 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Mule deer historic range in Mexico has declined dramatically in the last decade. Forage availability and quality at the Chihuahuan Desert may play an important role sustaining populations at the southern end of their current distribution. We evaluated forage availability and quality at the end of a 3-year drought at two different localities in the Chihuahuan Desert: Old Net and Pulpit, we measured plant availability and quality, diet content and calculated diet preference indices. Vegetation at Old Net consisted primarily of both succulents (47%) and trees-shrubs (42%) while Pulpit had more grasses (60%) and herbs (24%) (P < 0.005). Feces collected at the Pulpit presented a high proportion of herbs (44%), while those collected at Old Net contained more trees and shrubs (53%). Preference index suggests that mule deer prefer herbs and overlook grasses, but there is no strong selection for any particular plant. Our results suggest that forage is appropriate to sustain mule deer populations, however, we found considerable variation in both localities suggesting a patchy landscape. More information regarding forage nutritional status and diet preferences can enhance our understanding on mule deer population dynamics in the Chihuahuan Desert.
PUBLIC INTEREST STATEMENT
What do mule deer eat in the desert? What do they eat during a 3-year-drought? Much of the reported research on mule deer has been conducted in the USA, we ventured in Mexico to the middle of the Chihuahuan Desert for two summers at the end of a 3-year-drought to study mule deer diet. We collected feces, plants and measured the landscape. We found that the Chihuahuan Desert, even after a 3-year drought is providing with enough quality to mule deer. Both food and shelter are adequate in the Chihuahuan Desert and we identify important regions and plants that are the key in the appropriate sustainment of Mexican mule deer populations.
Competing Interests
The authors declare no competing interests.
Species distributions are constantly changing under different pressures; mule deer historic distribution has dramatically shrunk in the last decade in Mexico (Baker, Citation1956; Sánchez-Rojas & Gallina, Citation2007; Weber & Galindo, Citation2005) and North America (Anthony & Smith, Citation1977; Gill, Citation2001; McNay & Voller, Citation1995). In Mexico, mule deer were found all the way to San Luis Potosi, Mexico. At present, we find mule deer in the states of Chihuahua, Sonora, Coahuila, Durango and Baja California yielding a considerable territory loss. Mule deer (Odocoileus hemionus) historic range has contracted since the late 1950s giving place to many ecological and management concerns (Carpenter, Citation1998; Gill, Citation2001). While mule deer in the Chihuahuan Desert are under constant thermal, nutritional and competitional stress, mule deer at higher latitudes migrate to lower altitudes during winter looking for better food quality (Anderson et al., Citation2012; Monteith et al., Citation2011). Studies have also shown that ungulates living in seasonal locations reflect seasonality in their feeding habits (Hofmann, Citation1989; Sherlock & Fairley, Citation1993; Zweifel-Schielly, Leuenberger, Kreuzer, & Suter, Citation2012). Plant quality and quantity influence growth, fecundity, and survival of individuals (Andersen & Linnell, Citation2000; Armstrong, Davidson, Perrott, Roygard, & Buchanan, Citation2005; Loison & Langvatn, Citation1998). Plant quality can be described in terms of protein, minerals, and fiber content among others (Robbins, Citation1983; Verheyden et al., Citation2011). In general, deer are highly selective of plant species when resources are limited (Lashley & Harper, Citation2012). Previous studies in the Chihuahuan Desert suggest that mule deer use preferentially areas with an irregular slope and stay closer to water sources during dry seasons (Perez-Solano et al. Citation2017; Sánchez-Rojas & Gallina, Citation2000a, Citation2000b) further research suggests that mule deer prefer slopes with northwest elevations (Perez-Solano et al. Citation2016) as a strategy to minimize thermal challenges. Furthermore, mule deer in the Chihuahuan Desert are willing to trade off between food resources and predation risk and this decision is strongly affected by both resource availability and precipitation (Esparza-Carlos, Laundré, & Sosa, Citation2011). Specifically, depending on precipitation food resource may explain mule deer habitat use during dry conditions, however during wetter years predations risk explains habitat use (Esparza-Carlos et al., Citation2011). Proximity to hills seems to be an important characteristic when mule deer assess predation risk increasing their feeding apprehension (Esparza-Carlos, Laundré, Hernández, & Iñiguez-Dávalos, Citation2016).
With temperatures reaching 40 degrees Celsius and scarce precipitation, animals in the desert struggle to satisfy their food requirements and to find the appropriate cover that works either as cover from predators or shelter from climate challenges (Feldhamer, Drickamer, Vessey, & Merritt, Citation1999). The summer months in the Chihuahuan Desert bring important challenges to animals that inhabit it. Droughts, a period of 25% below average precipitation, are periodic and some years it is common for droughts to extend over a 10-month period in the desert. This further compromise forage quality and quantity available for wildlife and competing-livestock. Periods of days without rain have increased in length from 1910 to 2010 (Petriere, Collins, Gutzler, & Moore, Citation2014) and, therefore, it is becoming more important to understand the dynamics of habitat use in desert living wildlife. Vegetation change, extreme weather, and increased anthropogenic disturbances are among the culprit of the population decline (Bender, Boren, Halbritter, & Cox, Citation2013; Clements & Young, Citation1997; Gill, Citation2001; Heffelfinger & Messmer, Citation2003; Laliberte & Ripple, Citation2004). To assess habitat appropriateness in the south end of the mule deer distribution range, we look at forage availability, quality diet selection for mule deer in the Chihuahuan Desert after a 3-year drought. Knowledge about both vegetation structure and forage quality available for mule deer can provide important information for management and conservation actions.
