Abstract
Objective: Type 2 Diabetes Mellitus is one of the most serious and common public health problems and metabolic disorder in both developed and developing countries. Diabetic patient may suffer from imbalanced electrolyte and lipid profile due to complications of diabetes mellitus and the medication they receive. Electrolytes and lipids have noteworthy roles, and changes in their concentrations provide significant signs of disease progression in a number of non-communicable diseases like diabetes. As there is a very few study of electrolyte and lipid profile in young Bangladeshi female diabetic patients, we investigated to identify the status of serum electrolytes and lipid profile with fasting blood glucose levels in type 2 diabetic female subjects with age ranging from 20 to 30. Subjects: Thirty-five female type 2 diabetes mellitus patients and 15 non-diabetic healthy control female volunteers of 20–30 age were recruited to determine serum glucose, electrolytes (Na+, K+, Cl−, and HCO3−), and lipid profiles (total cholesterol, triglyceride, HDL, and LDL). Result: The mean levels of electrolytes except K+ and lipid profile except HDL were found significantly higher (p < 0.001) in comparison with control group. While, there was significant (p < 0.05) decrease in the serum levels of K + and HDL in all diabetics. Conclusion: These results presented some variations from other research having different age and sex. Therefore, it can be concluded that there might have an effect of age and sex differences on lipid and electrolyte profile. This abnormal biochemical profile could be a noteworthy sign of diabetes associated disease and may have great potential as a diagnostic tool in clinical practice.
Keywords:
Public Interest Statement
Type 2 diabetes is expected to become one of the major causes of death globally by 2030. It is a progressive condition in which the body can’t response to the normal effects of insulin and/or gradually loses the capacity to produce enough insulin in the pancreas. It usually develops in adults over the age of 45 years but is increasingly occurring in younger age groups including children, adolescents, and young adults. Generally, it occurs as a result of obesity and lack of exercise. Electrolytes and lipids have significant roles providing significant signs of disease progression in a number of non-communicable diseases specially in Diabetes. Therefore, we tried to find the biochemical status of electrolyte and lipid levels in type II diabetic young Bangladeshi female. We found significant biochemical profile that can be used as predictive diagnostic clinical tools.
Competing interests
The authors declare no competing interest.
1. Introduction
Diabetes Mellitus (DM) is defined by absolute or relative deficiencies in insulin secretion and/or insulin action associated with chronic hyperglycemia and disturbances of carbohydrate, lipid and protein metabolism (Abou-Seif & Youssef, Citation2004). Diabetes mellitus (DM) is one of the leading health problems, resulting in morbidity and mortality and adversely affecting both the quality and length of life (Ahmed, Citation2002). The frequency of type 2 diabetes has been rapidly rising worldwide and in Bangladesh it is 4.3% (Ahmed, Citation2002). The pathogenesis of type 2 diabetes mellitus is better understood. Asian populations develop diabetes at a younger age than Western populations (Katikireddi, Morling, & Bhopal, Citation2011). Though, racial variations are evident within Asian population with age-specific prevalence of diabetes. In the Chinese population prevalence of diabetes peaks at 79–89 years while in Asian Indian population, diabetes peaks at 60–69 years of age. Moreover, Indians also have a higher prevalence of Impaired Glucose Tolerance (IGT) at a younger age than the Chinese population. The reports of Pakistan (Shera, Jawad, & Maqsood, Citation2007) and Sri Lanka (Katulanda et al., Citation2008) shows the similar findings like India (Ramachandran, Mary, Yamuna, Murugesan, & Snehalatha, Citation2008). The ethnic contrasts in the predominance of diabetes and hindered glucose control may not be totally clarified by the living condition and geological areas, proposing a noteworthy part for hereditary factors too (Nakagami et al., Citation2003). The percentage of young people with diabetes is increasing with the growth of diabetes prevalence. China disclosed an 88% increase in prevalence in 35–44 years age group within a period of 6 years (Gu et al., Citation2003). In southern India, the prevalence of diabetes has increased from 25% to 36% of the total prevalence from 2000 to 2006 in persons under 44 years (Ramachandran et al., Citation2008 May). Asian people with young onset of diabetes have significant phenotypic heterogeneity, having positive family history, diminished beta cell function, no islet cell autoantibodies and with numerous cardio metabolic disorders (Ng et al., Citation2001; Ramachandran, Snehalatha, Satyavani, Sivasankari, & Vijay, Citation2003). The major source for the increasing prevalence of T2D in Asian young is the growing rate of obesity and reducing rate of physical activity, leading to insulin resistance (Gill, Citation2007).
