Abstract
Introduction: Pre-, post-menopausal, and male human osteoblasts (hObs) express estradiol-17β (E2) receptors α and β (ERα, ERβ), vitamin D receptor (VDR), and 25-hydroxy vitamin D3 1-α hydroxylase (1OHase) and produce 1,25 (OH)2D3 (1,25D). Pre-treatment with JKF (JKF 1624F2–2) up-regulated estrogenic responsiveness, via modulation of ERs expression and E2 binding. These estrogens induce VDR and 1OHase expression and 1,25D production. Purpose: Compared the effects of femarelle (F) to daidzein (D) and E2 by themselves and their modulation by JKF. Methods: hObs derived from human bones at different ages and sex were cultured, treated with different hormones and analyzed for the different parameters. Results: (1) F, D and E2 stimulated 3[H] thymidine incorporation (DNA) and creatine kinase specific activity (CK) in female but not in male hObs. The responses to E2 and to D unlike to F were up-regulated by JKF. (2) All hormones increased ERα and decreased ERβ mRNA in all hObs. (3) JKF modulated in different ways the expressions of ERα and ERβ mRNA in all hObs. (4) JKF did not significantly modulate the expressions of ERα and ERβ mRNA in all hObs. (5) JKF increased intracellular competitive binding of E2 by all hormones only in female hObs. (6) Pre-treatment with all hormones increased VDR and 1OHase expression and 1,25D formation in pre- and post-, but only JKF modulated it in male hObs. Conclusions: F, D and E2 increase different parameters in hObs. However, pre-treatment with JKF modulates the effect of E2 and D but not of F. F reciprocally modulates mRNA expression and activity of 1OHase which in turn up-regulate ERs expression and activity. These finding may contribute to F’s beneficial role in treatment of post-menopausal bone loss even in vitamin D deficiency.
Public Interest Statement
In the last years a lot of interest is in the use of phytoestrogens and their derivatives in human biology and medicine. Since there are a lot of health problems in the use of either native estrogen or native phytoestrogens, it is important to use new compounds. We therefore decided to analyze our new compound femarelle which is a synthetic new phytoestrogens in our experiments. We used it before in animal models showing its importance, and now in human bone cells. We can see how unique it is in its estrogenic effects and its activity which is not affected by other hormones as the estrogen itself.
We therefore believe in its special importance for bone biology and other effects as shown before.
Competing Interests
The authors declare no competing interest.
1. Introduction
Phytoestrogens are heterogeneous group of plant-derived compounds, some of which are selective estrogen receptor modulators (SERMs). They are all polyphenolic compounds with structural similarities to natural and synthetic estrogens and they bind to estrogen receptors (ERs) with much lower affinity compared to E2 (Miksicek, Citation1994). Phytoestrogens have estrogenic activity in bone and in the cardiovascular system but have anti-estrogenic activity in the breast and in the uterus (Brzezinski & Debi, Citation1999). They have been proposed to prevent bone resorption and to promote bone formation and increased bone density (Setchell & Lydeking-Olsen, Citation2003; Tham, Gardner, & Haskell, Citation1998; Yoles et al., Citation2003). Femarelle (F), a chemical derivative of the phytoestrogen daidzein (D), which is a standardized extract derived from soybeans was shown to increase bone mineral density in post-menopausal women (Yoles et al., Citation2004) and to relieve vasomotor symptoms with no effect on sex hormone levels or endometrial thickness (Somjen & Yoles, Citation2003a). It has also properties as selective estrogen receptor modulator (SERM) as was shown previously both in rat in vivo and in different cultured cells in vitro (Eriksen & Glerup, Citation2002; Somjen, Katzburg, Lieberherr, Hendel, & Yoles, Citation2006; Somjen & Yoles, Citation2003b). It has an estrogen-like activity and activates human-derived female-cultured bone cells (hObs) which express receptors for E2 both ERα and ERβ and for vitamin D (VDR). Estrogens and Vitamin D metabolites and their analogs regulate cell proliferation (DNA) and energy metabolism through modulation of the specific activity of creatine kinase (CK). Pre-treatment with vitamin D less-calcemic analog: JKF 1624F2–2 (JKF) up-regulated responsiveness to E2 and to different estrogens, via modulation of ERs mRNA expression. Estrogens, in turn, induce VDR and 25-hydroxy vitamin D3 1-α hydroxylase (1OHase) expression and 1,25(OH) 2D3 (1,25D) synthesis.
Optimal bone growth and prevention of osteoporosis in post-menopausal women, requires adequate concentrations of vitamin D3 as well as estrogens (Somjen, Waisman, Weisman, & Kaye, Citation2000). We have studied previously the interaction between the vitamin D3 metabolite; 1,25D and its less-calcemic analogs with estrogens in a rat model (Reiss & Kaye, Citation1981; Somjen, Waisman, Lee, Posner, & Kaye, Citation2001) using the increase in CK as a response marker for hormonal treatment in cells containing biologically active ERs (Sömjen, Harell, Jaccard, Weisman, & Kaye, Citation1990; Somjen, Weisman, Harell, Berger, & Kaye, Citation1989).
