 4°C. After different days of postmortem storage, small skin samples were excised aseptically from inner side of the ear and chopped into ten small explants (2–3 mm2 size) and adhered onto two 60-mm-diameter dishes (Falcon, BD Biosciences, Oxnard, CA) for each animal individually. Explants were cultured in DMEM supplemented with 10% FBS, 50 units/mL of penicillin, 50 μg/mL of streptomycin and 2.5 μg/mL of fungizone at 37°C, 5% CO2 in a humidified environment. Media were changed once a week and the dishes were observed for any microbial or fungal contamination, explant dislodging, and for the outgrowth of fibroblast-like cells under an inverted microscope. Dislodged explants and contaminated dishes were removed as soon as observed. The presence or absence of the outgrowth around each explant was recorded. Any outgrowth containing a cluster of more than 50 cells was considered positive.
2.2. Establishment of secondary cultures and cryopreservation of cells
Primary outgrowing cells on dish surface were trypsinized at 70–90% confluence and secondary cultures established as described (Singh & Ma, Citation2014). Briefly, the cells in dishes were washed twice with 3.0 mL of the balanced salt solution without calcium and magnesium (Gibco, Carlsbad, CA) and incubated with 2.0 mL of 0.125% trypsin for 5–10 min at 37°C. The trypsinized cells were neutralized with 5 vol. of growth media, counted to assess cell viability using Trypan Blue Dye Exclusion Method (Strober, Citation2001), and pelleted at 200 × g for 7 min. The cells were resuspended in Synth-a-Freeze® (Life Technologies Corp., Carlsbad, CA) media, aliquoted into cryogenic storage vials (1.0 × 105 cells/vial) and frozen at −80°C o/n using Nalgene™ Cryo 1°C Freezing Container (Nalgene, Rochester, NY). The vials were transferred to liquid nitrogen tank after 24 h and stored till used. Representative cryovials were thawed and postfreezing cell-viability percentage was determined at least after a week of storage. To establish secondary cultures and to expand them, the frozen vials were quickly thawed at 37°C, mixed slowly with 10 vol. of the media, pelleted at 200 × g for 7 min, dissolved in growth media, and cultured in appropriate (T25 or T75) culture flasks as described (Singh & Ma, Citation2014).
2.3. Transfection and GFP expression
Transfection of 42 dpm cell line (p5) was performed as per the vender instructions using a transfectamine 3000 kit from Invitrogen (Life Technologies Inc., CA). GFP containing plasmid pcDNA3.1-GFP:NT was prepared using Endo-Free plasmid kit (Qiagen) and the plasmid DNA was quantified using Nanodrop 2000 (Fisher Scientific, Wilmington, DE, USA). GFP gene expression was observed under UV light in EVOS Cell Imaging System (Thermo Fisher Scientific, Atlanta, GA) using GFP filter and the appropriate images captured using their software.
2.4. Karyotype analysis
Bovine cells from 42 dpm cell line at p5 were processed for karyotyping using GTL Banding technique as previously established (Meisner & Johnson, Citation2008) at Cell-line Genetics (Madison, WI; www.clgenetics.com). Chromosome assignments were made according to the Atlas of Mammalian Cytogenetics (O’Brien, Menninger, & Nash, Citation2006).
2.5. Cell passaging
Bovine cell cultures were splitted (1:3) after each subculture at 70–80% confluence until the cells stopped growing as described earlier (Freshney, Citation2000). In brief, the spent culture media from the cell culture dish were aspirated and washed twice with phosphate-buffered saline (PBS). The cells on surface were treated with 1.0 mL of trypsin-EDTA and incubated for 5–10 min at 37°C till the cells detached completely from the dish surface. Once the cells detached as observed under microscope, the complete media were added to the dish. The volume was made to 10 mL, and the cells were harvested at 200 × g for 7 min. The pellet was dissolved in 0.5–1.0 mL of complete media, the cells counted if needed, and otherwise splitted into one-third and cultured in new dishes. This procedure was repeated till cells stopped growing.