We conducted a study at the end of a 3-year (2011–2013) drought in the Chihuahuan Desert evaluating both forage availability and quality, diet content and diet selection at two different localities during the summer months in the Chihuahuan Desert rangelands. Specifically, we measured, vegetation structure, plant protein content, diet content as indicated by pellet content and diet selection indices on two localities for two summers (2012, 2013). With crude protein content being negatively affected by extreme droughts and deer becoming more selective of plant species (Lashley & Harper, Citation2012), we expect to find low protein content in plants available for mule deer of the Chihuahuan Desert. Additionally, studies suggest that for prey species predation risk is an important factor in survival (Brown, Laundre, & Gurung, Citation1999; Lima & Dill, Citation1990) making vegetation structure an important factor. Plant density, diversity and visibility have been positively correlated with deer density (Sánchez-Rojas & Gallina, Citation2000a). However, given the mule deer population status in the Chihuahuan desert, we expect to find appropriate nutrient and cover levels in the area while having a strong variation in both localities.
1. Materials and methods
We worked at Los Gemelos ranch in Aldama County, found in the central sub-region of the Chihuahuan desert (Figure ). Gemelos is approximately 70 miles northeast of Chihuahua capital. The ranch extends over 10,000 ha with elevations ranging from 1350 m to 1560 m and is primarily managed for big game hunting and to raise livestock. Livestock is rotated periodically through the ranch, sometimes overlapping with deer. Big game hunting took place on designated winter dates during our study period. Two localities with distinct geographic differences were studied within the bounds of Gemelos Ranch. The ranch includes both open plains and range hills. The first study area, Old Net, characterized as desert scrub microphyll (National Statistics and Geography Institute [INEGI], Citation2013) with slopes ranging from 10° to 25° and peak height of 1600 m. Old Net is located predominantly in the range hills, composed mainly of shrub lands. The second study area, Pulpit, was primarily natural grassland with slopes of less than 1° and peak elevation of 1400 m (Figure ). Artificial water sources are evenly distributed over the ranch available all year. The Pulpit consists largely of plains, a mosaic of desert grasslands and plains-mesa sand scrub. Sosa, Galarza, Toutcha, Soto, and Puga (Citation2006) described plant communities’ associations in an area similar to Old Net and near to our study site as follows: Larrea tridentata associated with Fouquieria splendens and Prosopis glandulosa as co-dominants, and Opuntia sp., Parthenium incanum associated with Jatropa dioica. and, Acacia constricta associated with Prosopis glandulosa. For Aldama County (INEGI, Citation2013), the annual precipitation ranged between 200 and 300 mm, with a median of 49 rainy days per year, and the annual average temperature ranged from 14° to 18° C. Summer average temperature and precipitation for the collection periods were 23.3° C and from 40 to 70 mm, respectively. Chihuahua underwent a severe 3-year drought starting 2010 for Aldama county the average annual precipitation in 2010 was 58 mm, for 2011 it was 14.5 mm and for 2012 it was 31.65 mm while 2013 the average annual precipitation was 67.5 mm (Figure ).
Figure 1. Los Gemelos Ranch, Aldama, Chihuahua. Cartographic map with topography indicating vegetation differences among the two localities, old net and the pulpit.
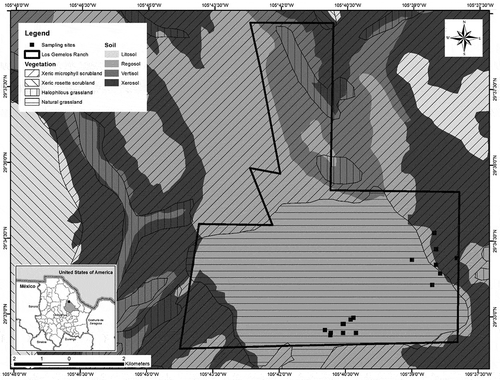
Figure 2. Annual average temperature and annual average precipitation during a 3-year drought for Aldama county, Chihuahua, Mexico. Data obtained from national meteorological service (Servicio Meteorologico Nacional 2018).
To assess forage availability, we determined plant communities’ composition during the summers of 2012 and 2013 using transect lines (Mostacedo & Fredericksen, Citation2000; Muller-Dumbois & Ellenberg, Citation1974). Using the point intercept method on transect lines, we determined plant communities; first, we established four transects in each locality to be used both years, we then recorded intercepting plants every 0.5 m in each of the eight 50 m transects for a total of 800 intercept points. To determine diet quality, we collected all plants present, gathering only the aerial part of the plants, including seed, fruit, if present, and leaves, for cactus, we collected only one part of the plant. The collection was used for taxonomic determination and crude protein analyses with a total of 158 and 218 plants for each summer, respectively. We classified plants into four forage classes: tree-shrub, grasses, herbs, and succulents. To calculate forage class percentage, we divided the total number of plants per forage class in each transect by the total number of plants of all classes. In addition, we estimated the Sorensen vegetation similarity index (IF) (Sorensen, Citation1948) using both localities as follows:
where C = number of similar species; A = species number on zone A; B = species number on zone B.