Dyslipidemia incorporates the changes in high density lipoprotein cholesterol (HDL-C), the size and density of low density lipoprotein cholesterol (LDL-C), total cholesterol, and triglyceride level (Goldberg, Citation2001; Mooradian, Citation2009; Smith & Lall, Citation2008). The abnormal lipid profiles are prevalent in diabetes mellitus since insulin resistance or deficiency affects key enzymes and pathways in lipid metabolism (Taskinen, Citation2002). Particularly, the following processes are affected: production of apoprotein, lipoprotein lipase regulation, cholesteryl ester action, transfer proteins, and hepatic and peripheral actions of insulin (Elinasri & Ahmed, Citation2008; Mooradian, Citation2009). The underlying association between atherosclerosis and dyslipidemia is well recognized. Hyperglycemia, obesity, and abnormal insulin functioning highly accelerate the progression to atherosclerosis (Regmi et al., Citation2009; Wexler, Grant, Meigs, & Nathan, Citation2005). Diabetic patients frequently develop a constellation of electrolyte disorders. These disturbances are particularly common in decompensated diabetics, especially in the context of diabetic ketoacidosis or non-ketotic hyperglycemic hyperosmolar syndrome (Liamis et al., Citation2013). Electrolytes play an important role in many body processes, such as controlling fluid levels, acid–base balance (pH), nerve conduction, blood clotting and muscle contraction. Sodium, potassium, chloride, and bicarbonate are all important for proper electrolyte balance. Electrolyte imbalance due to kidney failure, dehydration, fever, and vomiting has been suggested as one of the contributing factors toward complications observed in diabetes and other endocrine disorders (Husain, Arif Maan, Sheikh, Nawaz, & Jamil, Citation2009). Hyperglycemia sets the internal environment for osmotic diuresis while causing a dilutional effect on electrolyte concentrations.
Our present research aims to identify the status of serum electrolytes (sodium, potassium, chloride, and bicarbonate) and lipid profile with fasting blood glucose levels in type 2 diabetic female subjects with age ranging from 20 to 30. We selected this age range as to the best of our knowledge this study is the first to evaluate the serum electrolyte and lipid profiles in Bangladeshi young female adults with type 2 diabetes. We also selected sodium, potassium, chloride and bicarbonate as these are the most common macro electrolytes and have great correlation with Diabetes mellitus.
2. Patients and methods
2.1. Study area and design
The study was carried out at Chittagong Diabetic General Hospital, Chittagong, Bangladesh and was conducted in the clinical chemistry laboratory of the hospital. A cross-sectional non-probability sampling method was adopted after patient’s consent had been sought.
2.2. Ethical considerations
The study was approved by the ethical committee of the Department of Pharmacy, Noakhali Science and Technology University; Chittagong Diabetic General Hospital, Chittagong. Informed consent was obtained from all study participants who duly acknowledge by agreeing to the study. All procedures followed were in accordance with the ethical standards of Ministry of Health, Bangladesh as well as the Helsinki Declaration of 1975.
2.3. Patients selection and exclusion criteria
The study targeted medically diagnosed Type II Diabetes Mellitus female patients between 20 and 30 ages on diabetic treatment schedule to visit the hospital at regular intervals for routine medical review using the non-probability sampling method. Randomly selected female patients ages ranging from 20 to 30 with no history of diabetes of any type and not on Statins were used as controls. Male patients, pregnant women, age above 30, patients on statins for abnormal lipid treatment (both for Type II DM and Controls) were excluded.
2.4. Sample collection and storage
Five mL venous blood sample was collected from antecubital vein of each of the diabetic patients and control subjects in a metal-free sterile tube, between 7 and 8 am after an overnight fasting and after 2 h. The breakfast was similar for all patients maintaining the standard hospital meal consistently and defined same nutritional value for all patients. The blood was then allowed to clot at room temperature for 30 min and centrifuged at 3000 rpm for 15 min to extract the serum. The serum was taken in eppendorf tube and stored at −20 °C until analysis. Blood collection and serum separation were carried out in a dust-free environment.
2.5. Measurement of biochemical parameters
Fasting and after breakfast glucose level was determined by Glucose Oxidase–Peroxidase method (Vucic, Petrovic, Mesic, & Rocic, Citation1999), cholesterol by Cholesterol Oxidase–Peroxidase method (Barham & Trinder, Citation1972), triglycerides by Trinders Glycerol Phosphate Oxidase–Peroxidase method (Allain, Poon, Chan, Richmond, & Fu, Citation1974), HDL by Poly ethylene glycol [PEG] precipitation method (Bucolo & David, Citation1973), LDL cholesterol was calculated according to Freidewald Formula (Demacker, Hijmans, Vos-Janssen, Van’t Laar, & Jansen, Citation1980). The electrolytes were analyzed on Beckman Coulter DXC auto analyzer. Other biochemical parameters were determined by enzymatic process using assay kit those were adapted in hospital.