3. Results
3.1. Effect of postmortem time interval on in vitro culture of cells from tissues stored at 4°C
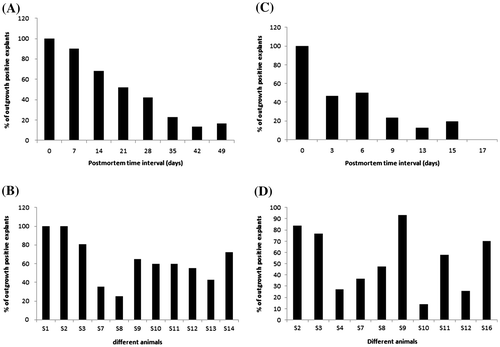
In order to test the time limit, within which proliferative cells can be recovered after animal death, we analyzed a total of 640 skin explants for in vitro culture. These explants were excised from 11 individual animal ears that were stored at 4°C. A subset of explants (n = 110) was cultured after different storage time intervals, i.e. 0, 7, 14, 21, 28, 35, 42 and 49 days postmortem. The outgrowing cells in cultured dishes were observed weekly until they reached > 50% confluence (Figure , panel B). The presence or absence of cell outgrowth around each adhered explant for each time interval was recorded. We observed outgrowth around tissues in all the time points studied including 16.67% of 49 dpm tissues (Table ). Out of 640 explants cultured, 567 (87.23%) adhered to dish surface, of which 333 (58.73%) exhibited fibroblast-like outgrowth. We never observed outgrowth around tissues dislodged from dish surface within first 3 days of culture initiation. Outgrowth ranged from 13.33 to 100% in tissues stored for various time intervals and show a reduction trend with increasing postmortem time interval (Figure , panel A). Percent outgrowth in tissues from different animals varied and ranged from 25 to 100% (Figure , panel B).
Table 1. Outgrowth of fibroblast-like cells around skin explants stored at 4°C for different days postmortem
Figure 1. Recovery of fibroblast-like cells from stored tissues at two different temperatures: (A) outgrowth after different days of storage at 4°C; (B) outgrowth in individual animal tissues stored at 4°C; (C) outgrowth after different days of storage at 25 ± 2°C; (D) outgrowth in individual animal tissues stored at 25 ± 2°C.
3.2. Effect of postmortem time interval on in vitro culture of cells from tissues stored at 25 ± 2°C
Similar to tissues stored at 4°C, we also studied effect of room temperature (25 ± 2°C) storage on outgrowth. Skin explants (n = 100) from 10 animals were cultured after 0, 3, 6, 9, 13, 15 and 17 days of postmortem. Out of 425 explants adhered, 204 (48%) exhibited outgrowth including 19.15% of 15 dpm tissues (Table ). Outgrowth ranged from 12.25 to 100% and exhibited a decreasing trend with increasing postmortem time interval as was observed for tissues stored at 4°C (Figure , panel C). Outgrowth in tissues from different animals varied and ranged from 14 to 93.33% (Figure , panel D).
Table 2. Outgrowth of fibroblast-like cells around skin explants stored at room temperature (25 ± 2°C) for different days postmortem
3.3. Cryopreservation, postfreezing cell morphology, and gene expression
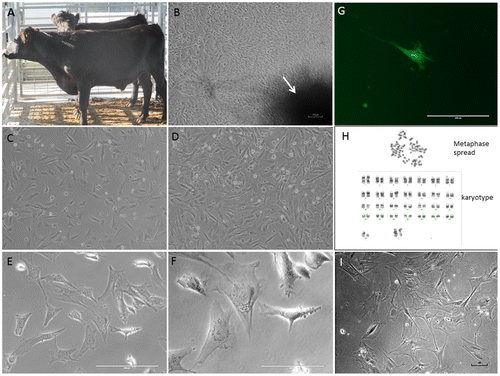
The dishes from selected time points were trypsinized at around 70% confluence to recover the primary cells and cryopreserved until used for further analysis. Secondary cultures from selected cell lines (0 dpm and 42 dpm) were then established by serial passaging. Secondary cultures of these cells grow much faster, as compared to the primary outgrowth, and reach 70–90% confluence in 5–7 days. The cells cryopreserved in DMEM with 10% DMSO retained >80% postfreezing cell viability and normal cell morphology, i.e. elongated, fibrous, and bipolar, which are typical characteristics of fibroblast cells (Figure , panel C–F). In order to test whether the postmortem tissue-derived cells have normal transcriptional machinery, we transfected 42 dpm cell line (p5) with pcDNA3.1-GFP: NT plasmid DNA vector. The transfected cells exhibited GFP gene expression when observed under UV light in an inverted fluorescent microscope (Figure , panel G).