We used microhistology (Alipayo, Valdez, Holechek, & Cárdenas, Citation1992; Holechek, Citation1982; Sparks & Malechek, Citation1968) and indexed nitrogen content for the diet study. We started with a botanical composition analysis on fecal pellets collected fresh on each vegetation transect and oven-dried them at 50 °C for 24 h. Dried samples were then ground to an even consistency and affixed to slides for plant identification. Slide samples were then examined under the microscope and compared with reference slides, microscopic photography or hand-drawn references. We analyzed two samples of 15 fecal groups as suggested by Anthony and Smith (Citation1974); eight and seven for Old Net and Pulpit, respectively, for both summers of 2012 and 2013. We prepared five slides for each composed sample assessing 20 fields under the microscope recording frequency counts and calculating plant percentage on diet for each locality separately. Furthermore, we indexed nitrogen content using Kjeldhal’s analysis (Bremmer & Mulvaney, Citation1982), and then multiplied nitrogen content by 6.25 to estimate crude protein (CP) for each plant found in both areas.
We characterized deer forage preference using results from the vegetation structure study as resource abundance and results from the diet study as resource use. To test for general preferences, we conducted a simple chi test, comparing resource abundance and use in both high (n = 117) and low density (n = 96) areas. For preference index, we use electivity index (Ivlev, Citation1961), commonly used because it is bounded between −1 and 1. Electivity index as follows:
where ri is resource abundance, pi is resource use.
We also report Manly’s preference index (Manly, Miller, & Cook, Citation1972);
where is Manly’s preference index for forage type i, pi is the proportion of available resource i, pj is the proportion of all forage types together remaining, and m is the number of forage types.
We conducted all our statistical analyses with SPSS 19.0 (SPSS Inc. 19). We tested for differences in forage classes (tree-shrub, grass, herbs and succulents) between areas with a chi-square test. We used a Kurskal-Wallis test to compare diet composition between study areas (each forage class*study area) and to compare forage class percentages (total number of individual plants N = 57) found in the fecal pellets collected both years. Finally, we also conducted an analysis of variance (ANOVA) (N = 59) and Tukey’s test to test for differences in crude protein (% CP) percentages for both study areas using forage groups (forage class *year) and employed residuals analysis to test for normality (Neter, Wasserman, & Kutner, Citation1990; Pierce, Bowyer, & Bleich, Citation2004).
2. Results
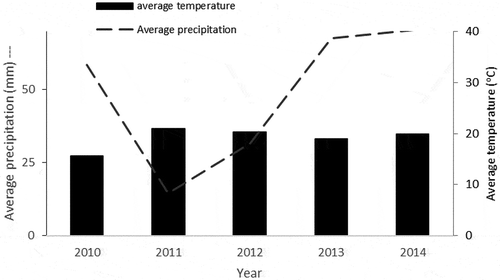
We found differences on forage availability in our two localities (N = 144, χ2 = 48.23, P < 0.001) (Figure ). The pulpit was mainly composed of grasses (40%), followed by herbs (24%) and succulents (23%), and finally trees and shrubs (13%). In contrast, Old Net had more succulents (47%) trees and shrubs (42%), followed by herbs (9%) and grasses (2%). Based on Sorensen index scores, both localities exhibited a nominal similarity of 35%, indicating that these two localities differed in forage availability.
Figure 3. Vegetation cover in two localities at Gemelos Ranch. Pulpit (black bars) contained primarily grasses (40%), herbs (24%) followed by succulents (23%), and finally trees and shrubs (13%). Old Net had more succulents (47%) followed by trees and shrubs (42%), and finally herbs (9%) and grasses (2%). Coverage composition between Old Net and Pulpit was statistically significant. Error bars denote one standard error.
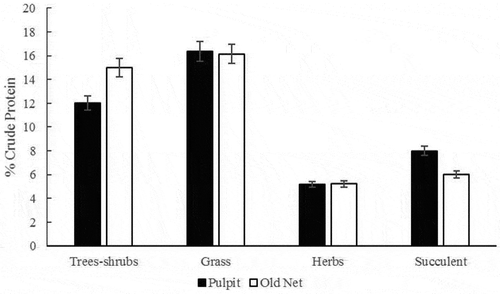
On average for both summers and both localities crude protein analysis showed that trees contained 13.31% CP; herbs 16.21% CP; grasses 5.201% CP and succulents 7.05% CP. Crude protein content means were not significantly different between years (F2,58 = 0.003, P = 0.958, N = 59), nor across localities (F = 0.977, P = 0.977, N = 61). Averaging both years for each locality, we found that Pulpit had 12% CP in trees and shrubs, 16.36% CP for herbs, grasses 5.18% CP, and 8% CP for succulent. For Old Net we found 15% CP in trees and shrubs; 16.14% CP for herbs, 5.23 % CP for grasses and 6% CP for succulents. Crude protein content results show the same pattern. Forage classes means differed significantly (F = 15.267, P = 0.000, N = 61) for both years (Figure ) (Table ).
Table 1. Crude protein (CP %) of native plants from northwest Mexico, in the Chihuahuan Desert
Figure 4. Plant quality, as measured by crude protein content, in the old net locality (white bars) locale was overall 1–3% higher than that at pulpit locality (black bars). Among forage classes, trees and shrubs presented the higher crude protein content. Error bars denote one standard error.