2.6. Statistical analysis
SPSS software package (version 16.0) was used to analyze the data. Descriptive statistics were calculated for all variables. Values were expressed as mean ± SEM. Comparison of data between patient and control groups was performed by one-way ANOVA analysis. p-values < 0.05 were considered statistically significant, p-value < 0.01 were considered statistically highly significant and p-value < 0.001 were considered statistically very highly significant.
3. Results
3.1. Demographic data and biophysical characteristics
The demographic data and biophysical characteristics of diabetic patients and control subjects are presented in Table . Total subjects of this study were 50 of female individuals aged between 20 and 30 years. Among the study subjects, 15 subjects were control group (group I) and 35 subjects were patient group with type 2 diabetes (group II). The age (years) as mean ± SEM was 24.38 ± 2.08, 25.81 ± 1.16 and the BMI (kg/m2) as mean ± SEM was 22.59 ± 0.73, 28.39 ± 1.04, respectively for group I and group II.
Table 1. Demographic data and biophysical characteristics of diabetic patients and control subjects
3.2. Evaluation of blood glucose level (fasting and after breakfast condition) in diabetic and control subjects
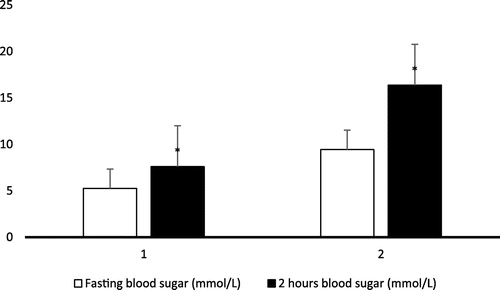
Fasting two hours blood glucose level of type II diabetic patients and control subjects are presented in Figure . From statistical analysis, it was found that the mean level of fasting blood sugar and two hours blood sugar were found significantly higher (*p < 0.001) in the serum of patient group (group II) when compared to the control group (group I).
3.3. Determination of electrolyte level and lipid profile in diabetic and control subjects
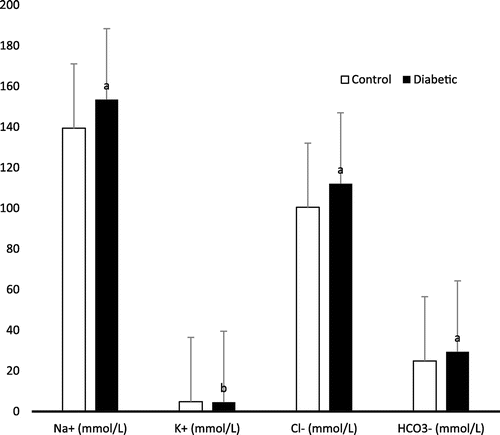
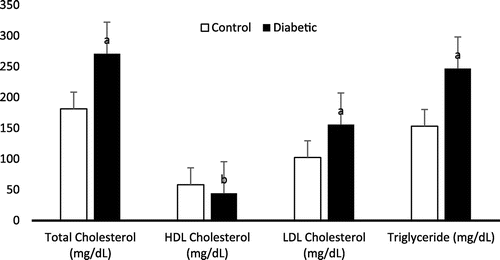
The mean levels of all electrolytes (Na+, Cl−, and HCO3−) except potassium (K+) were significantly higher (p < 0.001) in group II in comparison with group I. Figure shows the electrolyte level in diabetic patients and control subjects. The mean levels of total cholesterol, triglycerides and LDL were found very highly significant (p < 0.001), while the mean level of HDL were significantly lower (p < 0.05) in the serum of group II patients when compared to control subjects. Figure shows the lipid profile in diabetic patients and control subjects.