Figure 2. Postmortem recovery of fibroblast-like cells in cattle: (A) a sample of Black Angus just prior to slaughter; (B) migration of primary outgrowth around skin explant (arrow-marked dark-shaded area is explant) adhered on petridish surface after 10 days of culture (light microscopy, ×100 magnification); (C) & (D) low- (1 × 105 cells) and high (2 × 105 cells)-density postcryopreservation cultures of 42 dpm cell population (p5) after 24 h of culture (×100 magnifications); (E) & (F) ×200 and ×400 magnifications, respectively, of the cells in panel D; (G) GFP gene expression after 72 h of transfection in 42 dpm (p5) cultures (×200 magnification); (H) chromosome analysis at p5 level in 42 dpm fibroblast cell line (a representative metaphase cell spread and karyotype with 30 pairs of XX chromosomes are shown); (I) high passage (p25, on day 53) culture of 42 dpm cell line (×100 magnification but contrast correction of 56% to show better cell morphology).
3.4. Cytogenetic stability
In order to determine if there was any genetic change in the cells derived from stored postmortem tissues, we performed a cytogenetic analysis on a cell line derived from tissues stored at 4°C until 42 days postmortem. The diploid number of chromosomes determined for this cell line was 60, XX, which is consistent with earlier studies in bovine (Iannuzzi, King, & Di Berardino, Citation2009). Furthermore, 20 G-banded metaphase cells were also analyzed to see if there were any genetic aberrations. All 20 cells demonstrated normal female karyotype without any apparent genetic aberration (Figure , panel H).
3.5. Proliferative life span
In order to determine as how long the postmortem tissue-derived cell populations can last in culture, we chose three cell lines, (a) 42 dpm tissue derived (4°C stored tissue), (b) 15 dpm tissue derived (25°C stored tissues) and, (c) 0 dpm (control; fresh tissue derived). We observed that these cell lines proliferated well in initial passages giving about 70% confluence in 5–7 days. However, after passage 15 the growth slowed exhibiting 70% confluence in 2–3 weeks. The cells stopped proliferating around passage 23 to 27. An example of the cell morphology on day 53 of culture from 42 dpm cell line is shown (Figure , panel I). These cells at later passages look flat, thinner, highly branched and comparatively bigger sized (see Figure , panel C/D vs I, all of which are at 100x magnification).
4. Discussion
The development of animal cloning technology and induction of pluripotent stem cells (iPSCs) from somatic cells in last two decades has revolutionized not only the agricultural biotechnology but also biomedical and veterinary field, benefitting both agriculture and human health. However, for the proper utilization of somatic cells, it is essential that they retain some ability of proliferation ensuring the integrity of genetic material within the cell. Biopsies taken from live animals or immediately after animal death, cultured within few hours, are preferred somatic cells for cloning. However, in many instances, it is not possible to set up cultures within few hours. It is not precisely known as how long the individual cells in a biological tissue are alive and retain culture potential, after it is excised from mammalian body. Therefore, the objective of this study was to determine the postmortem survival limits of bovine tissues stored at two different temperatures, i.e. 25 and 4°C. To study this phenomenon, a simple in vitro culture procedure, previously optimized for goat and sheep skin tissue (Singh & Ma, Citation2014) was utilized. Here, tiny skin explants are adhered onto plastic dish surfaces and are cultured in fibroblast culture media. The outgrowing cells are measured as cell survival data points. Using this procedure, a comprehensive in vitro culture study was conducted on 11 Black Angus Steer tissues for various time intervals postmortem.
