?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Inflammation is one of the means the human body uses to defend itself in the event of infection, trauma, or exposure to toxic substances and it is closely associated with a number of disease symptoms. Steroidal and non-steroidal anti-inflammatory agents have been the drugs of choice for managing inflammation. However, reports of unpleasant side effects have necessitated a search for new anti-inflammatory agents which have minimal side effects. Marine-derived natural products continue to make significant contributions in the pharmaceutical, nutraceutical, and cosmeceutical industries and a number of extracts and compounds from marine origin have shown promise as anti-inflammatory agents. In Ghana, extracts that have been screened for their potential anti-inflammatory effects have almost exclusively come from plants. In this work, the anti-inflammatory activities of extracts from three different marine mollusks (Oliva sp., Patella rustica, and Littorina littorea) were evaluated. Extracts were obtained by cold maceration. The carrageenan-induced paw edema model in seven-day old chicks was used to evaluate anti-inflammatory potentials. Of the extracts tested, the ethyl acetate fraction of Oliva sp. was the most potent, with an ED50 of 10.16 mg/kg. The ethanol extract of L. littorea proved to be least effective in reducing inflammation, with an ED50 value of 119.80 mg/kg. When compared, extracts from Oliva sp. seemed to possess greater anti-inflammatory potentials than either P. rustica or L. littorea counterparts. The ethyl acetate fraction of Oliva sp. was a potent and promising anti-inflammatory agent and could be explored for anti-inflammatory lead compounds.
Public Interest Statement
The marine environment is emerging as an important contributor in providing lead compounds for various therapeutic areas in many drug development programs worldwide. In Ghana, attempts to utilize the sea in this regard have been few and far-fetched. Interestingly, less than 5% of non-fish organisms in Ghana’s marine ecosystem have been properly characterized. In this work, extracts from three mollusks commonly found along the shorelines of Ghana were tested for their ability to reduce inflammation. Current inflammation drugs have unwanted side effects that make their use unpleasant, especially over long periods. The results indicated that these mollusks harbor compounds that could potentially be exploited for pharmaceutical, nutraceutical, and cosmeceutical purposes.
Competing Interests
The authors declare no competing interest.
1. Background
It is widely known that inflammation is associated with most diseased conditions. Inflammation is usually characterized by redness, heat, pain, swellings, and disruption in normal physiological functions. The body uses inflammation as a protective response to infection, injury (or trauma), or chemical exposure. Inflammation is used as a mechanism by the body to inactivate invading organisms, remove irritants, and initiate tissue repair procedures (Mitchell & Cotran, Citation2003). Asthma, colitis, hepatitis, and arthritis, which are all inflammatory diseases, have been implicated as a leading cause of permanent disability and ultimately death in people worldwide (Emery, Citation2006). Usually, steroidal and non-steroidal anti-inflammatory agents are used to manage inflammation cases. Reported side effects associated with the use of these drugs have, however, been unpleasant. The side effects include gastric ulceration, bleeding, abnormal kidney function, hypertension, and immunosuppression (Chandra, Chatterjee, Dey, & Bhattacharya, Citation2012). The long term use of steroidal and non-steroidal anti-inflammatory drugs is understood to lead to drug-induced toxic effects and/or secondary adverse effects (Beg, Hasan, Hussain, Swain, & Barkat, Citation2011; Drew & Myers, Citation1997). The search for new anti-inflammatory agents with improved efficacy and limited side effects has therefore become the focus of global scientific research, with natural products leading the way.
The ocean is the largest biosphere of the earth and houses a colossal number of diverse organisms. The marine environment is made up of intricate ecologies, with many benthic and sessile organisms employing potent, biologically active molecules as the mainstay of their basic defense mechanisms (Thakur, Thakur, & Müller, Citation2005). The defense systems of these organisms are highly complex and toxic and the bioactive compounds play significant roles in these systems. Chemicals released into the aquatic ecosystem undergo rapid dilution. This means that such chemicals must be of high potency to be effective for use in chemical defense systems. Additionally, such chemicals must be water soluble and should be reasonably specific for the task required (Jimeno, Faircloth, Fernández Sousa-Faro, Scheuer, & Rinehart, Citation2004; Newman & Cragg, Citation2004). All these are good characteristics of potential drug candidates.