4. Discussion
The association among blood glucose, serum electrolytes, and lipid profile is multi factorial in which it is related to a number of other factors, which includes age and associated conditions (Liamis et al., Citation2013). Our study demonstrated that type 2 diabetic female subjects have electrolytes imbalance characterized by elevation of sodium, chloride, and bicarbonate ions accompanied with depletion of potassium ions – compared to control individual. The observed electrolyte imbalance might be the result of kidney dysfunction, diabetic nephropathy. This electrolyte imbalance might also occur due to inhibition of the rennin–angiotensin–aldosterone system, which plays a very significant role in the regulation of fluid and electrolyte balance. This enzyme system has been reported to be affected in many endocrine and cardiovascular diseases specially diabetes (Remuzzi, Perico, Macia, & Ruggenenti, Citation2005). There are several underlying mechanisms of dysnatremias in Diabetic patient (Liamis et al., Citation2008, 2013). In the development of hypernatremia, poorly controlled DM was implicated in one third of cases (34.5%) in a study of 113 hypernatremic patients (Liamis et al., Citation2008). The major causes of hypernatremia are associated with endocrine dysfunction. Study showed evidence that hypernatremia and hyperosmolarity are associated with impairment of both insulin-mediated glucose metabolism and glucagon-dependent glucose release in animals and man (Bratusch-Marrain & DeFronzo, Citation1983; Komjati, Kastner, Waldhäusl, & Bratusch-Marrain, Citation1988, 1989; Liamis, Milionis, & Elisaf, Citation2008). Therefore, hypernatremia and hyperosmolarity must be considered as causal factors of hyperglycemia in critically ill patients (Lindner & Funk, Citation2013). Moreover, hypernatremia is associated with the profound inhibition of gonadotrophin release in postmenopausal diabetic women with hyperglycemic and hyperosmolar syndrome (HHS). It is thought that hypernatremia causes a decrease in gonadotrophin-releasing hormone expression in GT1-7 neurons, although the underlying specific mechanisms remain unknown (Lado-Abeal, Lorenzo-Solar, Lago-Lestón, Palos-Paz, & Domingez-Gerpe, Citation2007). Again, gastrointestinal loss of K+ due to malabsorption syndromes (diabetic-induced motility disorders, bacterial overgrowth, chronic diarrheal states); redistribution of potassium ion [K+] from the extracellular to the intracellular fluid compartment (shift hypokalemia due to insulin administration); and renal loss of K+ (due to osmotic diuresis and/or coexistent hypomagnesemia) may cause hypokalemia in diabetics. Also, hypomagnesemia can occur hypokalemia since a low intracellular magnesium [Mg2+] concentration triggers the renal outer medullary K+ channel to secrete more K+ (Yang, Frindt, & Palmer, Citation2010). Again, exogenous insulin can cause mild hypokalemia because it promotes the entry of K+ into skeletal muscles and hepatic cells by increasing the activity of the Na+-K+-ATPase pump (Minaker & Rowe, Citation1982). Moreover, the increased secretion of epinephrine due to insulin-induced hypoglycemia also play a contributory role (Petersen, Schluter, & Kerp, Citation1982). During the treatment of severe hyperglycemia, insulin administration leads to hypokalemia. Hypokalemia is linked with disturbed insulin secretion and decreased peripheral glucose utilization resulting in carbohydrate intolerance and hyperglycemia (Wilcox, Citation1999). This is mostly challenging in diabetic patients causing a vicious circle where low serum K+ levels lead to poorly controlled DM and vice versa. Bicarbonate (HCO3−) is an ion that acts as a buffer to maintain the acidity (pH) in blood and other fluids in the body. Bicarbonate levels are measured to monitor the acidity of the blood and body fluids. The acidity may be affected by foods or medications that we ingest and the function of the kidneys and lungs. In other study, the value of serum Na+ and Cl− were significantly lower while potassium ion (K+) and bicarbonate (HCO3−) level did not show any statistical significant difference in the uncontrolled type 2 diabetic subjects in comparison with controlled type 2 diabetic subjects aged range from 30 to 60 (Peters, Citation2008). This difference from our present study might be due to the range of age and sex.
During the past decades, the relation between diabetes mellitus and serum lipid profile had been much studied (Elinasri & Ahmed, Citation2008; Lu et al., Citation2003; Mooradian, Citation2009; Peters, Citation2008). In our present study, it has been observed a significant increase (p < 0.001) in the serum levels of total cholesterol, LDL-cholesterol, triglycerides and a significant decrease in HDL-cholesterol in all diabetics in relation with all control. Research revealed that there is a trend toward a higher prevalence of dyslipidemia in T1DM in females than in males (Marcovecchio et al., Citation2009). Many studies have shown that microvascular changes and precursors of atherosclerosis are frequently observed in young patients early in the course of T1DM (Jarvisalo et al., Citation2002; Krantz et al., Citation2004). Both lipid profile and diabetes are the significant predictors for metabolic disturbances including dyslipidemia, hypertension, cardiovascular diseases, hyperinsulinemia (Goldberg, Citation2001). Dyslipidemia as a metabolic disorder is frequently related with diabetes mellitus. Depending on the type and severity of diabetes, glycaemic control, nutritional status, age, and other factors, its prevalence is variable. Earlier research indicated a strong clustering risk factor for coronary artery disease in diabetic subjects (Elinasri & Ahmed, Citation2008; Regmi et al., Citation2009). More than 70% patients with type 2 diabetes mellitus had one or more types of dyslipidemia (Elinasri & Ahmed, Citation2008). Similarly, our results reveal high prevalence of hypercholesterolemia, hypertriglyceridemia