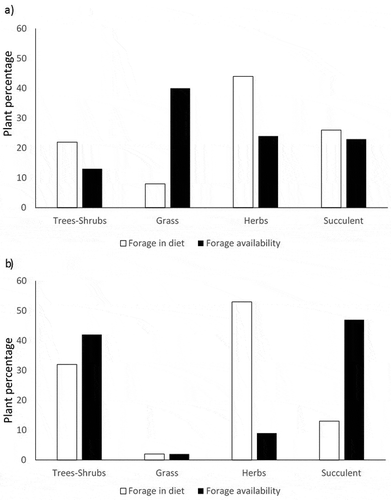
Microhistology analysis showed that feces collected at Pulpit contained 22% trees and shrubs, 44% herbs, 8% grasses, and 26% succulents; while feces collected at Old Net include 32% trees and shrubs, 53% herbs, 2% grasses and 13% of succulents. However, we found no significant difference for any forage class (X2(3) = 6.1, p = 0.1) between localities.
We identified a total of 25 plant species from the microhistology analysis. Ditaxis serrata (herbaceous) appeared at an unusually high percentage (36% for 2012 and 39% for 2013); other frequently occurring plants during summer 2012 included Opuntia (12.4%) and Flourensia cernua (15%), a succulent and shrub, respectively. We found a different pattern during 2013 when the plants with higher percentages included Yucca elata (succulent, 8%); Chilopsis linearis (tree), Flourensia cernua (shrub), Leucophyllum frutenscens (shrub), and the Prosopis glandulosa (tree), each with 5%, remaining plants were present at less than 2.5%.
Regarding diet preference, we found that deer present a higher selection to consume herbs at both Old Net and Pulpit (0.71, 0.29, respectively, Electivity index), while they show a low preference (Electivity index) for grasses at both locations (0.09, −0.67). The Manly selection index shows the same trend, with herbs presenting the higher selection index on both localities (0.58, 0.45 Old Net and Pulpit, respectively) and lowest selection index for grasses (0.09, 0.2, respectively). A chi-square analysis revealed a significant difference for both indices on both localities: Old Net (X2 = 27.204, df = 3, p < 0.001), Pulpit (X2 = 17.573, df = 3, p < 0.001). For plant analysis, we found that mule deer show a significant preference for Rhus mycrophylla (X2 = 32, df = 1, p < 0.001) at Old Net and literature suggests that CP level is 15% (Fraps & Gory ,1940), while we found no significant preferences at Pulpit (Table ).
Table 2. Selection index of native plants found in a mule deer diet study at two localities in the Chihuahuan Desert. Asterisk indicate significant selection index (forage use: forage availability)
3. Discussion
Landscape composition change may negatively influence animal populations (Wittmer et al., Citation2007), alterations to plant communities, loss of forage availability or changes in vegetation structure affect deer populations (Schaefer, Citation2003; Wittmer et al., Citation2005a; Wittmer, Sinclair, & McLellan, Citation2005b). Vegetation is not only a food source but plays an important role as predator cover. Studies in the Chihuahuan Desert suggest that vertical cover reduces predation risk, and mule deer are suggested to trade off food resources for predation risk (Esparza-Carlos et al., Citation2011; Grovenburg et al., Citation2010). Although many studies identify winter as the critical period for deer, decrease in quantity and quality of forage available during extended droughts, contribute to high deer mortality likewise (Anthony Citation1976). At the end of a 3-year drought in the Chihuahuan Desert, our results identify an important variation in cover structure for mule deer at two localities; we found a significantly higher proportion of trees and shrubs in Old Net than Pulpit. While forage quality analysis revealed that herbs and trees shrubs offer appropriate crude protein content (13.31% CP and 16.21% CP, respectively) over the minimal daily CP requirement for ruminants between 5 and 9% (Robbins, 1993), furthermore for deer an overall 7% CP has been suggested as minimum necessary for survival (Brown, Citation1994; Miller & Marchinton, Citation1995; Ramírez-Lozano, Citation2004).
During dry years, mule deer habitat use is explained by both food resources and variables related to predation risk, deer are expected to prefer cover from predators over food resources (Esparza-Carlos et al., Citation2011). At our first locality, Old Net, we found a higher percentage of trees and shrubs, specifically, Acacia berlandieri, Flourensia cernua, Fouquieria splendens, Larrea tridentata, Prosopis glandulosa, and Ziziphus obtusifolia. With more vertical cover, Old Net may offer better cover from both predators and hunters. Other studies support this finding suggesting that female mule deer in the Chihuahuan Desert prefer areas with plant associations including P. glandulosa and L. tridentata, and two more plants not present in our study location (Perez-Solano et al Citation2017). Furthermore, Old Net presents slopes ranging from 10º to 25º while Pulpit presents slopes of less than 1º. Game activity along with predator’s density at Gemelos ranch might be an important pressure source driving deer to localities like Old Net with more pronounced slopes. During 2012 and 2013, the ranch recorded an unusual high presence of cougars, Puma concolor, which is the main predator for mule deer in the area. Proximity to hills seems to be an important characteristic when mule deer assess predation risk increasing their feeding apprehension (Esparza-Carlos et al., Citation2016). Even when deer might prefer shallower slopes (Perez-Solano et al. Citation2017), mule deer do tend to select steep slopes (Marshal, Bleich, Krausman, Reed, & Adrew, Citation2006). Mule deer on this study reside in a game ranch and steeper slopes with vertical cover may appeal more to individuals in search of cover from hunters (Le Saout et al., Citation2014).