Results show outgrowth of cells around bovine explants up to 49 days of postmortem storage at 4°C and up to 15 days after storage at 25 ± 2°C. In an earlier study, bovine skin fibroblasts were cultured only up to 12 days of postmortem storage at 4°C (Silvestre et al., Citation2004), while muscle and cartilage were cultured only up to 9 days of postmortem storage at 4°C (Caputcu et al., Citation2012). To our knowledge, this is the first report of in vitro culture of mammalian cells from postmortem tissues stored for such a long period of 49 days at 4°C and 15 days at 25°C, after animal death. We observed that the numbers of explants exhibiting outgrowth decreased with increasing postmortem storage time interval in both the temperatures studied (Figure ). It is likely that the number of viable adult skin stem cells decreased in tissues with an increased postmortem time interval, which could explain the reduction with increasing postmortem time interval. These results are in agreement with a previous similar study in goats (Singh, Ma, & Sharma, Citation2012). Furthermore, it was interesting to learn that such a long postmortem time lapse did not affect cytogenetic stability of cells, since all 20 metaphase cell spreads analyzed had normal chromosomes. Postmortem stored tissue-derived cell populations were also competent for transfection and expressed GFP gene in their cytoplasm from a plasmid DNA, suggesting that the transcriptional and translational machinery of the cells are intact. These cell populations were recultured after cryopreservation in liquid nitrogen. Results show that they last in in vitro cultures for more than 20 passages suggesting that postmortem stored tissue-derived cells can be utilized for long-term storage and could benefit animal agriculture and biomedicine in future.
5. Conclusions
This study show that, (a) skin tissue of bovine stored at 25 ± 2°C and 4°C has potential for in vitro culture up to 15- and 49-day postmortem, respectively, (b) the number of explants with outgrowth decreases with increasing postmortem time interval, and (c) that the cell populations derived from tissues stored over a month of postmortem are genetically stable, have similar transcriptional and translational machinery, and can be easily re-cultured in vitro after cryopreservation. Future studies should reveal the potential of these cells for reprogramming, cloning of animals and for cell therapies.
Funding
This work was partly supported by a USDA-NIFA Capacity Building Teaching [grant number 2011-38821-30910] to MS.
Acknowledgements
This work is part of MS thesis of BW. We would like to thank NSF for graduate assistantship to BW through an educational grant. X. Ma and N. Degala are thankfully acknowledged for technical assistance. Part of this work was presented in the Society for In Vitro Biology Meeting held at San Diego, CA, June 13, 2016 (abstract No: A3000).
Additional information
Notes on contributors
Mahipal Singh
Mahipal Singh is associate professor of Animal Biotechnology at the Fort Valley. He teaches undergraduate and graduate students and has an active research program in animal biotechnology. His research interests include genetic engineering of livestock, stem cells, and animal cloning. The group utilizes molecular biological, reproductive, surgical and cell culture methods in their research. Recent focus of his laboratory has been to understand the time limits of cell/tissue survival after the death of an animal in various temperature regimes and their survival mechanisms. The interest is to recover live stem cells from postmortem tissues and their potential for cloning dead animals and to use for cellular therapies in humans. Recently, his laboratory has undertaken collaborative studies on understanding dynamics of peri-implantation phase of ruminant reproduction as relates to the pregnancy recognition genes. Brian Walcott is a former MS graduate student and is currently pursuing MD degree program.
References
- Briggs, H. M., & Briggs, D. M. (1980). Modern breeds of livestock (4th ed.). New York, NY: Macmillan publishing Company.
- Caputcu, A. T., Akkoc, T., Cetinkaya, G., & Arat, S. (2012). Tissue cryobanking for conservation programs: Effect of tissue type and storage time after death. Cell Tissue Bank, 14, 1–10.
- Erker, L., Azuma, H., Lee, A. Y., Guo, C., Orloff, S., Eaton, L., … Grompe, M. (2010). Therapeutic liver reconstitution with murine cells isolated long after death. Gastroenterology, 139, 1019–1029.10.1053/j.gastro.2010.05.082
- Freshney, R. I. (2000). Culture of animal cells: A manual of basic techniques. Hoboken, NJ: Wiley-Liss.
