Abstract
We report two cases of poliomyelitis in which an anterolateral thigh myocutaneous free flap was harvested from the paralytic limb for oral reconstruction. We observed a decrease in the pedicle diameter of the anterolateral thigh flap, but the blood supply to the skin paddle was adequate.
Introduction
Poliomyelitis was a widespread and feared condition during the first half of the twentieth century. Children were often afflicted with poliomyelitis sequelae that were characterized by flaccid muscle paralysis. Paralysis of one or both lower extremities is a common manifestation of late poliomyelitis sequelae. In this study, we report two cases with oral cancer and paralytic lower limbs secondary to poliomyelitis sequelae who each underwent anterolateral thigh (ALT)-free flap harvesting from their paralytic limbs for use in reconstructing oral defects that were present after a tumor ablation. This study provides details regarding our experience and the difficulties associated with flap harvesting.
Case reports
Case 1
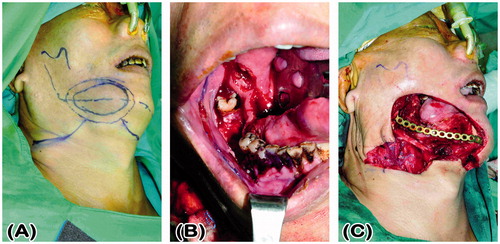
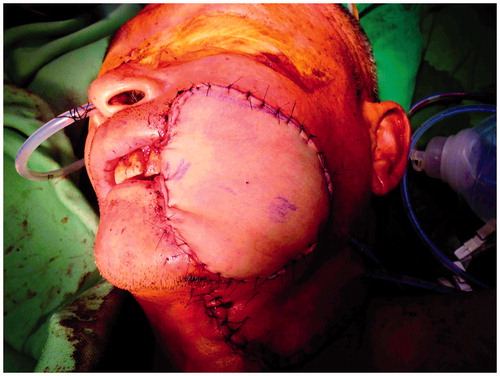
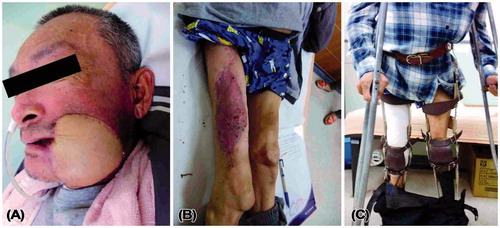
A 53-year-old wheelchair-bound man presented with a cT4aN0M0 right lower gingival squamous cell carcinoma (53 × 36 × 40 mm). He had suffered from childhood-onset poliomyelitis that was complicated with atrophy of both the lower extremities. His medical history included pure motor lacunar infarction with left hemiplegia for 5 years, with preoperative, bilateral, Grade 4 lower limb muscle power using the Medical Research Council’s grading system.[Citation1] Tumor ablation, segmental mandibulectomy from the symphysis to the right ramus, and a right supraomohyoid neck dissection were performed, resulting in a through-and-through right buccal defect. A reconstruction plate was used to reinforce the strength of the mandible (). The right buccal defect was reconstructed using a left ALT-free flap with a portion of the vastus lateralis muscle and six cutaneous perforators. The skin paddle for the mucosa defect measured 8 × 8 cm and for the cheek defect measured 11 × 9 cm in a snowman configuration. The descending branch of the lateral circumflex femoral artery was anastomosed to the right superior thyroid artery, and the two comitant veins of the descending branch of the lateral circumflex femoral artery were anastomosed to the right superior thyroid vein and a branch of right internal jugular vein. A split-thickness skin graft was harvested from the left medial thigh and was used to close the donor site defect. The flap survived, and the patient’s post-operative course was uneventful. The total duration of hospitalization was 11 days. After 2 years and 11 months of follow-up, the flap has a bulky appearance but there is no exposure of the reconstruction plate or trismus, and the grafted donor site is unobtrusive (). The bilateral lower limb muscle power remained at Grade 4.