Extended drought possibly decreases nutritional quality of forage available (Lashley & Harper, Citation2012) thus affecting deer plant selection. As others (reference), we found that deer consumed a high percentage of herbs and grasses in the 2013 diet (73%), specifically Ditaxis serrata (herbaceous) showed an unusually high frequency (39%). However, this plant was found in collected feces exclusively in its seed form; we found no plant epidermal tissue. Herbaceous plants with new growth leaves are relatively easy to digest, and some are further packaged with a high nutritional content (Feldhamer & McShea, Citation2012). D. serrata showed 8.0% CP, the lowest content of herbaceous plants, but still considered an acceptable level above the 7% suggested (Ramírez-Lozano, Citation2004). Deer being concentrate selectors (Zimmerman, Jenks, & Leslie, Citation2006) choosing the richest parts of consumed plants might be selecting specific parts of D. serrata that provide other nutrients not accounted for on this study.
Studies have suggested that protein content reflects seasonal and population patterns of diet quality (Verheyden et al., Citation2011). Our results suggest that forage quality is sufficient to maintain the nutritional requirements for deer; literature suggests that in general a 7% CP is necessary for minimal maintenance (Brown, Citation1994; Miller & Marchinton, Citation1995), while studies in white tailed deer suggest a CP content over 15% for more demanding life stages (Ramírez-Lozano, Citation2004). We observe a small tendency for higher percentage CP especially on tree and shrubs at Old Net (1–3%) compared with Pulpit’s. Trees and shrubs show high protein content for both years and both localities (13.31% CP); Old Net trees and shrubs presented a higher percentage (15% CP) than Pulpit’s (12% CP). During extreme drought, deer are more selective of plants to ensure meeting their nutritional requirements (Lashley & Harper, Citation2012). In general, plant CP is associated with growth stage, species, and environmental factors such as soil and rainfall (White, Citation2012). Studies found that higher CP values are usually observed during spring, but a decline is common through the summer and autumn (Campbell & Dotzenko, Citation1975; Tollefson, Shipley, Myers, & Dasgupta, Citation2011; van Soest, Citation1994; White, Citation1992). Our study was conducted during the summers 2012 and 2013 after a 3-year drought, our results might be showing a particularly low CP value for that region that is still sufficient to maintain healthy mule deer populations.
According to our forage preference and CP results, mule deer at our two localities are selecting herbs, which present higher protein content (16.21%). These results add to the body of evidence supporting the selective quality hypothesis, suggesting that deer are more selective of plant species when resources are limited (Lashley & Harper, Citation2012).
Considering that our study was conducted at the end of a 3-year drought crude protein content and forage availability provide both nutritional support (CP higher than 7% as suggested by literature) and appropriate cover (vertical) from predators and thermal challenges. Mule deer diet studies revealed a preference for herbs at both localities, suggesting that deer preferences are based on protein content. According to both anecdotal evidence from residents and local census studies, mule deer used more the Old Net locality which presents more tree-shrubs suggesting better shelter from both predators and climate challenges. These findings will help conservation agencies in the Chihuahuan Desert identify and conserve potential areas for mule deer optimal distribution; more studies are necessary that explore diet and mule deer habitat use during other seasons and to explore the role that vegetation plays in other seasons.
Additional information
Funding
Notes on contributors
Martha P. Olivas-Sánchez
DrMartha P. Olivas-Sánchez’s main work is on wildlife diet on the Chihuahuan Desert. She works at Universidad Autónoma de Ciudad Juárez, where she oversees several undergraduate and graduate theses that look into native plants and its relation to wildlife. Dr Olivas has participated in several book chapters about native plants, mushrooms and medicinal plants of the Chihuahuan Desert. Furthermore, she is part of the Animal Science masters program at UACJ on the wildlife terminal area.
References
- Alipayo, D., Valdez, R., Holechek, J. L., & Cárdenas, M. (1992). Evaluation of microhistological analysis for determining ruminant diet botanical composition. Journal of Range Management, 45, 148–152. doi:10.2307/4002773
- Andersen, R., & Linnell, J. D. (2000). Irruptive potential in roe deer: Density-dependent effects on body mass and fertility. Journal of Wildlife Management, 64, 698–706. doi:10.2307/3802739
- Anderson, E. D., Long, R. A., Atwood, M. P., Kie, J. G., Thomas, T. R., Zager, P., & Bower, R. T. (2012). Winter resource selection by female mule deer Odocoileus hemionus: Functional response to spatio-temporal changes in habitat. Wildlife Biology, 18, 153–163. doi:10.2981/11-048
- Anthony, R. G. (1976). Influence of drought on diets and numbers of desert deer. Journal of Wildlife Management, 40(1), 140–144. doi:10.2307/3800168
- Anthony, R. G., & Smith, N. S. (1974). Comparison of rumen and fecal analysis to describe deer diets. Journal of Wildlife Management, 38, 535–540. doi:10.2307/3800886
- Anthony, R. G., & Smith, N. S. (1977). Ecological relationship between mule deer and white-tailed deer in southeastern Arizona. Ecological Monographs, 47, 255–277. doi:10.2307/1942517
- Armstrong, D. P., Davidson, R. S., Perrott, J. K., Roygard, J., & Buchanan, L. (2005). Density-dependent population growth in a reintroduced population of North Island saddlebacks. Journal of Animals Ecology, 74, 160–170. doi:10.1111/j.1365-2656.2004.00908.x
- Baker, R. H. (1956). Remarks on the former distribution of animals in eastern Texas. Texas Journal Sciences, 8, 356–359.