- Hoshino, Y., Hayashi, N., Taniguchi, S., Kobayashi, N., Sakai, K., Otani, T., … Saeki, K. (2009). Resurrection of a bull by cloning from organs frozen without cryoprotectant in a −80°C freezer for a decade. PLoS ONE, 4, e4142.10.1371/journal.pone.0004142
- Iannuzzi, L., King, W. A., & Di Berardino, D. (2009). Chromosome evolution in domestic bovids as revealed by chromosome banding and fish-mapping techniques. Cytogenetic Genome Research, 126, 49–62.10.1159/000245906
- Latil, M., Rocheteau, P., Châtre, L., Sanulli, S., Mémet, S., Ricchetti, M., … Chrétien, F. (2012). Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nature Communications, 3, 903.10.1038/ncomms1890
- Mastromonaco, G. F., Perrault, S. D., Betts, D. H., & King, W. A. (2006). Role of chromosome stability and telomere length in the production of viable cell lines for somatic cell nuclear transfer. BMC Developmental Biology, 6, 41.10.1186/1471-213X-6-41
- Meisner, L. F., & Johnson, J. A. (2008). Protocols for cytogenetic studies of human embryonic stem cells. Methods, 45, 133–141.10.1016/j.ymeth.2008.03.005
- O’Brien, S. J., Menninger, J. C., & Nash, W. G. (2006). Atlas of mammalian chromosomes (pp. 653–655). Hoboken, NJ: Wiley.10.1002/0471779059
- Ogura, A., Inoue, K., & Wakayama, T. (2013). Recent advancements in cloning by somatic cell nuclear transfer. Philosophical Trans Royal Society of London B Biological Sciences, 368, 20110329.
- Okonkwo, C., & Singh, M. (2015). Recovery of fibroblast-like cells from refrigerated goat skin up to 41 d of animal death. In Vitro Cellular & Developmental Biology - Animal, 51, 463–469.10.1007/s11626-014-9856-9
- Palmer, T. D., Schwartz, P. H., Taupin, P., Kaspar, B., Stein, S. A., & Gage, F. H. (2001). Cell culture. Progenitor cells from human brain after death. Nature, 411, 42–43.10.1038/35075141
- Silvestre, M. A., Saeed, A. M., Cervera, R. P., Escribá, M. J., & Garcı́a-Ximénez, F. (2003). Rabbit and pig ear skin sample cryobanking: Effects of storage time and temperature of the whole ear extirpated immediately after death. Theriogenology, 59, 1469–1477.10.1016/S0093-691X(02)01185-8
- Silvestre, M. A., Sánchez, J. P., & Gómez, E. A. (2004). Vitrification of goat, sheep, and cattle skin samples from whole ear extirpated after death and maintained at different storage times and temperatures. Cryobiology, 49, 221–229.10.1016/j.cryobiol.2004.08.001
- Singh, M., & Ma, X. (2014). In vitro culture of fibroblast-like cells from sheep ear skin stored at 25–26°C for 10 days after animal death. International Journal of Biology, 6, 96–102.
- Singh, M., Ma, X., & Sharma, A. (2012). Effect of postmortem time interval on in vitro culture potential of goat skin tissues stored at room temperature. In Vitro Cellular and Developmental Biology- Animal, 48, 478–482.10.1007/s11626-012-9539-3
- Strober, W. (2001). Trypan blue exclusion test of cell viability. Current protocols in immunology. Appendix 3: Appendix 3B. doi:10.1002/0471142735.ima03bs111
- Viel, J. J., McManus, D. Q., Cady, C., Evans, M. S., & Brewer, G. J. (2001). Temperature and time interval for culture of postmortem neurons from adult rat cortex. Journal of Neuroscience Research, 64, 311–321.10.1002/(ISSN)1097-4547
- Wakayama, S., Ohta, H., Hikichi, T., Mizutani, E., Iwaki, T., Kanagawa, O., & Wakayama, T. (2008). Production of healthy cloned mice from bodies frozen at −20 C for 16 years. Proceedings of the National Academy of Sciences, 105, 17318–17322.10.1073/pnas.0806166105
- Xu, Y., Kimura, K., Matsumoto, N., & Ide, C. (2003). Isolation of neural stem cells from the forebrain of deceased early postnatal and adult rats with protracted post-mortem intervals. Journal of Neuroscience Research, 74, 533–540.10.1002/(ISSN)1097-4547