Moreover, pre-treatment with 1,25D up-regulated sex-specific responsiveness and sensitivity to E2 and to several SERMs in osteoblast-like cell lines and rat bone, as measured by both the stimulation of CK and the increase in DNA synthesis (Fournier, Haring, Kaye, & Somjen, Citation1996; Reiss & Kaye, Citation1981; Sömjen, Waisman, Weisman, & Kaye, Citation1998; Sömjen et al., Citation1990). This mutual interaction between E2 4 and vitamin D was also manifested by an increase in the expression of ERs after treatment with 1,25D (Kanis et al., Citation1979). However, the use of vitamin D3 metabolites is restricted by their hypercalcemic effects (Posner et al., Citation1998). We reported that multiple treatments with “less-calcemic” analogs of vitamin D, particularly JKF (Posner, Citation2004), stimulated osteoblast-like cells (Katzburg et al., Citation2005; Posner, Citation2004) and pre-treatment of skeletal-derived cells with these analogs, up-regulated both responsiveness and sensitivity to E2 (Fournier et al., Citation1996; Katzburg et al., Citation1999, Citation2005).
The present study was undertaken to measure age and sex specificities in the responses of cultured human female and male osteoblastss (hObs) to E2, to the phytoestrogen daidzein; D and to the synthethic phytoestrogen femarelle; F and their modulation by JKF. We sought to correlate these, VDR as β and ERαresponse changes with the expression of mRNA for ER well as 1OHase expression and activity resulting in the synthesis of the active metabolite 1,25D (Somjen et al., Citation2005), as well as the intracellular binding of E2.
2. Materials and methods
2.1. Reagents
Estradiol-17β (E2), Daidzein (D) and creatine kinase (CK) assay kit were purchased from Sigma Chemicals Co. (St. Louis, MO). Femarelle (F) was purchased from Se-Cure Pharmaceuticals (Dalton, Israel). JKF 1624F2–2 (JKF) was synthesized and donated by Dr G.H. Posner et al. (Citation1998). All other reagents were of analytical grade.
2.2. Cell Cultures
Human female osteoblasts (hObs) from pre- (age up to 45 years) and post-menopausal (age from 55 years) women or males (age 45–65 years) were prepared from bone explants of healthy individuals which were not taking any medicines, by a non-enzymatic method as described previously (Katzburg et al., Citation1999). Each culture was prepared from each donor separately. Shortly: samples of the trabecular surface of the iliac crest or long bones were cut into 1 mm3 pieces and repeatedly washed with phosphate buffered saline (PBS) to remove blood components. The explants were incubated in DMEM medium without calcium (to avoid fibroblastic growth) containing 10% fetal calf serum (FCS) and antibiotics. First passage cells were seeded at a density of 3 × 105 cells/35 mm tissue culture dish, in phenol red- free DMEM with 10% charcoal stripped FCS, and incubated at 37oC in 5% CO2.
2.3. Hormonal treatments
Cells were treated either for 24 h with the different hormones or daily with vehicle (0.01% ethanol in medium) or JKF at 1nM final concentration (Reiss & Kaye, Citation1981), for 3 days, starting on day 1 after seeding and on day 4 after seeding, the cultures were then treated for additional 24 h with 200 ng/ml F, 300nM D or 300nM E2, followed by harvesting for CK assay or DNA synthesis. In some experiments, cells were pre-treated with the different estrogenic compounds in similar pattern and the changes in the vitamin D system was assayed (Somjen et al., Citation2005).
2.4. Creatine kinase extraction and assay in hObs
Cells were treated for 24 h with the various agents as specified, scraped off and homogenized by freezing and thawing three times in an extraction buffer, and CK was extracted and assayed as previously described (Reiss & Kaye, Citation1981; Somjen et al., Citation1989, Citation2001). Protein was determined by Coomasie blue dye binding, using bovine serum albumin (BSA) as the standard.
2.5. DNA synthesis assay in hobs
Cells were grown until sub-confluence and then treated with various hormones or agents as indicated. Twenty-two hours following the exposure to these agents, 3[H]thymidine was added for 2 h and the 3[H]thymidine incorporation into DNA was determined (Katzburg et al., Citation1999).
2.6. Determination of ERα and ERβ mRNA by real time PCR in hObs
RNA was extracted from cultured human bone cells, shown previously (Eriksen & Glerup, Citation2002; Somjen et al., Citation2005) and subjected to reverse transcription as previously described (Katzburg et al., Citation1999; Somjen et al., Citation2005). ER standard controls and compared to RNAse P as internal control for mRNA.
2.7. Competitive binding assay for intracellular estrogenic binding sites in hObs
Cells with and without pre-treatment with JKF, were incubated for 60 min at 37OC with 3[H]E2 with and without excess of unlabelled compounds as described. Binding was terminated by four successive washes with ice-cold binding medium, and cellular content of 3[H]E2 was measured in a scintillation counter (Katzburg et al., Citation1999; Somjen et al., Citation2005).