- Bender, L. C., Boren, J. C., Halbritter, H., & Cox, S. (2013). Factors influencing survival and productivity of pronghorn in a semiarid grassland-woodland in east-central New Mexico. Human-Wildlife Interactions, 7(2), 313.
- Bowyer, R. T., Kie, J. G., & Van Ballenberhe, V. (1998). Habitat selection by neonatal black-tailed deer: Climate, forage or risk of predation? Journal of Mammalogy, 79, 415–425. doi:10.2307/1382972
- Bremmer, L., & Mulvaney, C. (1982). Total nitrogen. In R. H. Miller & D. R. Keeney (Eds.), Methods of soil analysis. Part 2. Chemical and microbiological properties (pp. 595–634). Madison, WI.
- Brown, J. S., Laundre, J. W., & Gurung, M. (1999). The ecology of fear; optimal foraging, game theory, and trophic interactions. Journal Mammal, 80, 385–399. doi:10.2307/1383287
- Brown, R. D. (1994). The nutrients for survival and growth. In D. Gerlach, S. Atwater, & J. Schnell (Eds.), Deer (pp. 203–207). Mechanicsburg, USA: Stackpole Books.
- Campbell, I. S., & Dotzenko, A. D. (1975). Evaluating forage quality of pastures. Journal of Range Management, 149–151. doi:10.2307/3897449
- Carpenter, L. H. 1998. Deer in the west. In: J. C. deVos Jr. [ED.]. Proceedings of the 1997 Deer/Elk Workshop Rio Rico, Arizona. Phoenix, AZ: Arizona Game and Fish Department. p 1–10.
- Clements, C. D., & Young, J. A. (1997). A viewpoint: Rangeland health and mule deer habitat. Journal of Range Management, 50, 129–138. doi:10.2307/4002369
- Creel, S., Winnie, J., Jr, Maxwell, B., Hamlin, K., & Creel, M. (2005). Elk alter habitat selection as an antipredator response to wolves. Ecology, 86, 3387–3397. doi:10.1890/05-0032
- Esparza-Carlos, J. P., Laundré, J. W., Hernández, L., & Iñiguez-Dávalos, L. I. (2016). Apprehension affecting foraging patterns and landscape use of mule deer in arid environments. Mammalian Biological, 81(6), 543–550. doi:10.1016/j.mambio.2016.07.006
- Esparza-Carlos, J. P., Laundré, J. W., & Sosa, V. J. (2011). Precipitation impacts on mule deer habitat use in the Chihuahuan Desert of Mexico. Journal of Arid Environment, 75(11), 1008–1015. doi:10.1016/j.jaridenv.2011.04.030
- Feldhamer, G. A., Drickamer, L. C., Vessey, S. H., & Merritt, J. F. (1999). Mammalogy: Adaptation, diversity, and ecology. Boston, MA, USA: WCB McGraw-Hill.
- Feldhamer, G. A., & McShea, J. W. (2012). Deer: The Animal Answer Guide. The Johns Hopkins Univ. Press.
- Fraps, G. S., & Cory, V. L. (1940). Composition and utilization of range vegetation of Sutton and Edwards counties. Bulletin , 58, 39. College Station, TX: Texas Agricultural Experiment Station.
- Gill, R. B. (2001). Declining mule deer populations in Colorado: Reasons and responses. Special Report, 77, 36. Denver, CO: Colorado Division of Wildlife.
- Godvik, I. M., Loe, L. E., Vik, J. O., Veiberg, V., Langvatn, R., & Mysterud, A. (2009). Temporal scales, tradeoffs, and functional responses in red deer habitat selection. Ecology, 90, 699–710. doi:10.1890/08-0576.1
- Goldsmith, A. E. (1990). Vigilance behavior of pronghorns in different habitats. Journal Mammal, 71, 460–462. doi:10.2307/1381961
- Grovenburg, T. W., Jacques, C. N., Klaver, R. W., & Jenks, J. A. (2010). Bed site selection by neonate deer in grassland habitats on the northern great plains. Journal of Wildlife Management, 74(6), 1250–1256. doi:10.1111/j.1937-2817.2010.tb01245.x
- Heffelfinger, J. R., & Messmer, T. A. (2003). Introduction. In J. C. deVos Jr., M. R. Conover, & N. E. Headrick (Eds.), Mule deer conservation: Issues and management strategies (pp. 1–12). Logan, UT: Berryman Institute Press.
- Hofmann, R. R. (1989). Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologica, 78(4), 443–457. doi:10.1007/BF00378733
- Holechek, J. L. (1982). Evaluation of microhistological analyses for determining ruminant diet botanical composition. Journal of Range Management, 36, 305–311.
- Instituto Nacional de Estadística y Geografía [INEGI]. 2013. INEGI homepage. Retrieved May 7, 2013 from http://www.inegi.org.mx/
- Ivlev, V. S. (1961). Experimental ecology of the feeding of fishes. New Haven: Yale University Press.