Marine invertebrates have proven to be valuable sources of pharmacologically active compounds. Many crude extracts and compounds isolated from the marine organisms have been found to possess antitumor, antifouling, antibacterial, antifungal, antiviral, and anti-inflammatory effects (Blunt et al., Citation2014; Cragg & Newman, Citation2013; Faulkner, Citation2000; Fusetani, Citation2011; Martins, Vieira, Gaspar, & Santos, Citation2014). Most compounds isolated have been classified as polyketides, terpenes, steroidal or triterpene saponins, carbohydrates, aliphatic compounds, amino acids, alkaloids, peptides, lipopeptides, and proteins. Of the many compounds isolated thus far, a number of them are either on the market as approved drugs or in various phases of clinical trials. In 2015, trabectedin (Pharmamar, trademark: Yondelis), an alkaloid that was isolated from a tunicate, was approved for use in the management of soft tissue sarcoma and ovarian cancer (Allavena et al., Citation2005; National Cancer Institute, Citation2015; U.S. Food & Drug Administration, Citation2015). Debromohymenialdisine, a secondary metabolite isolated from a marine sponge has been explored for use in the management of rheumatoid arthritis and osteoarthritis (Mayer & Lehmann, Citation2001). Other compounds whose anti-inflammatory properties have been investigated include manoalide, pseudopterosins, topsentins, and scytonemin (Cragg & Newman, Citation2013; Martins et al., Citation2014; Mayer, Rodriguezn, Berlinck, & Hamann, Citation2010). Secondary metabolites from marine sources therefore provide a promising avenue to prospect for new anti-inflammatory agents.
In this work, the anti-inflammatory activity of crude extracts from Oliva sp, Patella rustica, and Littorina littorea were investigated in vivo using the carrageenan-induced foot edema model of inflammation in chicks. Oliva sp., is a sand-borer and usually found in the beach sand, whereas P. rustica and L. littorea are usually found on rocks in the sea. P. rustica, Oliva sp., and L. littorea are gastropods belonging to the families Patellidae, Olividae, and Littorinidae, respectively. Our investigations indicate that the extracts of these marine mollusks possess significant anti-inflammatory activities. The active principles could therefore be isolated and explored as potential leads in drug discovery programs.
2. Methods
2.1. Sample collection
Samples of Oliva sp. were collected from Eikwe in the Western Region, while P. rustica and L. littorea specimens were collected from Labadi in the Greater Accra Region, all in Ghana. All samples were collected between November 2015 and February 2016. Samples were transported on ice to the laboratory. The body tissues were removed from the shell and washed with copious amount of distilled water, then stored at 0°C until use. Body tissues were used within 24 h of removal. A sample of each mollusk was sent to the Department of Fisheries and Marine Sciences, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana for authentication.
2.2. Solvent extraction
The body tissues were washed with distilled water and homogenized with a blender. To 100 g of blended body tissue was added 100 mL of appropriate solvent (methanol, 70% ethanol, or ethyl acetate) and kept for 24 h at room temperature with intermittent shaking. The mixture was centrifuged at 5000 rpm (SciSpin ONE, UK) for 15 min. The supernatant was transferred into a round-bottom flask and concentrated in vacuo (Cole Parmer SB-1200, Shanghai, China). Extracts were stored in vials at 4°C until use. Extracts obtained were labeled OSE (Oliva sp., 70% ethanol extract), OSM (Oliva sp., Methanol extract), OSEA (Oliva sp., ethyl acetate extract), PRE (P. rustica 70% ethanol extract), PREA (P. rustica ethyl acetate extract), LLE (L. littorea 70% ethanol extract), LLM (L. littorea methanol extract), and LLEA (L. littorea ethyl acetate extract).
2.3. Anti-inflammatory assay
2.3.1. Animals
One-day post-hatch cockerels (Gallus gallus, strain Shaver 5790) were acquired from Akate Farms (Kumasi, Ghana) and maintained in the Animal House of the Department of Pharmacology, KNUST in stainless steel cages (34 × 57 × 18 cm3) at a population density of 12–13 chicks per cage. The temperature in the cages was maintained between 26 and 29°C, with a 12-h light–dark cycle. Food and water was available ad libitum via 1-quart gravity-fed feeders and water troughs. Cages were maintained daily during the first quarter of the light cycle. Seven-day old chicks were used for the assay. Group sample sizes of 4–5 were employed throughout the study. Handling of the chicks and experimental protocols were in accordance with the National Institute of Health guidelines for the care and use of laboratory animals.