and high LDL-C levels, which are well-known risk factors for cardiovascular diseases among patients. These patients are on high-risk without complications but already had significant dyslipidemia, which develops the risk of cardiovascular events, certainly required therapeutic intervention. In diabetes, many factors are involved in the interrelationship between carbohydrates and lipid metabolism. Therefore, carbohydrate metabolism disorder may lead to disorder in lipid metabolism and vice versa (Chatterjee & Shinde, Citation2005). Insulin resistance is the major defects in the majority of with type 2 diabetes. In non-diabetic individuals, insulin resistance in combination with hyperinsulinemia has a significant role for future development of type 2 diabetes (Haffner, Mykkanen, & Festa, Citation2000). Several research disclosed that insulin affects the production of liver apolipoprotein and controls the enzymatic activity of lipoprotein lipase and cholesterol ester transport protein, which grounds dyslipidemia in diabetes mellitus. Moreover, insulin deficiency lessens hepatic lipase activity and slows several steps in the production of biologically active lipoprotein lipase (Elinasri & Ahmed, Citation2008; Mooradian, Citation2009; Smith & Lall, Citation2008). The result of our present study indicates that the most common recognized abnormality is hypertriglyceridaemia. Other researchers also linked the high triglyceride level to the poor glycaemic control of diabetes and obesity (Mooradian, Citation2009; Nau & Mallya, Citation2005). Abbate and Brunzell reported that the increase in triglycerides in poorly controlled patients is related to the decrease of adipose tissue and muscle lipoprotein lipase activity (Abbate & Brunzell, Citation1990). Packard et al., reported reduced HDL-C as a powerful predicator for premature coronary heart diseases (Packard, Nunn, & Hobbs, Citation2002). Goldberg reported that hyperglycemia progressively increases the transfer of cholesterol esters from HDL-C to VLDL-C particles, hence, denser LDL particles attain a large proportion of these HDL esters, further diminish the HDL-C level (Goldberg, Citation2001). In addition, HDL-C is a ready substrate for hepatic lipase which converts it into smaller particles, which are readily cleared from the plasma (Elinasri & Ahmed, Citation2008; Mooradian, Citation2009). Poor insulinization causes increased lipolysis in adipocytes. The resulting increase in fatty acid transport to the liver, which is a common abnormality in type 2 diabetes, may result in increased VLDL-C. Several authors reported a positive improvement in lipid profile with fair glycaemic control (Gimeno-Orna, Faure-Nogueras, & Sancho-Serrano, Citation2005; Roberto, Dodesini, & Lepore, Citation2006). Study found that increased testosterone levels is negatively associated with TC, LDL-C, and HDL-C levels (Garces et al., Citation2010). In another study, as the testosterone/estradiol ratio diminished, LDL-C, and HDL-C levels increased (Laskarzewski, Morrison, Gutai, Khoury, & Glueck, Citation1983). In uncontrolled diabetic patients, it has been stated that the activity of lipoprotein lipase and clearance of VLDL-C in the circulation is diminished due to insulin resistance. The level of total cholesterol is usually normal or near normal if glycaemic control is adequate, and worsening of control raises the level (Andersen et al., Citation1983). Therefore, improving glycaemic control can substantially reduce the risk of cardiovascular events in diabetic patients. Our present findings might be explained by the different degrees of insulin resistance in different ages and sexes or a direct effect of hormonal status on enzymes implicated in acid–base balance and lipoprotein metabolism (Jenkins et al., Citation2003; Schwab et al., Citation2006).
5. Conclusions
In conclusion, our study is the first report on lipid and electrolyte data in Bangladeshi young female adults with T2DM. It can be concluded that there might be an effect of age and sex differences on lipid and electrolyte profile. Therefore, further large prospective studies are warranted to assess the effect of age and sex as a noteworthy diagnostic tool of diabetes since early diagnosis, good glycemic control, and dietary modification are usually enough for prevention and treating complications in diabetes mellitus.
Limitations
| (1) | Small sample size with non-probability sampling. | ||||
| (2) | Narrow sampling of the general population (20–30 years old female). | ||||
| (3) | Some clinical parameters to dictate the physiological conditions of the patients (blood pressure, HbA1c, medications of T2D) were not measured. | ||||
Author Contribution
MMB – participated in experiments, study design. SMMR, NA – participated in statistics analysis. MSH – Supervising and directing the project. MMB, MSH – manuscript preparation, checked the grammatical mistakes and corrected the final manuscript. All authors read and approved the final version of the manuscript.
Funding
This project was funded by The Ministry of Science and Technology, Government of the People’s Republic of Bangladesh [grant number 39.012.002.01.03.019.2013-281(145)-130].
Acknowledgment
The Authors thank Chittagong Diabetic General Hospital, and Department of Pharmacy, Noakhali Science and Technology University, Chittagong, Bangladesh for their valuable support during the course of research work. The Authors also thank all participants for taking part in the study.
Additional information
Notes on contributors
Md. Mustahsan Billah
Md. Mustahsan Billah is a lecturer at the Department of Pharmacy, Noakhali Science and Technology University, Bangladesh. He is currently doing his PhD on Paternal Obesity at the School of Medical Science, Faculty of Medicine, The University of New South Wales, Australia. His research interests focus on obesity and diabetes and he published a number of peer-reviewed articles in the area of Pharmacology.