Case 2
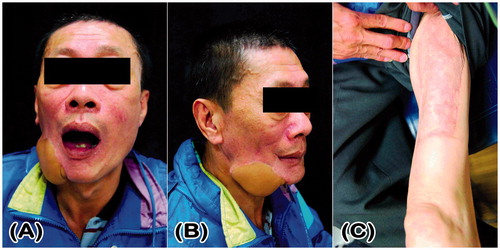
A 51-year-old man with atrophy of the muscles in both the lower extremities () presented with a cT4aN0M0 left buccal squamous cell carcinoma (35 × 23 × 20 mm) with skin invasion. He had suffered from poliomyelitis in his childhood, which was followed by a flexion contracture of both the hips and knees. He underwent operations to release the flexion contracture of his hips and knees approximately 40 years ago and could walk well with calipers and crutches. The preoperative muscle power in his right and left lower limbs was Grade 0 and 1, respectively. Wide tumor excision, marginal mandibulectomy, and left supraomohyoid neck dissection were performed, resulting in a through-and-through left buccal defect. An ALT-free flap that included a portion of the vastus lateralis muscle was harvested from his right lower limb to reconstruct the buccal defect. The skin island measured 20 × 10 cm and included two cutaneous perforators (). The descending branches of the lateral circumflex femoral vessels were anastomosed in an end-to-end fashion to the left superior thyroid artery and two comitant veins. The donor site was resurfaced using a split-thickness skin graft that was harvested from the right medial thigh. Arterial vasospasm occurred intraoperatively and was subdued using 2% xylocaine irrigation. The flap became pink in color, and the capillary refill returned to a normal level (). Post-operatively, intravenous infusion of prostaglandin E1 was prescribed to promote skin flap blood flow. The flap survived, and the patient’s post-operative course after 17 days of hospitalization was uneventful. At the 3-month follow-up, the patient was satisfied with the reconstructed cheek and there were no donor site complications. The muscle power in the right lower limb remained at Grade 0 and he could walk well with calipers and crutches ().
Figure 3. Clinical photograph of Case 2 showing flaccid paralysis with muscle atrophy in both the lower extremities.
Operative technique
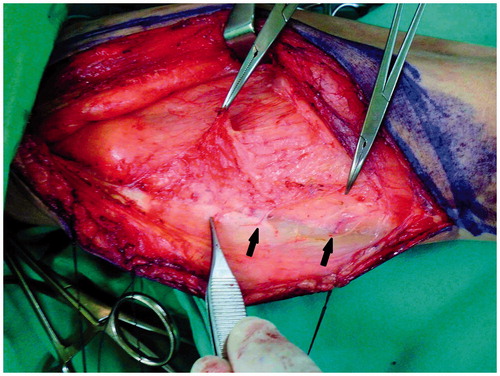
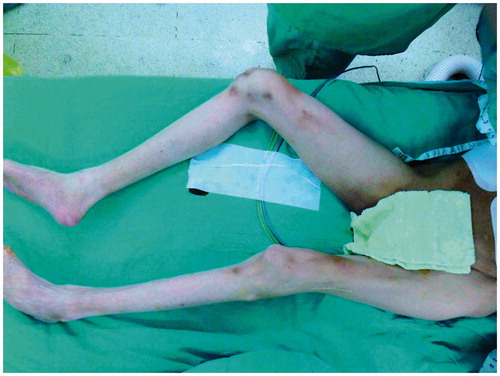
A line was drawn between the anterior superior iliac spine and superolateral corner of the patella, and a longitudinal skin incision of approximately 3–4 cm was medially made using normal procedures. A skin incision down to and through the thigh fascia will expose the rectus femoris in the normal lower limb but may encounter the vastus medialis muscle in a patient with poliomyelitis because of significant muscle atrophy. The quadriceps muscle had a pale, yellowish, fat-like color (), and the intermuscular septum was not clear. Inexperienced surgeons may mistake the vastus medialis muscle for the rectus femoris muscle. In a patient who has had poliomyelitis, a lateral 1–2 cm subfascial dissection is required to clearly identify the rectus femoris muscle. Following identification, the dissection proceeded along a subfascial plane