2.4. Carrageenan-induced foot edema in chicks
The anti-inflammatory activity of the extracts of Oliva sp., P. rustica, and L. littorea was evaluated using the carrageenan-induced foot edema model of inflammation in the seven-day old chicks with slight modifications (Roach & Sufka, Citation2003; Woode et al., Citation2007). Diclofenac (25 mg/kg) was used as the positive control. The various extracts were suspended in sterile distilled water. Two percent of tween-20 was added to enhance the suspension of the extracts. Carrageenan (10 μL of a 2% suspension in saline) was injected sub-plantar into the right footpads of the chicks to induce edema. Extracts were orally administered at different concentrations (50, 100, and 150 mg/kg) 1 h after edema induction. A digital Vernier Caliper was used to measure the foot volume before injection and at various time points after injection. The control animals received only tween-20 in sterile water, serving as the negative control. All drugs and extracts were orally administered in volumes not exceeding 100 mL/kg.
2.5. Data analysis
The raw scores for foot volume increase at each time interval for each chick was normalized as the percentage difference from the initial foot volume at time zero and then averaged for each treatment group. Increase in foot volume was computed using the equation below
Total foot volume for each treatment group was calculated in arbitrary unit as the area under the curve (AUC). Percentage inhibition of edema for each treatment group was then determined as follows:
One-way analysis of variance (ANOVA) followed by Holm–Sidak’s post hoc test was used to analyze differences in AUCs. ED50 values (dose responsible for 50% of maximal effect) for each extract were determined using an iterative computer least square method with the following nonlinear regression (three-parameter logistics) equation.
where X is the logarithm of dose and Y is the response. Y starts at a (the bottom) and goes to b (the top) with a sigmoid shape. Graphpad Prism for windows version 7.01 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses and ED50 determinations. p < 0.05 was considered statistically significant.
3. Results
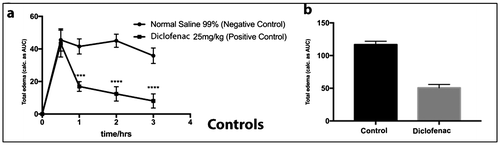
To evaluate the anti-inflammatory activities of the extracts, acute inflammation was induced in chicks using carrageenan. Subcutaneous administration of carrageenan resulted in a noticeable increase in paw size of the chicks. In control chicks, maximum inflammation was observed after 30 min and this persisted until the 2-h mark. This reduced very slowly to the third hour, an indication of the body’s own ability to combat inflammation (Figure (a), Control).
Oral administration of diclofenac (the positive control) or the extracts significantly reduced inflammation in the chicks and at a faster rate than the negative control group (Figures –). In general, all extracts examined in this study significantly reduced inflammation in a dose-dependent manner. One-way ANOVA data treatment followed by Dunnett’s post hoc test indicated that effect of extracts on edema were significant (p < 0.05). OSEA (at all concentrations tested; 50, 100, 150 mg/kg) had a significant effect in reducing inflammation (p < 0.05), similar to the standard drug, diclofenac.
Figure 1. Effect of LLE, LLEA, and LLM (50–150 mg kg−1) on time course curve (a, a′, & a″) and the total edema response in carrageenan-induced foot edema in chicks (b, b′, & b″). Values are means ± SEM (n = 4). p > 0.05 (ns), p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****) compared to vehicle-treated group. (One-way ANOVA followed by Dunnett post hoc test). p > 0.05(ns), p < 0.05 (ϕ), p < 0.01 (ϕϕ), p < 0.001 (ϕϕϕ), p < 0.0001 (ϕϕϕϕ) compared to vehicle-treated group (one-way ANOVA followed by Holm–Sidak’s post hoc test). LLE (Litorrina littorea 70% ethanol extract), LLEA (Litorrina littorea ethyl acetate extract) and LLM (Litorrina littorea methanol extract).