2.8. Determination of vitamin D receptor (VDR) and 25-hydroxy vitamin D3 1-hydroxylase (1OHase) mRNA by real time PCR in hObs
RNA was extracted from cultured cells and subjected to reverse transcription as previously described (Katzburg et al., Citation1999; Somjen et al., Citation2005). RNAse P expression served as an internal control.
2.9. Assay of 1OHase activity in hObs
1OHase activity was measured by the level of 1,25D generated in hObs within 60 min after the addition of 25(OH)D3 (200 ng/ml) to culture, using the 1,25D 125I RIA kit from Dia Sorin, Mn, USA (Somjen et al., Citation2005). Protein of the cellular layer was assayed by the Bradford method.
2.10. Statistical analysis
Differences between the mean values of experimental and control groups were evaluated by analysis of variance (ANOVA); p values less than 0.05 were considered significant.
3. Results
3.1. The effects of F, D, or E2 with and without JKF on stimulation of DNA synthesis in hObs
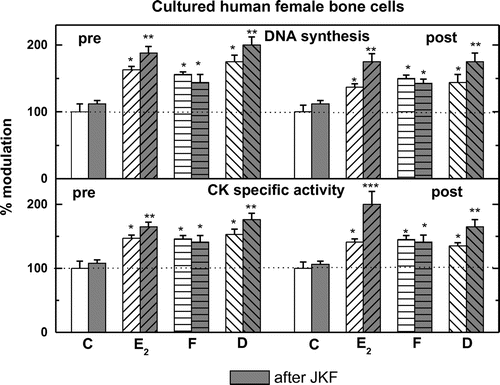
All cells analyzed pre-, post- and male hObs have basal DNA synthesis with lower activity in post-hObs (4,250 + 300 in pre- vs. 3,000 + 375 in post- and 4,450 + 400 dpm/well in male hObs) (Figure ). Addition of different hormones except JKF stimulated significantly 3[H] thymidine incorporation into all female but not in male hObs, with higher responses in pre-hObs compared to post-hObs 305 + 11% vs. 155 + 10% by E2, 167 + 11% vs. 150 + 14% by D and 173 + 14% vs. 159 + 12% by F (Figure ). Pre-treatment with JKF did not change the stimulation of DNA synthesis by F 159 + 5% vs. 141 + 12% in pre- and 159 + 4% vs. 141 + 5% in post-hObs, but did up-regulate the stimulation by E2 (165 + 6% vs. 188 + 12% in pre- and 135 + 4% vs. 176 + 12% in post-hObs) and by D (175 + 12% vs. 200 + 13% in pre- and 141 + 12% vs. 176 + 13% in post-hObs) (Figure ). No significant changes in the age dependent responsiveness to F unlike to E2 or to D were observed (Figures and ).
Figure 1. Basal levels of DNA synthesis and CK specific activity in pre-menopausal (pre), in post-menopausal human osteoblasts (post) and male human cultured bone cells.
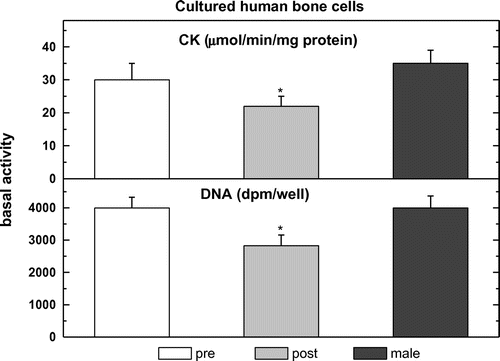
Figure 2. Stimulation of DNA synthesis or CK specific activity by F, D or E2 as well as JKF in hObs from pre- and post-menopausal women or male.
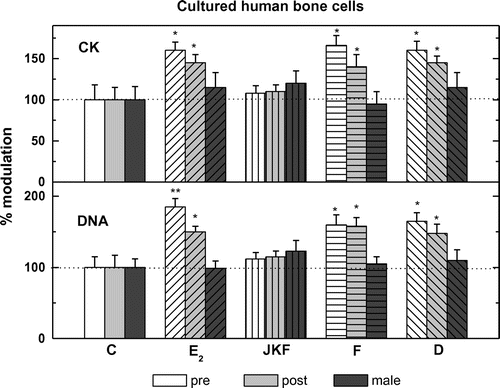
Figure 3. Stimulation of DNA synthesis or CK specific activity by F, D, or E2 in hObs from pre- and post-menopausal women after pre-treatment with JKF (light gray bars).
3.2. The effects of F, D or E2 with and without JKF on stimulation of creatine kinase (CK) specific activity in hObs
All cells analyzed pre-, post- and male hObs have basal CK specific activity with lower activity in post- hObs (32.5 + 0.5 in pre- vs. 24.5 + 3.0 in post- and 32.5 + 0.5 μmol/min/mg protein in male hObs) (Figure ). Addition of the hormones except JKF stimulated significantly CK in female hObs at different ages but not in male, with higher responses in pre- compared to post-hObs (155 + 9% vs. 141 + 9% by E2, 155 + 12% vs. 141 + 9% by D and 161 + 10% vs. 136 + 11% by F) (Figure ). Pre-treatment with JKF did not change the stimulation of CK by F (150 + 6% vs. 138 + 10% in pre- and 150 + 5% vs. 144 + 12% in post-hObs) but did up-regulate the stimulation by E2 (150 + 5% vs. 169 + 11% in pre- and 144 + 5% vs. 200 + 25% in post-hObs) and by D (156 + 11% vs. 175 + 12% in pre- and 138 + 5% vs. 175 + 12% in post-hObs) (Figure ). No significant changes in the age dependent responsiveness to F unlike to E2 or to D were observed (Figures and ).