laterally toward the intermuscular septum between the rectus femoris and vastus lateralis muscles. Medial retraction of the rectus femoris muscle revealed the vascular pedicle with a descending branch of the lateral circumflex femoral vessels. In our experience, the diameter of the vascular pedicle is less than 1 mm and smaller in patients with a history of poliomyelitis compared with patients without a history of poliomyelitis. The thigh skin was then raised and laterally retracted by a subfascial plane dissection, exposing the septocutaneous and/or musculocutaneous perforators. The number and size of cutaneous perforators were similar to normal ambulating limbs. Distal to proximal intramuscular dissection of the musculocutaneous perforator was then initiated. The intramuscular dissection was easier and more rapid than in a normal limb because there were fewer vastus lateralis muscle branches and less muscle bleeding. The distal end of the descending branch of the lateral circumflex femoral vessels was ligated, and a thigh fasciocutaneous or myocutaneous ALT flap was well prepared for harvesting.
Histological examination
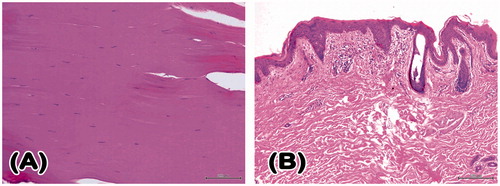
On pathological examination of the paralytic limbs in Case 2, the thigh skin tissue revealed chronic inflammatory cell infiltration and fibrosis. As expected, the evaluation of the vastus lateralis muscle revealed degeneration and fibrosis ().
Figure 7. Histopathological examination of a vastus lateralis muscle specimen from Case 2’s paralytic lower limb revealed denervation muscle fiber atrophy with striation loss and collagen fiber accumulation (H&E 200×) (A). Histological sections of paralytic thigh skin showed the presence of chronic perivascular inflammatory cell infiltration and fibrosis. (H&E 100×) (B). H&E: haematoxylin and eosin.
Results
All flaps survived, and both the patients had uneventful recoveries with no complications.
Discussion
Poliomyelitis is caused by an infection with poliovirus, which colonizes the gastrointestinal tract and more specifically the intestine and oropharynx. The disease is highly transmitted by fecal–oral (intestinal source) and oral–oral (oropharyngeal source) contamination. However, the mechanism of the spread of poliovirus to the central nervous system is not well understood. Virus invasion causes motor neuron death, particularly in the anterior horn cells of the spine. When a spinal motor neuron dies, Wallerian degeneration occurs, leading to paralysis and atrophy of the muscle that is innervated by this motor neuron. Lower limbs are more often affected than upper limbs, and proximal muscles are more frequently affected than distal ones. The patients experience symptoms of progressive muscle weakness, pain, fatigue, atrophy, and decreased ambulatory abilities.[Citation2] This progressive deterioration is called post-polio syndrome and generally occurs approximately 35 years after the acute onset of poliomyelitis.