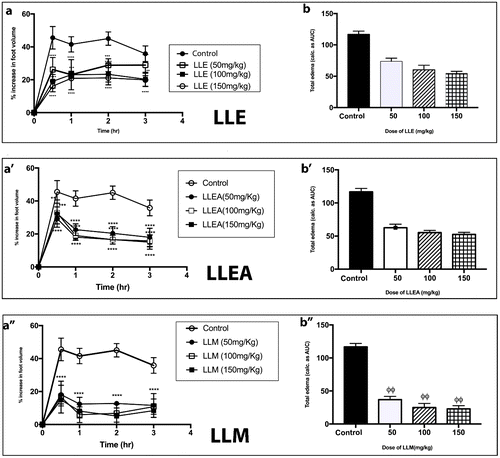
Figure 2. Effect of OSE, OSEA, and OSM (50–150 mg kg−1) on time course curve (c, c′, & c″) and the total edema response in carrageenan-induced foot edema in chicks (d, d′,& d″). Values are means ± SEM (n = 4). p > 0.05 (ns), p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****) compared to vehicle-treated group. (One-way ANOVA followed by Dunnett post hoc test). p > 0.05(ns), p < 0.01 (ϕ), p < 0.001 (ϕϕ), p < 0.0002 (ϕϕϕ), p < 0.0001 (ϕϕϕϕ) compared to vehicle-treated group (one-way ANOVA followed by Holm–Sidak’s post hoc test). OSE (Oliva sp., 70% ethanol extract), OSEA (Oliva sp., ethyl acetate extract), and OSM (Oliva sp., Methanol extract).
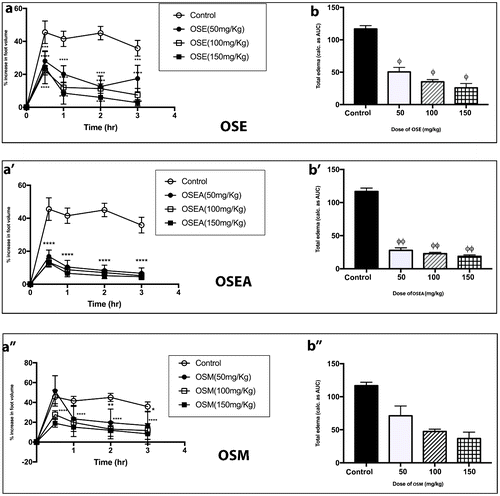
Figure 3. Effect of PRE and PREA (50–150 mg kg−1) on time course curve (e & e′) and the total edema response in carrageenan-induced foot edema in chicks (f & f′). Values are means ± SEM (n = 4). p > 0.05 (ns), p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****) compared to vehicle-treated group. (One-way ANOVA followed by Dunnett’s post hoc test). p > 0.05 (ns), p < 0.05 (ϕ), p < 0.01 (ϕϕ), p < 0.004 (ϕϕϕ), p < 0.0001 (ϕϕϕϕ) compared to vehicle-treated group (one-way ANOVA followed by Holm–Sidak’s post hoc test). PRE (Patella rustica ethanol extract), PREA (Patella rustica ethyl acetate extract).
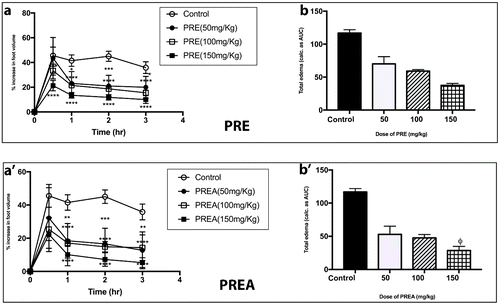
Figure 4. Effect of Normal saline 99% and Diclofenac (25 mg/kg) on time course curve (a) and the total edema response in carrageenan-induced foot edema in chicks (b). Values are means ± SEM (n = 4). p > 0.05 (ns), p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****) compared to vehicle-treated group. (One-way ANOVA followed by Dunnett’s post hoc test).