S.M. Masud Rana
S.M. Masud Rana is doing investigations on Pharmaceutical products in one of the leading Pharmaceuticals (ACME Pharmaceutical Ltd) in Bangladesh.
Nahida Akter
Nahida Akter is a lecturer at the Department of Computer Science and Telecommunication Engineering, Noakhali Science and Technology University, Bangladesh. She is currently focusing on the application of data mining in diseases.
Mohammad Salim Hossain
Mohammad Salim Hossain, PhD is a professor at the Department of Pharmacy, Noakhali Science and Technology University, Bangladesh. His research areas include pharmacology, pharmaceutical technology and phytochemistry. He published many peer-reviewed articles and book in the areas of pharmacology, pharmaceutical technology and phytochemistry.
References
- Abbate, S. L., & Brunzell, J. D. (1990). Pathophysiology of hyperlipidemia in diabetes. Journal of Cardiovascular Pharmacology, 16, 1–7.10.1097/00005344-199000169-00002
- Abou-Seif, M. A., & Youssef, A. A. (2004, August 16). Evaluation of some biochemical changes in diabetic patients. Clinica Chimica Acta, 346(2), 161–170.10.1016/j.cccn.2004.03.030
- Ahmed, A. M. (2002). History of diabetes mellitus. Saudi Medical Journal, 23(4), 373–378.
- Allain, C. C., Poon, L. S., Chan, C. S. G., Richmond, W., & Fu, P. C. (1974). Enzymatic determination of total serum cholesterol. Clinical Chemistry, 20(4), 470–475.
- Andersen, G. E., Christiansen, J. S., Mortensen, H. B., Christiansen, K. M., Predersen-Bjerguard, L., & Kastrup, K. W. (1983). Plasma lipid and lipoprotein in type 1 diabetic children and adolescent in relation to metabolic regulation, obesity and genetic hyperlipoprotenimia. Acta Paediatrica, 72, 361–365.10.1111/apa.1983.72.issue-3
- Barham, D., & Trinder, P. (1972). An improved color reagent for the determination of blood glucose by the oxidase system. Analyst, 97(151), 142–145.10.1039/an9729700142
- Bratusch-Marrain, P. R., & DeFronzo, R. A. (1983). Impairment of insulin-mediated glucose metabolism by hyperosmolality in man. Diabetes, 32, 1028–1034.10.2337/diab.32.11.1028
- Bucolo, G., & David, H. (1973). Quantitative determination of serum triglycerides by the use of enzymes. Clinical Chemistry, 19(5), 476–482.
- Chatterjee, M. N., & Shinde, R. (2005). Text book of medical laboratory technology. In Metabolism of carbohydrates (Vol. 6, pp. 266–330). Delhi: Jaypee Brothers Medical Publisher.
- Demacker, P. N., Hijmans, A. G., Vos-Janssen, H. E., Van’t Laar, A., & Jansen, A. P. (1980). A study of the use of polyethylene glycol in estimating cholesterol in high-density lipoprotein. Clinical Chemistry, 26(13), 1775–1779.
- Elinasri, H. A., & Ahmed, A. M. (2008). Patterns of lipid changes among type 2 diabetes patients in Sudan. Eastern Mediterranean Health Journal, 14(2), 314–324.
- Garces, C., Oya, Id., Lasuncion, M. A., Lopez-Simon, L., Cano, B., & de Oya, M. (2010). Sex hormone-binding globulin and lipid profile in pubertal children. Metabolism, 59, 166–171.10.1016/j.metabol.2009.06.033
- Gill, T. (2007). Young people with diabetes and obesity in Asia: A growing epidemic. Diabetes Voice, 52, 20–22.
- Gimeno-Orna, J. A., Faure-Nogueras, E., & Sancho-Serrano, M. A. (2005). Usefulness of total cholesterol/HDL-cholesterol ratio in the management of diabetic dyslipidemia. Diabetic Medicine, 22, 26–31.10.1111/dme.2005.22.issue-1
- Goldberg, I. J. (2001). Diabetic dyslipidemia: Causes and consequences. Journal of Clinical Endocrinology & Metabolism, 8(3), 965–971.10.1210/jcem.86.3.7304
- Gu, D., Reynolds, K., Duan, X., Xin, X., Chen, J., & Wu, X. (2003, September). Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International collaborative study of cardiovascular disease in Asia. Diabetologia, 46(9), 1190–1198.10.1007/s00125-003-1167-8
- Haffner, S. M., Mykkanen, L., & Festa, A. (2000). Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects. Circulation, 101, 975–980.10.1161/01.CIR.101.9.975
- Husain, F., Arif Maan, M., Sheikh, M. A., Nawaz, H., & Jamil, A. (2009). Trace elements status in type 2 diabetes. Bangladesh Journal of Medical Science, 8, 52–56.