3.3. The expression and its modulation by JKF, F, D or E2 of ERα and ERβ in hObs
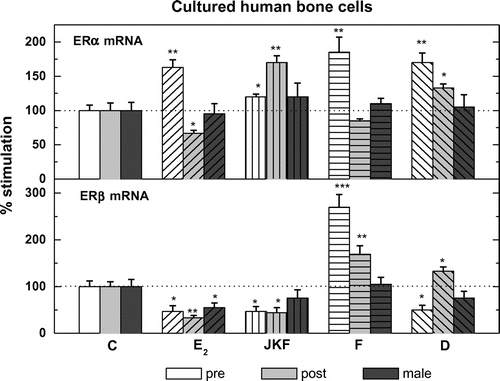
All cells analyzed; pre-, post- and male hObs expressed mRNA for both ERα and ERβ as measured by real time PCR, corrected for RNAse P mRNA. In pre-ERα expression was 0.094 + 0.007 vs. 0.076 + 0.006 in post- and 0.100 + 0.008 2−∆CT in male hObs, whereas ERβ in pre- 0.00085 + 0.00012 vs. 0.00098 + 0.00011 in post- and 0.00094 + 0.00008 2−∆CT in male hObs. The ratio of ERα to ERβ was 121:1 in pre-, 78:1 in post- and 105:1 in male hObs (Figure ). Pre-treatment with the different hormones as well as JKF modulated the expression of ERα and ERβ to different extents in both female age groups and to less extent in male hObs (in ERα 117 + 20% in pre- and 167 + 10% in post-hObs and 117 + 20% in male hObs and in ERβ 50 + 9% in pre-, 49 + 15% in post- and 67 + 17% in male hObs) (Figure ). On the other hand E2 increased ERα by 156 + 11 in pre- but not in post- and in male hObs (67 + 15% and 99 + 12% respectively). D increased ERα (by 167 + 12% in pre-, by 133 + 5% in post- and 101 + 21% in male hObs). F increased ERα by 178 + 22% in pre-, but not in post- (78 + 5%) and in male (106 + 10%) hObs. D increased ERβ by 267 + 30% in pre-, by 167 + 18% in post- and by 100 + 10% in male hObs. E2 did not increase ERβ in pre- (50 + 15%), or in post- (33 + 5%) and in male (67 + 10%) hObs, JKF increased ERα in pre- and post-hObs and decreased ERβ in pre- and post-hObs with no effect on male hObs (Figure ).
Figure 4. The expression of estrogen receptors ERα and ERβ in pre-menopausal human osteoblasts (pre), in post-menopausal human osteoblasts (post) and human males (male).
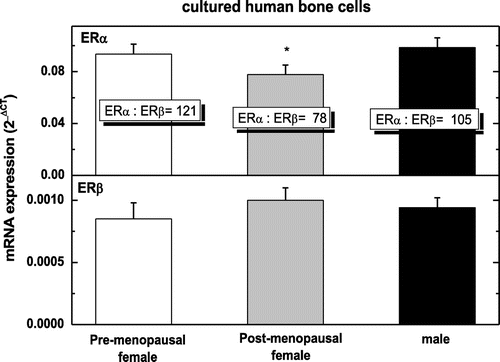
Figure 5. Modulation by pre-treatment with E2, D, F or JKF on the expression of ER mRNA in hObs obtained from pre- and post-menopausal women.
3.4. The intracellular binding of E2 and its modulation by JKF, F, D or E2 in hObs
Both pre- and post-menopausal female but not male-derived hObs demonstrated E2 specific binding of 3[H]E2 (Figure ), presumably predominantly nuclear under these conditions (37° for 60 min). All compounds tested for competition with 3[H]E2 i.e. E2, F and D, but not JKF showed significant binding in both age groups but not in male hObs (Figure ). Pre-treatment of hObs with JKF increased only slightly non significantly the specific binding of 3[H]E2 in female cells from both age groups by all hormones studied (Figure ).
Figure 6. The effect of E2, D, F or JKF on intracellular binding of E2 in pre- or post-menopausal female hObs, as measured by competition of the binding of 3 [H] E2. The effects of treatment with JKF (3 days with 1nM) or E2, D and F on intracellular binding of E2 in pre- or post-menopausal female hObs, as measured by competition of the binding of 3 [H] E2.