When the total edema over the experimental period for all extracts were expressed as AUCs (in arbitrary units) over the time course curves, a dose-dependent effect in decreasing total edema was also observed. The highest % inhibition of edema (83.90%) was recorded again by OSEA at a dose of 150 mg/kg (Figure ), whereas the least % inhibition of 33.42% was recorded by LLE for its 50 mg/kg dose (Figure ). The dose-dependent effect was also evident in the % inhibitions of all the extracts studied. As an example, PRE had % inhibitions of 46.69, 57.58, and 79.26 for 50, 100, and 150 mg/kg doses, respectively (Figure ). In comparison, the % inhibition of diclofenac at 25 mg/kg was 65.48%.
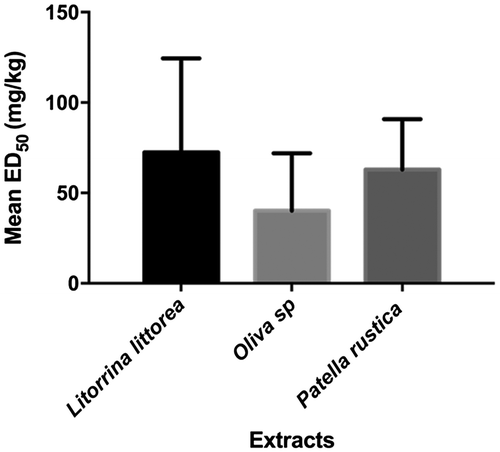
The ED50 values were computed via the three-parameter logistics equation. The most potent extract from this study was OSEA with an ED50 of 10.16 mg/kg. This was over 10 times better than LLE, whose ED50 value of 119.80 mg/kg was the highest and hence least potent (Table ). To compare how potent extracts from a specific mollusk was against another, the ED50 value of each extract from that particular mollusk were added and the mean computed (Figure ). Oliva sp. extracts were the most potent with a mean ED50 of 40.15 ± 18.35. L. littorea extracts were the least potent. One-way ANOVA treatment of data followed by Holm–Sidak’s post hoc test, however, revealed no statistically significant difference in potency between the extracts obtained from these three organisms (p > 0.05).
Table 1. Effect of extracts on carrageenan-induced edema in seven-day old chicks
4. Discussion
Two distinct phases have been associated with the carrageenan-induced paw edema model. The first phase (0–60 min) is mediated by mast cell degranulation with histamine and serotonin release followed by a late phase where inflammatory mediators such as prostaglandins, proteases, and lysosomes are produced (Eddouks, Chattopadhyay, & Zeggwagh, Citation2012; Posadas et al., Citation2004; Vinegar, Schreiber, & Hugo, Citation1969). It has also been suggested that prostaglandin release may be attributed to the cyclooxygenase (COX) induction in tissues (Di Rosa, Citation1972). All extracts used in this study profoundly inhibited carrageenan-induced acute edema in chick foot. The ethyl acetate extracts of Oliva sp. (OSEA), in particular, exhibited impressive potency among all extracts examined.
Even though the exact mechanism by which the extracts inhibited carrageenan-induced inflammation was not explored in this study, it is possible that reduction in inflammation occurs via the inhibition of the many inflammatory mediators released during the carrageenan-induced acute inflammation. For most of the extracts, reduction of inflammation was prominent after 30 min and seemed to plateau from 60 min till the end of the experiment (Figures and ). This suggests that the extracts were probably interacting with the inflammatory mediators that are released in the first phase of carrageenan-induced paw edema (i.e. histamine and serotonin). LLE did not seem to follow this route (Figure (a)), with no discernable reduction in inflammation after the first hour. It is, however, interesting to note that the highest level of inflammation which was achieved after 30 min for LLE is significantly lower than that of the control group (Figure (a)), indicating some level of potency. It has been suggested that the late phase are particularly susceptible to most anti-inflammatory agents (Di Rosa & Willoughby, Citation1971; Vinegar et al., Citation1969). The results obtained for diclofenac (Figure (a)) supports this notion. OSEA, in particular, had a time course profile (Figure (a′)) similar to that of the standard drug, diclofenac (Figure (a)).
The aquatic environment has proven to be a viable source of biologically active extracts and compounds and results from this study corroborates this notion. Many compounds isolated from the marine environment have shown impressive activities in various therapeutic areas. Antimicrobial and antitumor compounds accounts for most of these activities. However, others with antimalarial, antiviral, anti-inflammatory, anti-helminthic, and antioxidant potentials have also being unearthed (Blunt et al., Citation2014; Borquaye, Darko, Oklu, Anson-Yevu, & Ababio, Citation2016; Mayer et al., Citation2010).