- Klein, D. R. (1985). Population ecology: The interaction between deer and their food supply. Biology of deer populations.
- LaGory, K. E. (1987). The influence of habitat and group characteristics on the alarm and flight response of white-tailed deer. Animals Behavior, 35, 20–25. doi:10.1016/S0003-3472(87)80206-3
- Laliberte, A. S., & Ripple, W. J. (2004). Range contractions of North American carnivores and ungulates. AIBS Bulletin, 54(2), 123–138.
- Lashley, M. A., & Harper, C. A. (2012). The effects of extreme drought on native forage nutritional quality and white-tailed deer diet selection. Southeastern Natural, 11(4), 699–710. doi:10.1656/058.011.0409
- Le Saout, S., Padié, S., Chamaillé-Jammes, S., Côté, S., Morellet, N., Pattison, J., … Martin, J.-L. (2014). Short-term effects of hunting on naïve black-tailed deer (Odocoileus hemionus sitkensis): Behavioural response and consequences on vegetation growth. Canada Journal Zoological, 92(11), 915–925. doi:10.1139/cjz-2014-0122
- Lendrum, P. E., Charles, J., Anderson, R., Long, R. A., Kie, J. G., & Bowyer, R. T. (2012). Habitat selection by mule deer during migration: Effects of landscape structure and natural-gas development. Ecosphere, 5(9), 117.
- Lima, S. L., & Dill, L. M. (1990). Behavioral decisions made under the risk of predation: A review and prospectus. Canada Journal Zoological, 68, 619–640. doi:10.1139/z90-092
- Loison, A., & Langvatn, R. (1998). Short- and long-term effects of winter and spring weather on growth and survival of red deer in Norway. Oecologia, 116(4), 489–500. doi:10.1007/s004420050614
- Long, E. S., Diefenbach, D. R., Rosenberry, C. S., Wallingford, B. D., & Grund, M. D. (2005). Forest cover influences dispersal distance of white-tailed deer. Journal of Mammalogy, 86, 623–629. doi:10.1644/1545-1542(2005)86[623:FCIDDO]2.0.CO;2
- Manly, B., Miller, P., & Cook, L. (1972). Analysis of a selective predation experiment. American Natural, 106, 719–736. doi:10.1086/282808
- Marshal, J. P., Bleich, V. C., Krausman, P. R., Reed, M. L., & Adrew, N. G. (2006). Factors affecting habitat use and distribution of desert mule deer in an arid environment. Wild Social Bulletin, 34(3), 609–619. doi:10.2193/0091-7648(2006)34[609:FAHUAD]2.0.CO;2
- McNay, R. S., & Voller, J. M. (1995). Mortality causes and survival estimates for adult female Columbian black-tailed deer. The Journal of Wildlife Management, 59, 138–146. doi:10.2307/3809126
- Miller, K. V., & Marchinton, R. L. (1995). Quality whitetails: The why and how of quality deer management. Mechanicsburg.
- Monteith, K. L., Bleich, V. C., Stephenson, T. R., Pierce, B. M., Conner, M. M., Klaver, R. W., & Bowyer, R. T. (2011). Timing of seasonal migration in mule deer: Effects of climate, plant phenology, and life-history characteristics. Ecosphere., 2(4), 47. doi:10.1890/ES10-00096.1
- Mostacedo, B., & Fredericksen, T. (2000). Manual of basic methods manual of sampling and analysis. Plant ecology. Universidad de Santa Cruz Bolivia Press.
- Muller-Dumbois, D., & Ellenberg, H. (1974). Aims and methods of vegetation. New York, USA: John Wiley.
- Mysterud, A., & Østbye, E. (1999). Cover as a habitat element for temperate ungulates: Effects on habitat selection and demography. Wildlife Society Bulletin, 27, 385–394.
- Neter, J., Wasserman, W., & Kutner, M. H. (1990). Applied linear statistical models. Homewood, IL: Richard D. Irwin.
- Pérez-Solano, L. A., Gallina-Tessaro, S., & Sánchez-Rojas, G. (2016). Individual variation in mule deer (Odocoileus hemionus) habitat and home range in the Chihuahuan Desert, Mexico. Journal Mammalian, 97(4), 1228–1237. doi:10.1093/jmammal/gyw075
- Pérez-Solano, L. A., García-Feria, L. M., & Gallina-Tessaro, S. (2017). Factors affecting the selection of and displacement within core areas by female mule deer (Odocoileus hemionus) in the Chihuahuan Desert, Mexico. Mammalian Biological, 87, 152–159. doi:10.1016/j.mambio.2017.08.005
- Petriere, M. D., Collins, S. L., Gutzler, D. S., & Moore, D. M. (2014). Regional trends and local variability in monsoon precipitation in the northern Chihuahuan Desert, USA. Journal of Arid Environment, 103, 63–70. doi:10.1016/j.jaridenv.2014.01.005
- Pierce, B. M., Bowyer, R. T., & Bleich, V. C. (2004). Habitat selection by mule deer: Forage benefits or risk of predation? The Journal of Wildlife Management, 68, 533–541. doi:10.2193/0022-541X(2004)068[0533:HSBMDF]2.0.CO;2
- Ramírez-Lozano, R. G. (2004). Nutrición del Venado Cola Blanca [White tailed deer nutrition]. Universidad Autónoma de Nuevo León. Unión Ganadera Regional de Nuevo León, Fundación Produce Nuevo León, A.C. Monterrey. Press.