Progressive wasting and weakness commonly affects one or both the lower limbs. It is important to preserve the healthy upper extremities and trunk to minimize any potential functional loss, and the use of the paralyzed and dysfunctional lower extremities as the donor site for flap harvesting is considered a logical strategy. ALT, posterior tibial, and fibular flaps can be harvested from paralytic lower limbs for head and neck defect reconstruction without additional functional loss. A large skin paddle that incorporates the vastus lateralis muscle of the ALT flap is suitable for reconstructing various buccal through-and-through defects. However, an ALT flap is difficult to harvest from patients with frog-leg positioning because of severe flexion contracture of the hip and knee. A posterior tibial flap may serve as an alternative to overcome this problem. Drs. Ulusal [Citation3] and Mardini [Citation4] reported one and three cases, respectively, using posterior tibial flaps from the paralyzed side of patients with late poliomyelitis sequelae for use in head and neck reconstruction. Mardini et al. [Citation4] indicated that the perforators and vascular pedicles of the posterior tibial flap are much smaller and the muscles are more fibrotic, making the dissection of musculocutaneous perforators more difficult than with a limb with normal ambulation. Three cases of head and neck defect reconstruction using free ALT flaps that were harvested from paralytic limbs have been described.[Citation3,Citation5,Citation6] In each case, the perforators and vascular pedicles were of normal size. However, in both our cases involving harvesting an ALT flap from a paralytic limb, the diameter of the pedicle was decreased. The objective measurements of pedicle diameter in paralytic limbs were less than 1 mm, and are at least 2 mm in normal patients without any muscle disease.[Citation7] We observed a reduced vascular supply to an atrophied vastus lateralis muscle, and fewer intramuscular branches were encountered during the dissection of the musculocutaneous perforators of the ALT flaps. It appears reasonable that the vascular pedicle was smaller in size, which contributed to the reduced blood supply for a reduced muscle volume. However, the size and number of cutaneous perforators was not reduced when compared with a normal limb, and the blood supply to the skin paddle was adequate. Moreover, we observed that the dissection of the musculocutaneous perforators was smoother and more rapid than that of a normal limb because of the presence of fewer perforator intramuscular branches and minimal muscle bleeding.
Another concern with ALT flap harvesting from a paralytic limb is that of a donor site morbidity, such as donor site complications, knee extension weakness and joint instability, and lateral thigh sensory loss. Donor site-related complications may include seroma, hematoma, infection, wound dehiscence, and partial skin graft loss. Previous studies have indicated that damage to the lower motor neurons results in the downregulation of T-cell-mediated cutaneous inflammation [Citation8] and sympathetic tone loss resulting in the dilation and engorgement of the cutaneous venous capacitance beds.[Citation9] Theoretically, the incidence of delayed wound healing should be higher in the paralytic limbs of patients with poliomyelitis. However, among the limited published case reports [Citation3–6] and in our study, no donor site complications were observed among patients with poliomyelitis (). The second issue dealt with altered knee stability and knee extension weakness after removing the vastus lateralis muscle. The quadriceps muscle comprises four muscles that meet just above the patella to form the quadriceps tendon, the vastus lateralis being the largest muscle of the quadriceps femoris. Knee stability could deteriorate after removing the vastus lateralis. However, Kuo et al. [Citation10] revealed no statistically significant decrease in knee stability, except mild knee extension weakness after ALT myocutaneous flap transfer in limbs with normal ambulation. Maximum functional preservation would be achieved with totally muscle-sparing techniques, and sacrifice of any muscle of the involved limb may theoretically increase the disability and deteriorate the knee stability. For head and neck reconstruction, using an ALT perforator (muscle-sparing) flap is better than a myocutaneous flap in an ambulatory poliomyelitis patients to preserve maximum muscle function and minimize ambulatory disability after surgery. In our cases with through-and-through buccal defect and segmental mandibulectomy status post reconstruction plate reinforcement, the ALT flap incorporation of a section of the vastus lateralis muscle can provide more bulk to augment the cheek, obliterate the dead space, and overcome plate exposure. There was no consideration of ambulatory ability in Case 1 because he is a wheelchair-bound man, and the donor site is nonfunctional. The use of a myocutaneous flap must be carefully evaluated in ambulatory patients. In Case 2, no muscle movement of the donor site was observed preoperatively, and sacrifice of a portion of the vastus lateralis muscle in an already paralytic lower extremity appeared to be harmless for his ambulatory ability after surgery. Until now, very few studies have used this approach with paralytic limbs. In our observation, no additional post-operative functional loss was found in Case 2, and the patient was able to walk well with calipers and crutches.