The anti-inflammatory potentials of solvent extracts of some marine invertebrates were investigated by Herencia et al. (Citation1998). Extracts of Coscinasterias tenuispina and Holothuria tubulosa were shown to dose dependently inhibit edema, with lower elastase activity and decreased PGE2 levels measured in homogenates from inflamed paws, without affecting the levels of this prostanoid present in stomach homogenates. The sesquiterpenoid derivatives, avarol and avarone isolated from the Mediterranean sponge, Dysidea avara, have been shown to potently inhibit paw edema induced by carrageenan in mice (Ferrándiz et al., Citation1994). Other marine-derived compounds such as bolinaquinone and petrosiaspongiolide M have been shown to inhibit neutrophilic infiltration, interleukin-1β, prostaglandin E2 levels, and cyclooxygenase 2 (COX2) protein expression in vivo. This has led to plans to further develop these compounds for “protective strategies” against intestinal inflammatory diseases (Busserolles, Payá, & D’Auria, Citation2005). These examples, and many others, are indicative of the huge potential of marine natural products.
In Ghana, many crude extracts with substantial anti-inflammatory activities have been recorded (Abotsi, Ainooson, & Woode, Citation2012; Fleischer, Annan, Dickson, Mensah, & Sarpong, Citation2013; Mensah, Donkor, & Fleischer, Citation2011; Mensah, Mireku, & Okwuonu, Citation2014; Woode et al., Citation2009). The anti-inflammatory effects of Ficus exasperata (sandpaper tree) extracts have been evaluated to confirm their traditional use in pain management (Fleischer et al., Citation2013; Woode et al., Citation2009). However, investigations into the use of extracts from marine sources in such studies are negligible. We have shown earlier that extracts from P. rustica and L. littorea possess significant antimicrobial and antioxidant activities (Borquaye, Darko, Ocansey, & Ankomah, Citation2015; Borquaye et al., Citation2016). This work further shows that these mollusks harbor important compounds that could be beneficial in our search for new therapeutic agents.
5. Conclusion
In this study, the solvent extracts of Oliva sp, P. rustica, and L. littorea were shown to reduce inflammation in carrageenan-induced paw edema in chicks in a dose-dependent fashion. Oliva sp. extracts were most potent. Isolation and characterization of the pure compounds that elicited these anti-inflammatory activities is currently in progress in our laboratories. This work shows the immense utility of the marine environment as an important source of bioactive compounds for various therapeutic uses.
Author contributions
LSB and GD conceived the study and helped draft the manuscript. All experiments were designed by LSB, GD, and MKL. Samples were collected by RB, VR, MKL, and ENG. VR, RB, and ENG carried out all the experiments. Data analysis was by LSB, GD, and MKL. All authors read and approved the final manuscript.
Funding
The authors received no direct funding for this research.
Acknowledgment
The authors are grateful to the Departments of Chemistry and Pharmacology of the Kwame Nkrumah University of Science and Technology, Kumasi, for the use of its facilities for this study. We are also grateful to Dr. Nathaniel Owusu Boadi, Department of Chemistry for helpful discussions as well as Mr. Godwin Darku of the Animal House, Department of Pharmacology for technical support.
Additional information
Notes on contributors
Lawrence Sheringham Borquaye
Lawrence Sheringham Borquaye is a bioorganic chemist with a research focus on science at the interface of chemistry and biology. He’s been exploring biologically active natural products from marine organisms (mollusks, bacteria, and fungi) and from plant sources. Other research interests include the development of methods for the analysis of pharmaceutical and personal care products in the environment and the characterization of volatiles (essential oils) from plants.
Godfred Darko
Godfred Darko’s research interests spans environmental chemistry (www.sheathe.org), analytical chemistry, nanochemistry, and natural products.
Michael Konney Laryea
Michael Konney Laryea is a graduate student in the Borquaye Lab working on the isolation of biologically active natural products from plant and marine sources.
Victor Roberts
Richmond Boateng and Victor Roberts are undergraduate researchers in the Borquaye Lab. Visit us at www.borquayelab.com for detailed profiles of all authors.