- Jarvisalo, M. J., Putto-Laurila, A., Jartti, L., Lehtimaki, T., Solakivi, T., & Ronnemaa, T. (2002). Carotid artery intima-media thickness in children with type 1 diabetes. Diabetes, 51, 493–498.10.2337/diabetes.51.2.493
- Jenkins, A. J., Lyons, T. J., Zheng, D., Otvos, J. D., Lackland, D. T., & Mcgee, D. (2003). Lipoproteins in the DCCT/EDIC cohort: Associations with diabetic nephropathy. Kidney International, 64, 817–828.10.1046/j.1523-1755.2003.00164.x
- Katikireddi, S. V., Morling, J. R., & Bhopal, R. (2011, December). Is there a divergence in time trends in the prevalence of impaired glucose tolerance and diabetes? A systematic review in South Asian populations. International Journal of Epidemiology, 40(6), 1542–1553.10.1093/ije/dyr159
- Katulanda, P., Constantine, G. R., Mahesh, J. G., Sheriff, R., Seneviratne, R. D., Wijeratne, S., … Matthews, D. R. (2008, September). Prevalence and projections of diabetes and pre-diabetes in adults in Sri Lanka-Sri Lanka Diabetes, Cardiovascular Study (SLDCS). Diabetic Medicine, 25(9), 1062–1069.10.1111/dme.2008.25.issue-9
- Komjati, M., Kastner, G., Waldhäusl, W., & Bratusch-Marrain, P. (1988). Detrimental effect of hyperosmolality on insulin-stimulated glucose metabolism in adipose and muscle tissue in vitro. Biochemical Medicine and Metabolic Biology, 39, 312–318.10.1016/0885-4505(88)90091-6
- Komjati, M., Kastner, G., Waldhäusl, W., & Bratusch-Marrain, P. (1989). Effect of hyperosmolality on basal and hormone-stimulated hepatic glucose metabolism in vitro. European Journal of Clinical Investigation, 19, 128–134.10.1111/j.1365-2362.1989.tb00206.x
- Krantz, J. S., Mack, W. J., Hodis, H. N., Liu, C. R., Liu, C. H., & Kaufman, F. R. (2004). Early onset of subclinical atherosclerosis in young persons with type 1 diabetes. The Journal of Pediatrics, 145, 452–457.10.1016/j.jpeds.2004.06.042
- Lado-Abeal, J., Lorenzo-Solar, M., Lago-Lestón, R., Palos-Paz, F., & Domingez-Gerpe, L. (2007). Hyperglycaemic hyperosmolar nonketotic state as a cause of low gonadotrophin levels in postmenopausal diabetic women: A role for severe hypernatraemia. Journal of Neuroendocrinology, 19, 983–987.10.1111/j.1365-2826.2007.01614.x
- Laskarzewski, P. M., Morrison, J. A., Gutai, J., Khoury, P. R., & Glueck, C. J. (1983). Longitudinal relationships among endogenous testosterone, estradiol, and quetelet index with high and low density lipoprotein cholesterols in adolescent boys. Pediatric Research, 17, 689–698.10.1203/00006450-198308000-00018
- Liamis, G., Tsimihodimos, V., Doumas, M., Spyrou, A., Bairaktari, E., & Elisaf, M. (2008). Clinical and laboratory characteristics of hypernatraemia in an internal medicine clinic. Nephrology Dialysis Transplantation, 23, 136–143.
- Liamis, G., Milionis, H., & Elisaf, M. (2008). Blood pressure drug therapy and electrolyte disturbances. International Journal of Clinical Practice, 62, 1572–1580.10.1111/j.1742-1241.2008.01860.x
- Liamis, G., Rodenburg, E. M., Hofman, A., Zietse, R., Stricker, B. H., & Hoorn, E. J. (2013). Electrolyte disorders in community subjects: Prevalence and risk factors. The American Journal of Medicine, 126, 256–263.10.1016/j.amjmed.2012.06.037
- Lindner, G., & Funk, G. C. (2013). Hypernatremia in critically ill patients. Journal of Critical Care, 28(216), e11–216.e20.
- Marcovecchio, M. L., Dalton, R. N., Prevost, A. T., Acerini, C. L., Barrett, T. G., & Cooper, J. D. (2009). Prevalence of abnormal lipid profiles and the relationship with the development of microalbuminuria in adolescents with type 1 diabetes. Diabetes Care, 32, 658–663.10.2337/dc08-1641
- Lu, W., Resnick, H. E., Jablonski, K. A., Jones, K. L., Jain, A. K., & Howard, W. J. (2003). Non-HDL cholesterol as a predictor of cardiovascular disease in type 2 diabetes: The strong heart study. Diabetes Care, 26, 16–23.10.2337/diacare.26.1.16
- Minaker, K. L., & Rowe, J. W. (1982). Potassium homeostasis during hyperinsulinemia: Effect of insulin level, beta-blockade, and age. American Journal of Physiology, 242, E373–E377.