![Figure 6. The effect of E2, D, F or JKF on intracellular binding of E2 in pre- or post-menopausal female hObs, as measured by competition of the binding of 3 [H] E2. The effects of treatment with JKF (3 days with 1nM) or E2, D and F on intracellular binding of E2 in pre- or post-menopausal female hObs, as measured by competition of the binding of 3 [H] E2.](/cms/asset/57d5e3e2-4fa3-4417-aa91-fbba8b1c92e1/oabi_a_1374919_f0006_b.gif)
Figure 7. Modulation of the specific intracellular binding of 3 [H] E2 binding in pre-menopausal human osteoblasts (pre-), in post-menopausal human osteoblasts (post-) and males (male) by 30nM E2, 3,000 nM D or 200 ng/ml F after three daily treatments with 1nM JKF.
![Figure 7. Modulation of the specific intracellular binding of 3 [H] E2 binding in pre-menopausal human osteoblasts (pre-), in post-menopausal human osteoblasts (post-) and males (male) by 30nM E2, 3,000 nM D or 200 ng/ml F after three daily treatments with 1nM JKF.](/cms/asset/57704696-083f-4c97-ac25-39143687b767/oabi_a_1374919_f0007_b.gif)
3.5. The modulation by JKF, F, D or E2 of VDR and 1OHase mRNA expression in hObs
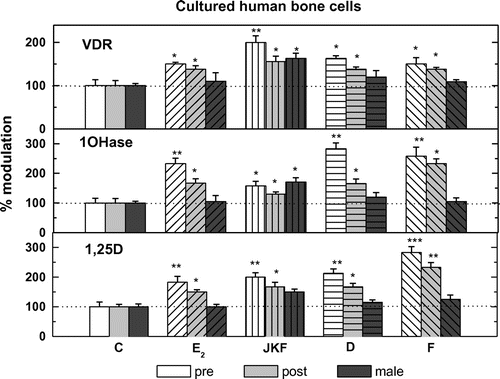
Female-derived bone cells from both ages and male hObs expressed mRNA for VDR and 1OHase as measured by real time PCR, corrected for RNAse P mRNA (VDR 0.8 + 0.033 in pre-, 0.41 + 0.067 in post- and 0.50 + 0.133 2−∆CT in male hObs, 1OHase 0.188 + 0.025 in pre-, 0.25 + 0.02 in post- and 0.075 + 0.002 2−∆CT in male hObs) (Figure ). Treatment with all hormones tested increased the expression of 1OHase and VDR by about 35–165%, in both age groups but only JKF increased the expressions in male hObs by about 50% (Figure ).
Figure 8. The expression of VDR or 1OHase and 1,25D production in pre-menopausal human osteoblasts (pre-), in post-menopausal human osteoblasts (post-) and in human male osteoblasts (male).
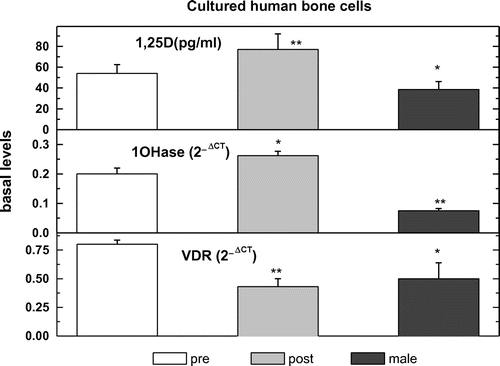
Figure 9. The effects of treatment with E2, D, F, or JKF on 1,25D production (lower panel) and 1OHase mRNA expression (middle panel), as well as on VDR mRNA expression (upper panel) in pre- and post-menopausal and male hObs.
3.6. The production of 1,25D and its modulation by JKF, F, D or E2 in hObs
Female-derived bone cells from both ages as well as male hObs produced 1,25D as measured by radio-immunoassay 53.7 + 7.1 in pre-, 72.8 + 14.3 in post- and 35.7 + 7.1 pg/ml in male hObs (Figure ). F, D and E2 treatment increased the activity of 1OHase by increasing the production of 1,25D by about 80–150%, in both age groups but not in male hObs (Figure ). JKF increased 1,25D production also in male hObs by about 40% as well as by about 180% in pre- and about 160% in post-hObs (Figure ).
4. Discussion
In our previous studies as well as in the present one, we found that F similar to D (its precursor) or E2 stimulates—age- and sex-dependently—both DNA synthesis and CK specific activity in primary cultures of human female hObs but not in human male hObs (Eriksen & Glerup, Citation2002). Pre-treatment with JKF emphasizes the difference between F and D or E2, whereas the stimulation of D or E2 on both parameters and in both female age groups were up-regulated by the vitamin D analog JKF, the effects of F were not significantly changed in either age groups by JKF pre-treatment (Figures , and ). We showed that up-regulation of the stimulation by vitamin D3 less-calcemic analogs occurs by modulation of ERα and ERβ mRNA in these cells. We have previously demonstrated, using western blot analysis (Kanis et al., Citation1979) that JKF increased the protein levels of ERα and ERβ with a greater increase of ERα than ERβ in pre-menopausal-derived cells and the opposite occurred in post-menopausal-derived cells (Sömjen, Weisman, & Kaye, Citation1995; Sömjen et al., Citation1990). The effects of JKF on nuclear binding (Katzburg et al., Citation1999; Somjen et al., Citation2005) are consistent with the increased responsiveness to E2 after JKF pre-treatment as well as changes in ER proteins (Fournier et al., Citation1996). In contrast, the membranal binding of E2 and all the phytoestrogens tested was abolished by JKF pre-treatment (Katzburg et al., Citation1999). Membranal binding is therefore not correlated with the up-regulation of DNA synthesis or CK specific activity stimulated by estrogens after JKF pre-treatment. This indicates that membranal processes are not involved in CK stimulation by estrogenic compounds tested in this study or others (Katzburg et al., Citation1999; Somjen & Yoles, Citation2003b; Somjen et al., Citation2006).