Edward Ntim Gasu
Edward Ntim Gasu is a research assistant at the KNUST Central Laboratory investigating the unique properties of antimicrobial peptides.
References
- Abotsi, W. K. M., Ainooson, G. K., & Woode, E. (2012). Anti-inflammatory and antioxidant effects of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. (Phytolaccaceae). African Journal of Traditional, Complementary and Alternative Medicines, 9, 138–152.
- Allavena, P., Signorelli, M., Chieppa, M., Erba, E., Bianchi, G., Marchesi, F., … D ‘incalci, M. (2005). Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): Inhibition of macrophage differentiation and cytokine production. Cancer Research, 65, 2964–2971.10.1158/0008-5472.CAN-04-4037
- Beg, S., Hasan, H., Hussain, M. S., Swain, S., & Barkat, Ma (2011). Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacognosy Reviews, 5, 120.10.4103/0973-7847.91102
- Blunt, J. W., Copp, B. R., Keyzers, R. A., Munro, M. H. G., Prinsep, M. R., Zatylny-Gaudin, C., … Molgó, J. (2014). Marine natural products. Natural Product Reports, 31, 160.10.1039/c3np70117d
- Borquaye, L. S., Darko, G., Ocansey, E., & Ankomah, E. (2015). Antimicrobial and antioxidant properties of the crude peptide extracts of Galatea paradoxa and Patella rustica. SpringerPlus, 4, 1.10.1186/s40064-015-1266-2
- Borquaye, L. S., Darko, G., Oklu, N., Anson-Yevu, C., & Ababio, A. (2016). Antimicrobial and antioxidant activities of ethyl acetate and methanol extracts of Littorina littorea and Galatea paradoxa. Cogent Chemistry, 2, 1161865.
- Busserolles, J., Payá, M., & D’Auria, M. V. (2005). Protection against 2, 4, 6-trinitrobenzenesulphonic acid-induced colonic inflammation in mice by the marine products bolinaquinone and petrosaspongiolide M. Biochemical Pharmacology, 69, 1433–1440.10.1016/j.bcp.2005.01.020
- Chandra, S., Chatterjee, P., Dey, P., & Bhattacharya, S. (2012). Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pacific Journal of Tropical Biomedicine, 2, S178–S180.10.1016/S2221-1691(12)60154-3
- Cragg, G. M., & Newman, D. J. (2013). Natural products: A continuing source of novel drug leads. Biochimica et Biophysica Acta (BBA) - General Subjects, 1830, 3670–3695.10.1016/j.bbagen.2013.02.008
- Di Rosa, M. (1972). Biological properties of carrageenan. Journal of Pharmacy and Pharmacology, 24, 89–102.10.1111/jphp.1972.24.issue-2
- Di Rosa, M., & Willoughby, D. A. (1971). Screens for anti-inflammatory drugs. Journal of Pharmacy and Pharmacology, 23, 297–298.10.1111/jphp.1971.23.issue-4
- Drew, A. K., & Myers, S. P. (1997). Safety issues in herbal medicine: Implications for the health professions. The Medical Journal of Australia, 166, 538–541.
- Eddouks, M., Chattopadhyay, D., & Zeggwagh, N. A. (2012). Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evidence-Based Complementary and Alternative Medicine, 2012, 1–14.
- Emery, P. (2006). Treatment of rheumatoid arthritis. BMJ: British Medical Journal, 332, 152–155.10.1136/bmj.332.7534.152
- Faulkner, D. J. (2000). Highlights of marine natural products chemistry (1972–1999). Natural Product Reports, 17, 1–6.10.1039/a909113k
- Ferrándiz, M. L., Sanz, M. J., Bustos, G., Payá, M., Alcaraz, M. J., & De Rosa, S. (1994). Avarol and avarone, two new anti-inflammatory agents of marine origin. European Journal of Pharmacology, 253, 75–82.10.1016/0014-2999(94)90759-5
- Fleischer, T. C., Annan, K., Dickson, R. A., Mensah, A. Y., & Sarpong, F. M. (2013). Anti-inflammatory, antioxidant and antimicrobial activity of the stem bark extract and fractions of Ficus exasperata Vahl. (Moraceae). Journal of Pharmacognosy and Phytochemistry, 2, 38–44.