- Robbins, C. T. (1993). Wildlife feeding and nutrition. San Diego, CA: Academic Press.
- Sánchez-Rojas, G., & Gallina, S. (2000a). Factors affecting habitat use by mule deer (Odocoileus hemionus) in the central part of the Chihuahuan Desert, Mexico: An assessment with univariate and multivariate methods. Ethology Ecology Evolution, 12, 405–417. doi:10.1080/08927014.2000.9522795
- Sánchez-Rojas, G., & Gallina, S. (2000b). Mule deer (Odocoileus hemionus) density in a landscape element of the Chihuahuan Desert, Mexico. Journal of Arid Environments, 44, 357–368. doi:10.1006/jare.1999.0605
- Sánchez-Rojas, G., & Gallina, S. (2007). La metapoblación del venado bura en la reserva de la biosfera Mapimí, México: Consideraciones para su conservación. [Mule deer metapopulation in Mapimi Biosphere Reserve, Mexico: Conservation considerations]. Universidad de Alicante. Centro Iberoamericano de la Biodiversidad. Cuadernos de biodiversidad, 22, 7–15.
- Schaefer, J. A. (2003). Long-term range recession and the persistence of caribou in the Taiga. Conservation Biological, 17, 1435–1439. doi:10.1046/j.1523-1739.2003.02288.x
- Sherlock, M. G., & Fairley, J. S. (1993). Seasonal changes in the diet of red deer Cervus elaphus in the Connemara National Park. Biologic Environment Proceedings Royal Irish Academic, 93B(2), 85–90.
- Sorensen, T. (1948). A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Biologiske Skirfter, 5, 1–34.
- Sosa, M., Galarza, J. L., Toutcha, L., Soto, R., & Puga, S. (2006). Clasificación de las comunidades vegetales en la región árida del estado de Chihuahua. México [Vegetal communities classification in the Chihuahua arid region, Mexico]. Ecología Aplicada, 5:, 002. doi:10.21704/rea.v5i1-2.317
- Sparks, D., & Malechek, J. (1968). Estimating percentage dry weight in diets using a microscopic technique. Journal of Range Management, 21, 264–265. doi:10.2307/3895829
- Tollefson, T. N., Shipley, L. A., Myers, W. L., & Dasgupta, N. (2011). Forage quality’s influence on mule deer fawns. Journal of Wildlife Management, 75(4), 919–928. doi:10.1002/jwmg.113
- Uzal, A., Walls, S., Stillman, R. A., & Diaz, A. (2014). Sika deer distribution and habitat selection: The influence of the availability and distribution of food, cover, and threats. European Journal Wildlife Researcher, 59, 563–572. doi:10.1007/s10344-013-0705-z
- van Soest, P. J. (1994). Nutritional ecology of the ruminant. Itaca, NY: Cornell Univ. Press.
- Verheyden, H., Aubry, L., Merlet, J., Petibon, P., Chauveau-Duriot, B., Guillon, N., & Duncan, P. (2011). Faecal nitrogen, an index of diet quality in roe deer capreolus capreolus? Wildlife Biology, 17, 166–175. doi:10.2981/10-111
- Weber, M., & Galindo, C. (2005). Venado cola blanca. Los mamíferos silvestres de México. In G. Ceballos & G. Oliva (Eds.), Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (pp. 517–521). México, D. F: Fondo de Cultura Económica.
- White, R. G. (1992). Nutritional in relation to season, lactation, and growth of north temperate deer. In R. Brown (Ed.), The biology of deer (pp. 407–418). New York, NY: Springer.
- White, T. C. R. (2012). The inadequate environment: Nitrogen and the abundance of animals. New York, NY: Springer.
- Wittmer, H. U., McLellan, B. N., Seip, D. R., Young, J. A., Kinley, T. A., Watts, G. S., & Hamilton, D. (2005a). Population dynamics of the endangered mountain ecotype of woodland caribou (Rangifer tarandus caribou) in British Columbia, Canada. Canadian Journal of Zoology, 83, 407–418. doi:10.1139/z05-034
- Wittmer, H. U., Mclellan, B. N., Serrouya, R., & Apps, C. D. (2007). Changes in landscape composition influence the decline of a threatened woodland caribou population. The Journal of Animal Ecology, 76, 568–579. doi:10.1111/j.1365-2656.2007.01220.x
- Wittmer, H. U., Sinclair, A. R. E., & McLellan, B. N. (2005b). The role of predation in the decline and extirpation of woodland caribou. Oecologia, 144, 257–267. doi:10.1007/s00442-005-0055-y
- Zimmerman, T. J., Jenks, J. A., & Leslie, D. M., Jr. (2006). Gastrointestinal morphology of female white-tailed and mule deer: Effects of fire, reproduction, and feeding type. Journal Mammal, 87(3), 598–605. doi:10.1644/05-mamm-A-356R1.1
- Zweifel-Schielly, B., Leuenberger, Y., Kreuzer, M., & Suter, W. (2012). A herbivore’s food landscape: Seasonal dynamics and nutritional implications of diet selection by a red deer population in contrasting Alpine habitats. Journal of Zoology, 268, 68–80. doi:10.1111/j.1469-7998.2011.00853.x