It is important to notice that these results are seen in vitamin D deficient rats in which the response of bone to E2 is attenuated and is restored after treatment with sufficient amounts of vitamin D (Somjen et al., Citation2007).
ERα and ERβ mRNA were found in both ages of female-derived osteoblasts but while JKF increased ERα expression and decreased ERβ expression, E2 and D significantly down regulated ERβ without affecting ERα suggesting a negative effect of E2 on estrogenic responsiveness in cells from both age groups. On the other hand, F increased both ERs suggesting its positive effect on estrogenic responsiveness in human female-derived osteoblasts from both age groups.
The synthesis of 1,25D from its precursor 25-hydroxyvitamin D3 (25(OH) D3), is catalyzed by 1OHase in epithelial cells comprising various parts of the human nephron (Sömjen, Kaye, Harell, & Weisman, Citation1989; CitationZehnder, Bland, Walker, et al., 2001). Renal 1OHase is subject to tight systemic metabolic control by parathyroid hormone (PTH), calcium, phosphate and vitamin D3 metabolites, predominantly 1,25D itself (Reichel, Koeffler, & Norman, Citation1987). Renal 1OHase is the major source of circulating 1,25D, which controls systemic calcium homeostasis; nevertheless, external-renal expression of 1OHase and 1,25D is now well documented in various tissues and cell types such as prostate cells (Reichel et al., Citation1987; Sömjen et al., Citation1989; Zehnder, Bland, Walker, et al., Citation2001). Molecular studies indicate that the enzyme is expressed in both the kidney and non-renal tissues (Sömjen et al., Citation1989; Zehnder, Bland, Walker, et al., Citation2001) and is differentially regulated at least in some of these tissues (Feldman, Malloy, Goldschmidt, & Gross, Citation2001; Pryke, Duggan, White, Posen, & Mason, Citation1990; Zehnder, Bland, Williams, et al., Citation2001). In contrast to circulating 1,25D which controls systemic calcium homeostasis through its action on the intestinal mucosa, bone and kidney, accumulating data indicate that the 1,25D produced by extra-renal 1OHase in various tissues does not contribute to circulatory levels but rather appears to act in an autocrine and/or paracrine fashion by modulating cell proliferation, differentiation, apoptosis, immunoregulation and other functions at a local level (Holick, Citation2003; Walters, Citation1992) .
We have shown that human female osteoblasts express 1OHase mRNA and produces 1,25D both of which are modulated by different hormones including estrogenic compounds (Somjen et al., Citation2007). The synthesis of 1,25D in hObs is quantitatively significant at basal production rate of ~1.5 pmol/mg protein/hr, reaching ~4 pmol/mg protein/hr under saturating concentrations of its substrate 25(OH)D3 (Somjen et al., Citation2005), which might be important for the physiology of bone as was shown for other parameters.
The effects of F as well as D and E2 on 1OHase expression and activity remain obscure. The current study demonstrates that F modulates local 1OHase expression in hObs which might, in turn, modulate the effects of other estrogenic compounds on the cells. It is not known, however, whether this happens also in vivo. If it does, it might be used as a mixed new treatment for bone formation in post-menopausal osteoporosis.
The effect of the locally formed 1,25D might be involved in the modulation by vitamin D of the hormonal responses of hObs to the different estrogenic compounds. This might be due to the changes in the expression of estrogen receptors, which were reported to be changed with external vitamin D treatments as described previously (Holick, Citation2003; Somjen et al., Citation2005, Citation2007). This might be a defence mechanism in the pathophysiological conditions of absence of vitamin D in the body. The different parameters changed in hObs response to estrogens by vitamin D analogs show a high increase in CK, and a slight increase in intracellular estrogen binding as well as increased mRNA for ERα. On the other hand, both binding of membranal receptors and mRNA for ERβ were reduced. Whether this indicates a differential interaction of F with the different binding sites through which it exerts its biological activity, needs to be studied.
In conclusion, the present study provides evidence for the mutual interaction between F or other estrogenic compounds and the vitamin D3 system. The potential role of this system as an autocrine/ paracrine mechanism to modulate bone cell metabolism and physiology warrants further investigation. Of importance is the fact that since F is not up-regulated by vitamin D-like estrogens, it is also active in vitamin D deficient conditions, unlike estrogens. This might add to its advantage in hormone replacement therapy treatment over the use of E2 and other estrogens.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
D. Somjen
The authors of the paper are members of the Tel-Aviv medical center and other institutions as reported. We are interested in bone and vascular endocrinology and especially in synthetic estrogens and vitamin D analogs as reported in here and in other previous studies and papers. We have shown their importance and their use with much less side effects as other ones, we believe in their use in human health and hope to promote it, without any commercial benefits to us.