- Fusetani, N. (2011). Antifouling marine natural products. Natural Product Reports, 28, 400–410.10.1039/C0NP00034E
- Herencia, F., Ubeda, A., Ferrándiz, M. L., Terencio, M. C., Alcaraz, M. J., García-Carrascosa, M., … Payá, M. (1998). Anti-inflammatory activity in mice of extracts from Mediterranean marine invertebrates. Life Sciences, 62, 115–120.
- Jimeno, J., Faircloth, G., Fernández Sousa-Faro, J., Scheuer, P., & Rinehart, K. (2004). New Marine Derived Anticancer Therapeutics ─ A Journey from the Sea to Clinical Trials. Marine Drugs, 2, 14–29.10.3390/md201014
- Martins, A., Vieira, H., Gaspar, H., & Santos, S. (2014). Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Marine Drugs, 12, 1066–1101.10.3390/md12021066
- Mayer, A. M. S., & Lehmann, V. K. B. (2001). Marine pharmacology in 1999: Antitumor and cytotoxic compounds. Anticancer Research, 21, 2489–2500.
- Mayer, A. M. S., Rodriguezn, A. D., Berlinck, R. G. S., & Hamann, M. T. (2010). Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous syste. Biochimica et Biophysica Acta (BBA) - General Subjects, 1790, 283–308.
- Mensah, A. Y., Donkor, P. O., & Fleischer, T. C. (2011). Anti-inflammatory and antioxidant activities of the leaves of Wissadula amplissima var Rostrata. African Journal of Traditional, Complementary and Alternative Medicines, 8, 185–195.
- Mensah, A., Mireku, E., & Okwuonu, V. (2014). Anti-inflammatory and anti-oxidant activities of Secamone afzelii (Rhoem) Ascleipiadaceae. Journal of Medical and Biomedical Sciences, 3, 23–30.10.4314/jmbs.v3i1.4
- Mitchell, R., & Cotran, R. (2003). Acute and chronic inflammation. In R. L. Johnson (Ed.), Robbins basic pathology( Vol. 7, pp. 33–59). Philadelphia, PA: Saunders.
- National Cancer Institute. (2015). FDA approves trabectedin for sarcoma. Author. Retrieved March 12, 2017, from https://www.cancer.gov/news-events/cancer-currents-blog/2015/fda-trabectedin-sarcoma
- Newman, D. J., & Cragg, G. M. (2004). Marine natural products and related compounds in clinical and advanced preclinical trials. Journal of Natural Products, 67, 1216–1238.10.1021/np040031y
- Posadas, I., Bucci, M., Roviezzo, F., Rossi, A., Parente, L., Sautebin, L., & Cirino, G. (2004). Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. British Journal of Pharmacology, 142, 331–338.10.1038/sj.bjp.0705650
- Roach, J., & Sufka, K. (2003). Characterization of the chick carrageenan response. Brain Research, 994, 216–225.10.1016/j.brainres.2003.09.038
- Thakur, N. L., Thakur, A. N., & Müller, W. E. G. (2005). Marine natural products in drug discovery. Natural Product Radiance, 4, 471–477.
- U.S. Food and Drug Administration. (2015). Press announcements - FDA approves new therapy for certain types of advanced soft tissue sarcoma. Retrieved March 12, 2017, from https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm468832.htm
- Vinegar, R., Schreiber, W., & Hugo, R. (1969). Biphasic development of carrageenin edema in rats. Journal of Pharmacology and Experimental Therapeutics, 166, 96–103.
- Woode, E., Ansah, C., Ainooson, G. K., Abotsi, W. M. K., Mensah, A. Y., & Duwiejua, M. (2007). Anti-inflammatory and antioxidant properties of the root extract of Carissa edulis (Forsk.) Vahl (Apocynaceae. Journal of Science and Technology, 27, 5–15.
- Woode, E., Poku, R. A., Ainooson, G. K., Boakye-Gya, E., Abotsi, W. K. M., Mensah, T. L., & Amoh-Barim, A. K. (2009). An evaluation of the anti-inflammatory, antipyretic and antinociceptive effects of Ficus exasperata (Vahl) Leaf Extract. Journal of Pharmacology and Toxicology, 4, 138–151.10.3923/jpt.2009.138.151