- Mooradian, A. D. (2009, March). Dyslipidemia in type 2 diabetes mellitus. Nature Clinical Practice Endocrinology & Metabolism, 5(3), 150–159.10.1038/ncpendmet1066
- Nakagami, T., Qiao, Q., Carstensen, B., Nhr-Hansen, C., Hu, G., Tuomilehto, J., … Borch-Johnsen, K. (2003, August). Age, body mass index and Type 2 diabetes-associations modified by ethnicity. A Study Group. Diabetologia, 46(8), 1063–1070.
- Nau, D. P., & Mallya, U. (2005). Sex disparity in the management of dyslipidemia among patients with type 2 diabetes mellitus in a managed care organization. The American Journal of Managed Care, 11, 69–73.
- Ng, M. C., Lee, S. C., Ko, G. T., Li, J. K., So, W. Y., Hashim, Y., … Chan, J. C. (2001, April). Familial early-onset type 2 diabetes in chinese patients: Obesity and genetics have more significant roles than autoimmunity. Diabetes Care, 24(4), 663–671.10.2337/diacare.24.4.663
- Packard, C., Nunn, A., & Hobbs, R. (2002). High density lipoprotein: Guardian of the vascular system. International Journal of Clinical Practice, 56, 761–771.
- Peters, A. L. (2008). Clinical relevance of non-HDL cholesterol in patients with diabetes. Clinical Diabetes, 26, 3–7.10.2337/diaclin.26.1.3
- Petersen, K. G., Schluter, K. J., & Kerp, L. (1982). Regulation of serum potassium during insulin-induced hypoglycemia. Diabetes, 31, 615–617.10.2337/diab.31.7.615
- Ramachandran, A., Snehalatha, C., Satyavani, K., Sivasankari, S., & Vijay, V. (2003, April). Type 2 diabetes in Asian-Indian urban children. Diabetes Care, 26(4), 1022–1025.10.2337/diacare.26.4.1022
- Ramachandran, A., Mary, S., Yamuna, A., Murugesan, N., & Snehalatha, C. (2008, May). High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care, 31(5), 893–898.10.2337/dc07-1207
- Regmi, P., Gyawali, P., Shrestha, R., Sigdel, M., Mehta, K. D., & Majhi, S. (2009). Pattern of dyslipidemia in type-2 diabetic subjects in Eastern Nepal. Journal of Nepal Medical Association, 10, 11–13.
- Remuzzi, G., Perico, N., Macia, M., & Ruggenenti, P. (2005, December). The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney International, 99, S57–S65.10.1111/j.1523-1755.2005.09911.x
- Roberto, T., Dodesini, A. R., & Lepore, G. (2006). Lipid and Renal disease. Journal of the American Society of Nephrology, 17, S145–S147.
- Schwab, K. O., Doerfer, J., Hecker, W., Grulich-Henn, J., Wiemann, D., Kordonouri, O., … Holl, R. W. (2006). Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type 1 diabetes: Cross-sectional data from the German diabetes documentation and quality management system (DPV). Diabetes Care, 29, 218–225.10.2337/diacare.29.02.06.dc05-0724
- Shera, A. S., Jawad, F., & Maqsood, A. (2007, May). Prevalence of diabetes in Pakistan. Diabetes Research and Clinical Practice, 76(2), 219–222.10.1016/j.diabres.2006.08.011
- Smith, S., & Lall, A. M. (2008). A study on lipid profile levels of diabetics and non-diabetics among Naini region of Allahabad. Turkish Journal of Biochemistry, 33(4), 138–141.
- Taskinen, M. R. (2002). Diabetic dyslipidemia. Atherosclerosis Supplements, 3(1), 47–51.10.1016/S1567-5688(01)00006-X
- Vucic, M., Petrovic, S., Mesic, R., & Rocic, B. (1999). An automated immunoturbidimetric assay for HbA1c determination. Clinical Chemistry and Laboratory Medicine, 37, S199.
- Wexler, D. J., Grant, R. W., Meigs, J. B., & Nathan, D. M. (2005). Cagliero: Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care, 28, 514–520. doi:10.2337/diacare.28.3.514
- Wilcox, C. S. (1999). Metabolic and adverse effects of diuretics. Seminars in Nephrology, 19, 557–568.
- Yang, L., Frindt, G., & Palmer, L. G. (2010). Magnesium modulates ROMK channel-mediated potassium secretion. Journal of the American Society of Nephrology, 21, 2109–2116.10.1681/ASN.2010060617