References
- Brzezinski, A., & Debi, A. (1999). Phytoestrogens: The “natural” selective estrogen receptor modulators? European Journal of Obstetrics & Gynecology and Reproductive Biology, 85, 47–51.10.1016/S0301-2115(98)00281-4
- Eriksen, E. F., & Glerup, H. (2002). Vitamin D deficiency and aging: Implications for general health and osteoporosis. Biogerontology, 3, 73–77.10.1023/A:1015263514765
- Feldman, D., Malloy, P. J., Goldschmidt, D., & Gross, C. (2001). Vitamin D: Biology, action and clinical implications. In R. Marcus, D. Feldman, & J. Kelsey (Eds.), Osteoporosis (Vol 1, pp. 230–257). San Diego, CA: Academic Press.
- Fournier, B., Haring, S., Kaye, A. M., & Somjen, D. (1996). Stimulation of creatine kinase specific activity in human osteoblast and endometrial cells by estrogens and antiestrogens and its modulation by calciotropic hormones. Journal of Endocrinology, 150, 275–285.10.1677/joe.0.1500275
- Holick, M. F. (2003). Prostatic 25-hydroxy vitamin D-1-α-hydroxylase and its implication in prostate cancer. Journal of Cellular Biochemistry, 88, 315–322.
- Kanis, J. A., Cundy, T., Earnshaw, M., Henderson, R. G., Heynen, G., Naik, R., & Russell, R. G. G. (1979). Treatment of renal bone disease with 1α derivatives of vitamin D3. An International Journal of Medicine, 48, 289–322.
- Katzburg, S., Lieberherr, M., Ornoy, A., Klein, B. Y., Hendel, D., & Somjen, D. (1999). Isolation and hormonal responsiveness of primary cultures of human bonederived cells: Gender and age differences. Bone, 25, 667–673.10.1016/S8756-3282(99)00225-2
- Katzburg, S., Hendel, D., Waisman, A., Posner, G. H., Kaye, A. M., & Somjen, D. (2005). Treatment with “non-hypercalcemic” analogs of 1,25 dihydroxy vitamin D3 increases responsiveness to 17-β estradiol, dihydrotestosterone or raloxifene in primary human osteoblasts. The Journal of Steroid Biochemistry and Molecular Biology, 88, 213–219.
- Miksicek, R. J. (1994). Interaction of urally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. The Journal of Steroid Biochemistry and Molecular Biology, 49, 153–160.10.1016/0960-0760(94)90005-1
- Posner, G. H. (2004). Modulation of response to estrogens in cultured human female bone cells by A Non- calcemic vitamin D analog: Changes in nuclear and membranal binding. The Journal of Steroid Biochemistry and Molecular Biology, 89–90, 393–395.
- Posner, G. H., Lee, J. K., Wang, Q., Peleg, S., Burke, M., Brom, H., … Kensler, T. W. (1998). lcemic, antiproliferative, transcriptionally active, 24- fluorinated hybrid analogues of the hormone 1α, 25- dihydroxyvitamin D3. Synthesis and preliminary biological evaluation. Journal of Medicinal Chemistry, 41, 3008–3014.
- Pryke, A. M., Duggan, C., White, C. P., Posen, S., & Mason, R. S. (1990). Tumor necrosis factor-α induces vitamin D-1-hydroxylase activity in normal human alveolar macrophages. Journal of Cellular Physiology, 142, 652–656.10.1002/(ISSN)1097-4652
- Reichel, H., Koeffler, H. P., & Norman, A. W. (1987). Synthesis in vitro of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 by interferon–stimulated normal human bone marrow and alveolar macrophages. Journal of Cellular Biochemistry, 262, 10931–10937.
- Reiss, N., & Kaye, A. M. (1981). Identification of the major component of the estrogen induced protein of rat uterus as the BB isozyme of creatine kinase. The Journal of Biological Chemistry, 256, 5741–5749.
- Setchell, K. D., & Lydeking-Olsen, E. (2003). Dietary phytoestrogens and their effect on bone: Evidence from in vitro and in vivo, human observational, and dietary intervention studies. The American Journal of Clinical Nutrition, 78, 593S–609S.