Table 1. Poliomyelitis patients’ summary for free anterolateral thigh flap harvested from paralytic limbs.
At a minimum, sensation was not affected in patients with poliomyelitis because the sensory neurons are not infected by the poliovirus. Harvesting an ALT flap from a patient with poliomyelitis may cause the loss of lateral thigh sensation if the lateral cutaneous femoral nerve (LCFN) is damaged during flap harvesting. Hanasono et al. [Citation11] reported that the incidence of sensory loss after flap harvesting is as high as 84%, with only 5% of patients regaining sensation. Corujo et al. preoperatively performed LCFN blocks using a 22-gauge, 5 cm insulated needle under ultrasound guidance to verify the indemnity of the cutaneous innervation of the thigh.[Citation12] We did not perform preoperative sensitive testing of the ALT area to determine the LCFN territory of distribution. This does not appear to be a critical problem because the majority of the ALT skin is innervated by LCFN, which is included in the harvested ALT flap for extensive head and neck defect reconstructions. However, for small flaps, sparing the LCFN is necessary to avoid loss of sensation to a wide area of the lateral thigh.
We believe that harvesting an ALT flap from a paralytic lower limb is easier and more rapid than harvesting from normal limbs. Although the diameter of the vascular pedicles of the ALT flap is smaller in patients with post-polio syndrome, anastomosis of these small vessels is not difficult under microscope magnification and the blood supply to the flap does not appear to be compromised. In summary, harvesting an ALT flap from a paralytic or nonfunctional extremity appears to be a safe and feasible procedure.
Disclosure statement
The authors have no financial interest to declare in relation to the content of this article.
References
- Medical Research Council. Aids to the examination of the peripheral nervous system, Memorandum no. 45. London: Her Majesty’s Stationery Office; 1981.
- Howard RS. Poliomyelitis and the postpolio syndrome. BMJ. 2005;330:1314–1318.
- Ulusal BG, Ulusal AE, Yeh CJ, et al. Free flaps harvested from paralytic lower extremities in patients with late polio sequel. Plast Reconstr Surg. 2006;118:5e–7e. Discussion 8e–9e.
- Mardini S, Salgado CJ, Chen HC, et al. Posterior tibial artery flap in poliomyelitis patients with lower extremity paralysis. Plast Reconstr Surg. 2006;117:640–645.
- Chan RC, Liu HL, Chan YW. Free-flap harvesting from paralytic limbs of poliomyelitis patients—a safe and feasible option. J Plast Reconstr Aesthet Surg. 2012;65:821–823.
- Valentini V, Terenzi V, Cassoni A, et al. Anterolateral thigh flap harvested from paralytic lower extremity in a patient with late polio sequel. J Craniomaxillofac Surg. 2012;40:e5–e7.
- Arik Z, Wei FC, Lin CH, et al. Anterolateral thigh perforator flaps in head and neck reconstruction. Semin Plast Surg. 2006;20:064–072.
- Tarkowski E, Naver H, Wallin BG, et al. Lateralization of cutaneous inflammatory responses in patients with unilateral paresis after poliomyelitis. J Neuroimmunol. 1996;67:1e6.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865–869.
- Kuo YR, Jeng SF, Kuo MH, et al. Free anterolateral thigh flap for extremity reconstruction: clinical experience and functional assessment of donor site. Plast Reconstr Surg. 2001;107:1766–1771.
- Hanasono MM, Skoracki RJ, Yu P. A prospective study of donor-site morbidity after anterolateral thigh fasciocutaneous and myocutaneous free flap harvest in 220 patients. Plast Reconstr Surg. 2010;125:209–214.
- Corujo A, Franco CD, Williams JM. The sensory territory of the lateral cutaneous nerve of the thigh as determined by anatomic dissections and ultrasound-guided blocks. Reg Anesth Pain Med. 2012;37:561–564.