- Sömjen, D., Harell, A., Jaccard, N., Weisman, Y., & Kaye, A. M. (1990). Reciprocal modulation by sex steroids and calciotropic hormones of skeletal cell proliferation. The Journal of Steroid Biochemistry and Molecular Biology, 37, 491–499.10.1016/0960-0760(90)90392-X
- Somjen, D., Katzburg, S., Lieberherr, M., Hendel, D., & Yoles, I. (2006). DT56a stimulates gender- specific human cultured bone cells in vitro. The Journal of Steroid Biochemistry and Molecular Biology, 98, 90–96.10.1016/j.jsbmb.2005.08.002
- Somjen, D., Katzburg, S., Stern, N., Kohen, F., Sharon, O., Limor, R., … Weisman, Y. (2007). 25 hydroxy-vitamin D3-1α hydroxylase expression and activity in cultured human osteoblasts and their modulation by parathyroid hormone, estrogenic compounds and dihydrotestosterone. The Journal of Steroid Biochemistry and Molecular Biology, 107, 238–244.10.1016/j.jsbmb.2007.03.048
- Sömjen, D., Kaye, A. M., Harell, A., & Weisman, Y. (1989). Modulation by vitamin D status of the responsiveness of rat bone to gonadal steroids. Endocrinology, 125, 1870–1876.10.1210/endo-125-4-1870
- Somjen, D., Kohen, F., Lieberherr, M., Gayer, B., Schejter, E., Katzburg, S., … Stern, N. (2005). The effects of phytoestrogens, and their carboxy derivatives on human vascular and bone cells: New insights based on studies with carboxy- biochainin A. The Journal of Steroid Biochemistry and Molecular Biology, 93, 293–303.10.1016/j.jsbmb.2004.12.029
- Somjen, D., Weisman, Y., Harell, A., Berger, E., & Kaye, A. M. (1989). Direct and sex specific stimulation by sex steroids of creatine kinase activity and DNA synthesis in rat bone. Proceedings of the National Academy of Sciences, 86, 3361–3365.10.1073/pnas.86.9.3361
- Somjen, D., Waisman, A., Lee, J.-K., Posner, G. H., & Kaye, A. M. (2001). A noncalcemic, 25 dihydroxy vitamin D3 (JKF) up regulates the induction of creatine kinase B in osteoblast-like ROS 17/2.8 cells and in rat diaphysis by estradiol-17β. The Journal of Steroid Biochemistry and Molecular Biology, 77, 205–212.10.1016/S0960-0760(01)00065-6
- Sömjen, D., Waisman, A., Weisman, J., & Kaye, A. M. (1998). Nonhypercalcemic analogs of vitamin D stimulate creatine kinase activity in osteoblast-like ROS 17/2.8 cells and upregulate their responsiveness to estrogens. Steroids, 63, 340–343.10.1016/S0039-128X(98)00026-9
- Somjen, D., Waisman, A., Weisman, Y., & Kaye, A. M. (2000). “Nonhypercalcemic” analogs of, 25 dihydroxy vitamin D augment the induction of creatine kinase B by selective estrogen receptor modulators (SERMS) in osteoblast-like cells and rat skeletal organs. The Journal of Steroid Biochemistry and Molecular Biology, 72, 79–88.10.1016/S0960-0760(00)00028-5
- Sömjen, D., Weisman, Y., & Kaye, A. M. (1995). Pretreatment with 1,25 (OH)2 vitamin D or 24,25 (OH)2 vitamin D increases synergistically responsiveness to sex steroids in skeletal derived cells. The Journal of Steroid Biochemistry and Molecular Biology, 55, 211–217.10.1016/0960-0760(95)00175-Y
- Somjen, D., & Yoles, I. (2003a). DT56a (Tofupill/Femarelle) selectively stimulates creatine kinase specific activity in skeletal tissues of rats but not in the uterus. The Journal of Steroid Biochemistry and Molecular Biology, 86, 93–98.10.1016/S0960-0760(03)00252-8
- Somjen, D., & Yoles, I. (2003b). DT56a stimulates creatine kinase specific activity in vascular tissues of rats. Journal of Endocrinological Investigation, 26, 966–971.10.1007/BF03348193
- Tham, D. M., Gardner, C. D., & Haskell, W. L. (1998). Potential health benefits of dietary Phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. The Journal of Clinical Endocrinology & Metabolism, 83, 2223–2235.
- Walters, M. R. (1992). Newly identified actions of the vitamin D endocrine system. Endocr Rev, 13, 719–764.
- Yoles, I., Yogev, Y., Frenkel, Y., Hirsch, M., Nahum, R., & Kaplan, B. (2004). Efficacy and safety of standard versus low-dose Femarelle (DT56a) for the treatment of menopausal symptoms. Clinical and Experimental Obstetrics and Gynecology, 31, 123–126.
- Yoles, I., Yogev, Y., Frenkel, Y., Nahum, R., Hirsch, M., & Kaplan, B. (2003). Tofupill/femarelle (DT56a): A new phyto-selective estrogen receptor modulator-like substance for the treatment of postmenopausal bone loss. Menopause, 10, 522–525.10.1097/01.GME.0000064864.58809.77
- Zehnder, D., Bland, R., Walker, E. A., Bradwell, A. R., Howie, A. J., Hewison, M., & Stewart, P. M. (2001). Expression of 25 - hydroxyvitamin D3-1 the human kidney. Journal of the American Society of Nephrology, 10, 2465–2473.
- Zehnder, D., Bland, R., Williams, M. C., McNinch, R. W., Howie, A. J., Stewart, P. M., & Hewison, M. (2001). Extrarenal expression of 25-hydroxyvitamin d(3)-1 α-hydroxylase. The Journal of Clinical Endocrinology & Metabolism, 86, 888–894.
